Abstract
Study Objectives:
Sarcopenia, or loss of muscle mass, occurs with aging and results in frailty, disability, cardiovascular disease, and insulin resistance. Recently, researchers have asserted that sarcopenia is not an inevitable process, but is a modifiable condition. Adequate sleep duration is also important to maintain good physical and mental health. Therefore, the aim of our study was to examine the association between sleep duration and sarcopenia in Korean adults.
Methods:
Data from 16,148 participants (7,158 men and 8,990 women) were analyzed from the 2008–2011 Korean National Health and Nutrition Examination Survey (KNHANES). We defined sarcopenia as one standard deviation below the sex-specific means of the appendicular skeletal muscle/height-squared values of a young reference group. Participants were categorized into 5 groups according to sleep duration. The odds ratios (OR) and 95% confidence intervals (95% CI) for sarcopenia according to sleep duration were calculated using multiple logistic regression analysis.
Results:
The prevalence of sarcopenia was 14.3% in the total population (males 18.7%, females 9.7%). Compared to the 7 hours of sleep group, the OR (95% CI) for sarcopenia of the long sleep duration group (9 hours or more) was 1.589 (1.100–2.295) after controlling for confounding factors. From the results of subgroup analysis, high-risk groups for sarcopenia are as follows: 40–64 years old (OR = 1.868), normal body mass index (OR = 1.516), smoking (OR = 2.219), no regular exercise (OR = 1.506) in long sleepers.
Conclusions:
Long sleep duration (9 hours or longer) is independently associated with sarcopenia in Korean adults.
Citation:
Kwon YJ, Jang SY, Park EC, Cho AR, Shim JY, Linton JA. Long sleep duration is associated with sarcopenia in Korean adults based on data from the 2008–2011 KNHANES. J Clin Sleep Med. 2017;13(9):1097–1104.
Keywords: inflammation, insulin resistance, sarcopenia, sleep duration
INTRODUCTION
Sarcopenia is a condition characterized by the loss of skeletal muscle mass, strength, and function.1 During normal aging in humans, muscle mass decreases by approximately 3% to 5% every 10 years after the age of 30 years.2 Especially in older people, sarcopenia is closely related to frailty, disability, and falls. Furthermore, emerging evidence suggests that sarcopenia is associated with many cardiometabolic diseases such as insulin resistance, diabetes mellitus, and cardiovascular disease.3–6 Although decline in muscle mass is an inevitable process with aging, recent studies suggest that sarcopenia is a modifiable condition, and the rate of decline of muscle mass can be slowed by adopting a healthy lifestyle. The pathophysiology of sarcopenia is multifactorial. Physical inactivity, low body weight, malnutrition, and a reduction in endocrine function have been identified as risk factors in several studies.7,8
An adequate amount of sleep plays an important role in physical health and quality of life. Therefore, researchers have tried to suggest an appropriate sleep duration. Although sleep duration varies across life span, the National Sleep Foundation has recommended that sleep duration be 7 to 8 hours.9 Many previous studies have established that both too little sleep and too much sleep are related to all-cause mortality,10 cardiovascular disease,11–13 diabetes,14 and metabolic syndrome.14,15
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although adequate sleep duration is an important factor of health management, few researchers have investigated the association between sleep duration and sarcopenia. We studied the association between sleep duration and sarcopenia.
Study Impact: We found that long sleep duration was associated with sarcopenia. In particular, this association was more apparent in middle-aged subjects.
Considering the aforementioned findings, sarcopenia may be a detrimental preclinical condition that affects the relationship between sleep duration and adverse health outcomes. The association between sleep duration and sarcopenia has not been fully examined. Thus, in this study, we aimed to investigate the association between sleep duration and sarcopenia in Korea using a nationally representative dataset.
METHODS
Study Population
The Korea National Health and Nutrition Examination Survey (KNHANES) is a national population-based survey assessing the health and nutrition status of Korean people. This survey has been conducted by the Korea Centers for Disease Control and Prevention (KCDC) since 1998 and is composed of a health interview, health examination, and nutrition survey.
Response rates in 2008, 2009, 2010, and 2011 KNHANES were 77.8%, 82.2%, 81.9%, and 80.0%, respectively; a total of 37,753 citizens (9,744 in 2008; 10,533 in 2009; 8,958 in 2010; 8,518 in 2011) participated in at least 1 of the 3 survey components. Survey items in KNHANES have partially been changed. Body composition and bone density were measured only during the period of July 2008 to May 2011 and checked in individuals who were 10 years and older.
Trained medical staff performed the health examination and interview at a mobile center for each primary sampling unit. The sampling design of this study was multistage clustered probability and the sample weights for participants were allocated to best represent all of Koreans after considering the nonresponse rate and poststratification.16
We excluded people who were not checked by dual-energy x-ray absorptiometry (younger than 10 years, n = 5,206), over the periods of January 2008 to June 2008 and June 2011 to December 2011 (n = 9,857). We also excluded patients who were younger than 20 years (n = 2,983) and 3,739 subjects who had missing data in at least one of the following components: health questionnaires, blood samples, and/or anthropometric variables. In the end, data from 16,148 participants (7,158 men and 8,990 women) were analyzed in this study. Figure 1 illustrated the data selection process. The KNHANES was approved by the Institutional Review Board of the KCDC (IRB No: 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06C), and written consent was obtained from all of the participants before this survey.
Figure 1. Data management of the study population from 2008–2011 KNHANES.
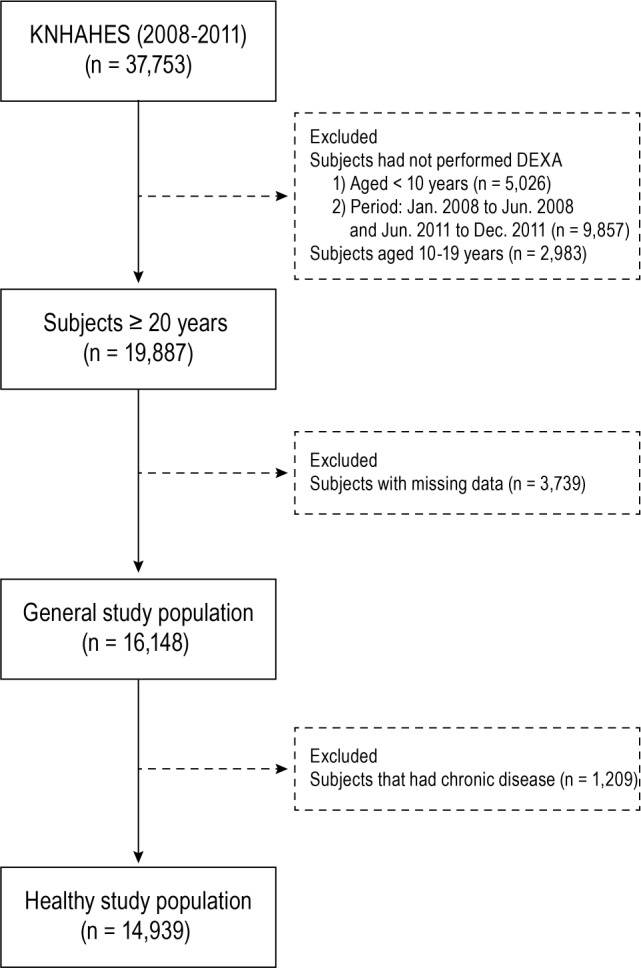
DEXA = dual-energy x-ray absorptiometry, KNHANES = Korean National Health Examination and Nutrition Survey.
Definition of Sarcopenia
In the KNHANES, appendicular skeletal mass (ASM) was measured using dual-energy x-ray absorptiometry (QDR 4500A; Hologic Inc., Bedford, Massachusetts, United States). This is one of the recommended tools for quantifying sarcopenia.17 ASM was calculated as the sum of the lean soft tissue mass (non-fat and non-bone mass) in the arms and legs, which was used to approximate skeletal muscle mass. To define the cutoff value for sarcopenia, we used the sex-specific mean and standard deviation of the ASM/height2 according to recommendations of the Asian Working Group for Sarcopenia.1,18 Also, we defined sarcopenia as 1 standard deviation below the sex-specific means of the ASM/height2 values of a young reference group (healthy men and women aged 20–39 years).1,19 The cutoff values for sarcopenia were 6.98 kg/m2 for males and 4.95 kg/m2 for females.
Sleep Duration
In this survey, sleep duration was assessed by self-report using the following question: “How long do you usually sleep a day?” Subjects were categorized into 5 groups: ≤ 5, 6, 7, 8, and more than 9 hours.
Covariates
Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. Participants wore light indoor clothes without shoes during measurement. Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2). BMI was categorized into 3 groups according to the World Health Organization and Korean Society for the Study of Obesity standards20,21: thin < 18.5 kg/m2, normal 18.5–24.9 kg/m2, and overweight/obese ≥ 25 kg/m2. Systolic blood pressure and diastolic blood pressure were measured 3 times in the right arm. Hypertension was defined as follows: systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or taking hypertension medication. Blood samples were collected in the morning after an overnight fast and analyzed using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). The subjects with diabetes were defined as follows: fasting blood glucose level ≥ 126 mg/dL or taking diabetes medication or insulin. Participants were also asked about their lifestyle behaviors, including cigarette smoking and alcohol consumption. Smoking status was categorized into “current smoking” and “no current smoking.” For alcohol consumption, we defined “alcohol intake” as drinking at least 7 servings for men and 5 servings for women 2 or more times a week. The International Physical Activity Questionnaire was adopted to determine the level of physical activity. “Regular exercise” was defined as ≥ 20 minutes of vigorous-intensity physical activity for ≥ 3 days a week, or ≥ 30 minutes of light- or moderate-intensity physical activity ≥ 5 days a week. Socioeconomic status was assessed with a face-to-face interview. Household income was grouped into quartiles and ranked from lowest to highest. Education level was categorized as follows: less than elementary school, middle school, high school, and college or more. Employment status was divided into 2 groups: employed and unemployed.
Work schedules were classified as daytime work (from 6:00 AM to 6:00 PM), evening work (from 2:00 PM to midnight), night work (from 9:00 PM to 8:00 AM), and shift work (including day-and-night rotating shift, 24-hour rotating shift, and split shift).
Statistical Analysis
Statistical analyses were applied to the sampling weights in order to represent the entire Korean population. Characteristics of the study population were analyzed using either a weighted chi-square test for categorical variables and weighted one-way analysis of variance for continuous variables. The odds ratios (OR) and 95% confidence intervals (CI) for sarcopenia were determined using multiple logistic regression analysis after adjusting confounding variables. To confirm our results, we analyzed multiple logistic regression models in both the general population and the apparently healthy population, after excluding patients with a diagnosis of cancer, chronic kidney disease, liver disease, thyroid disease, rheumatism, stroke, angina, or myocardial infarction (n = 1,209). We also analyzed multinomial logistic models to investigate the relationship between sarcopenia and sleep duration.
We further conducted subgroup analyses by sex, age, BMI, exercise, smoking, alcohol, and employment groups, and used Bonferroni correction for multiple testing. We also described the data that compare the demographic and physical information between analyzed subjects and those who were not (see Appendix 1 in the supplemental material). There was a low possibility of having selection bias in the participation rate, because we applied sampling weights to account for complex sampling.
All analyses were conducted using SPSS statistical software, version 23.0 (IBM Corp., Armonk, New York, United States). A value of P < .05 was considered statistically signifi-cant, and all statistical tests were two-sided.
RESULTS
Table 1 shows characteristics of the study population. Of the 16,148 participants included in this study, there were 7,158 men (44.3%) and 8,990 women (55.7%). The results are presented as mean ± standard error (SE) for continuous variables and numbers (percentage) for categorical variables. There were 13,843 participants (85.7%) without sarcopenia and 2,305 participants (14.3%) with sarcopenia. Of the men, 1,339 (18.7%) had sarcopenia. Of the women, 872 (9.7%) had sarcopenia. The mean ± SE of the age of the participants without sarcopenia and with sarcopenia was 44.1 ± 0.2 and 45.2 ± 0.5 years, respectively. The prevalence of sarcopenia according to sleep duration was as follows: 13.0% in those who slept ≤ 5 hours, 12.8% for 6 hours, 13.1% for 7 hours, 15.9% for 8 hours, and 20.8% for ≥ 9 hours. In this study population, the mean ± SE value of sleep duration was 6.9 ± 0.0. The associations between sleep duration and sarcopenia are presented in Table 2 and Appendix 2 in the supplemental material. Compared with the group who slept 7 hours, the corresponding OR of the group who slept more than 9 hours for sarcopenia was 1.589 (95% CI 1.100–2.295), after adjusting for age, sex, BMI, household income, regular exercise, current smoking, alcohol consumption, hypertension, work schedule, and employment status. In an apparently healthy population, these significant associations remained after adjusting the same confounding factors. In multinomial logistic regression analysis, the OR of sarcopenia group for long sleep duration (≥ 9 hours) was 1.621 (1.108–2.371) (see Appendix 2 in the supplemental material).
Table 1.
Characteristics of study population.
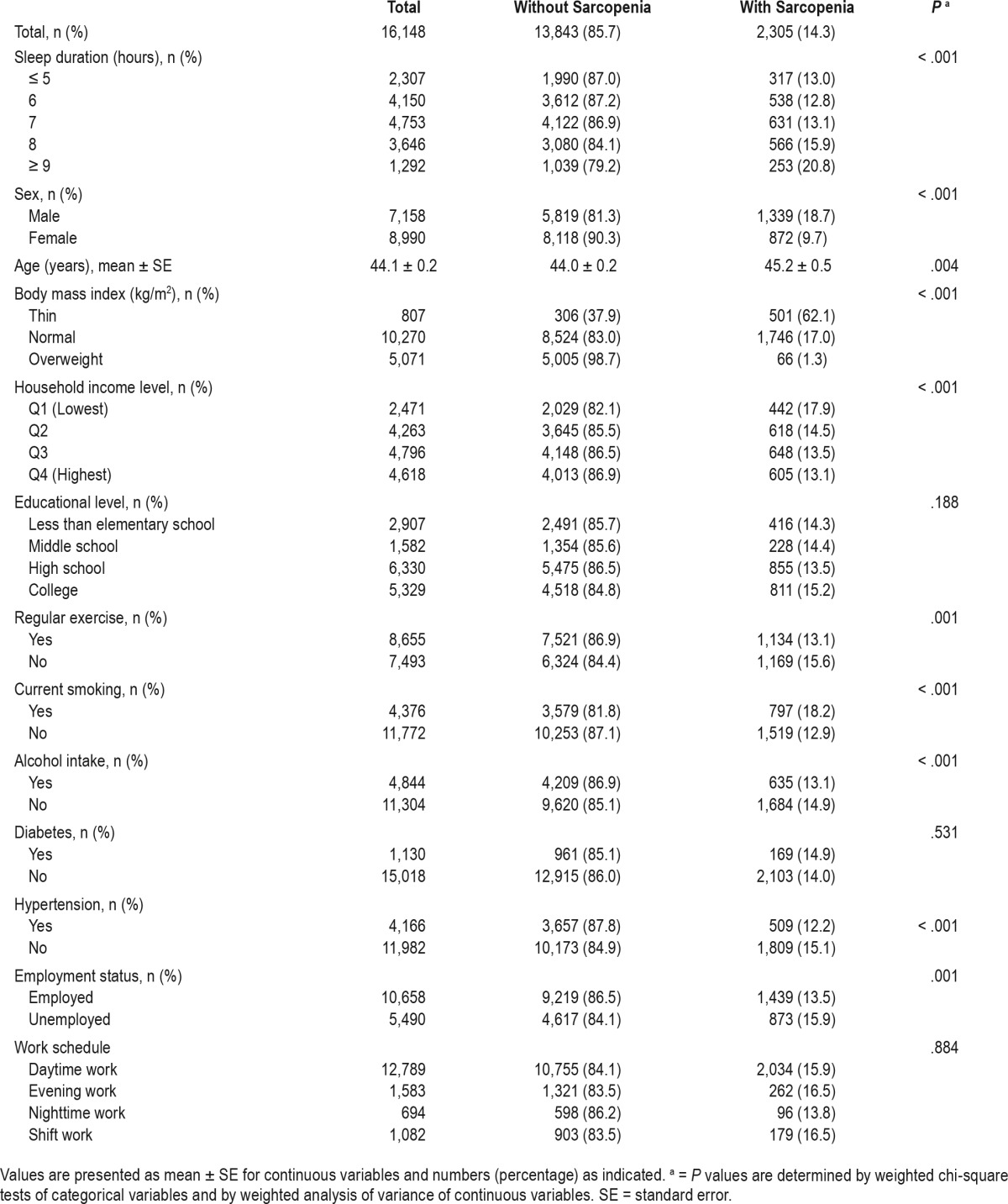
Table 2.
Adjusted odds ratios of sarcopenia from logistic regression analysis.
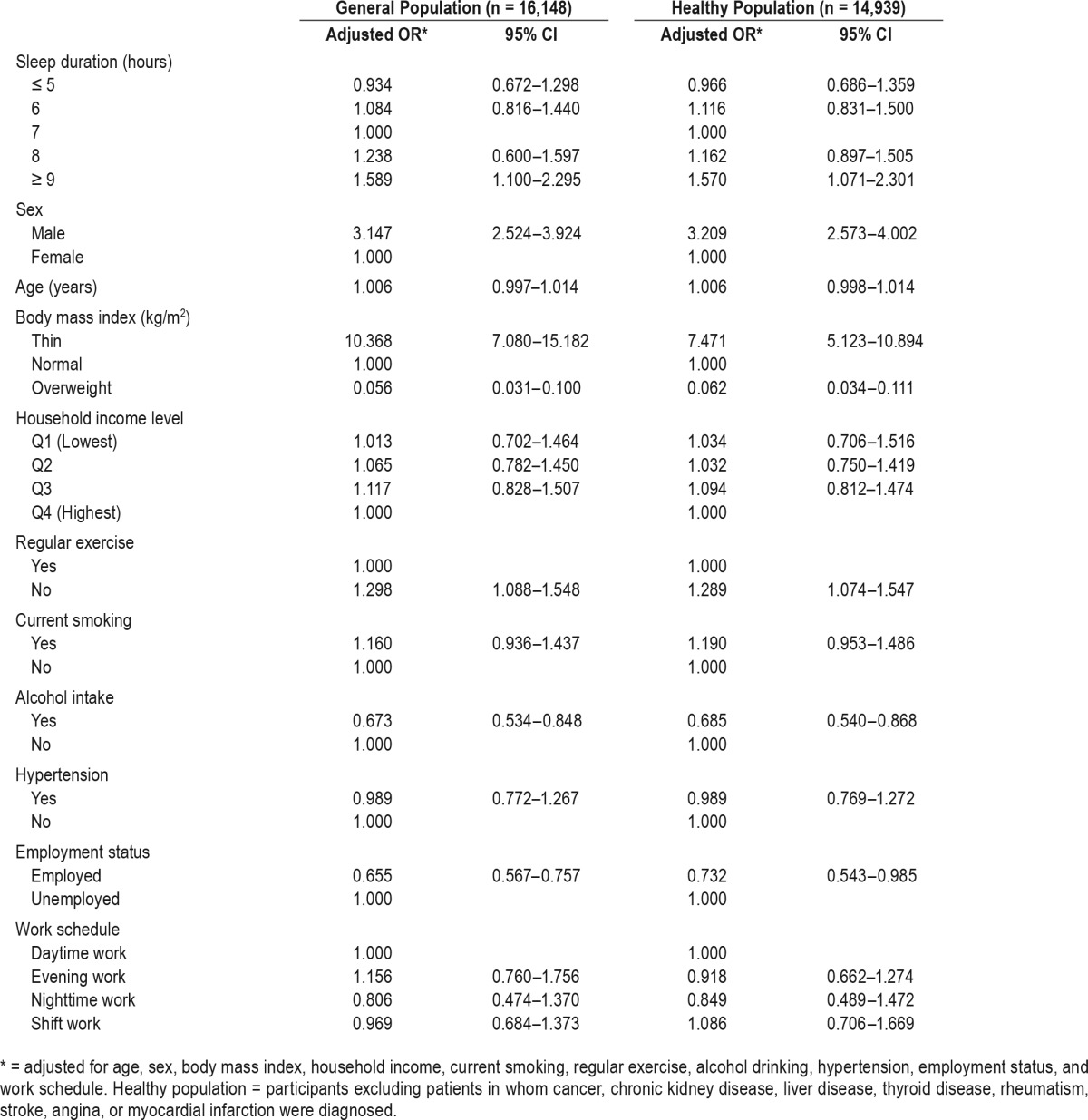
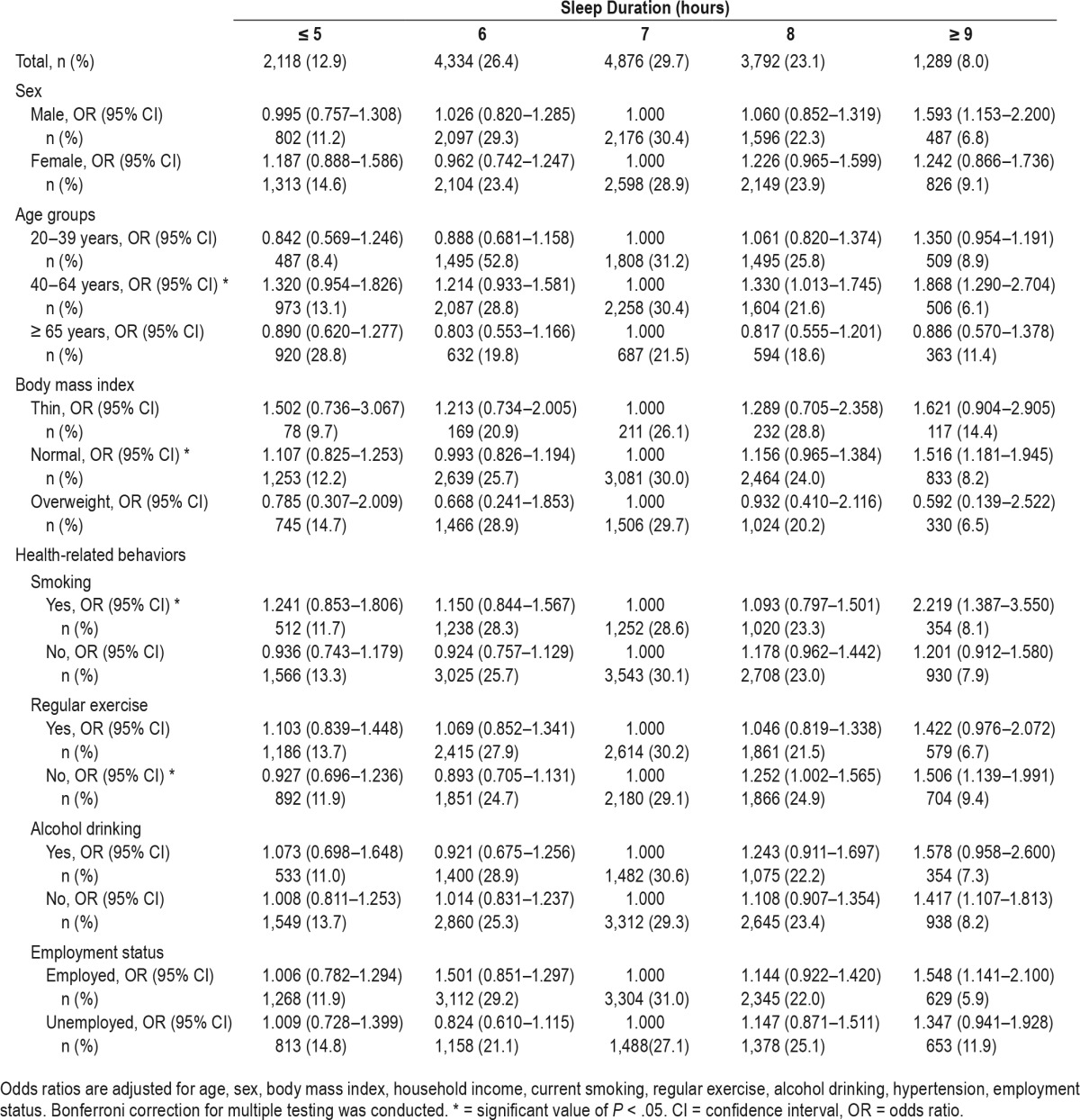
Table 3 shows the results of all adjusted logistic regression analyses between sleep duration and sarcopenia in subgroups. In subgroup analysis, when using the 7-hour sleep duration group as a reference, we found significantly positive associations between sleep duration and sarcopenia in participants with the following characteristics: middle-aged, with a 18.5 ≤ BMI < 25 kg/m2, current smokers, and did not exercise regularly.
Table 3.
Odds ratios and 95% confidence intervals of sarcopenia by sleep duration from subgroup analysis.
DISCUSSION
In the current study, we demonstrated that, compared to participants who sleep an average of 7 hours per day, those who sleep ≥ 9 hours per day were significantly more likely to have sarcopenia in the Korean population. This association was still significant after adjusting for health status (BMI, smoking, exercise, alcohol consumption, and hypertension) and socioeconomic status (age, sex, household income, employment, and work schedule). After adjusting for the same confounding factors, significant associations between sleep duration and sarcopenia were found in participants with the following characteristics: middle-aged, with 18.5 ≤ BMI < 25 kg/m2, current smokers, and did not exercise regularly. In addition, despite the lack of significant significance, U-shaped associations between sleep duration and sarcopenia were shown in most subgroups. Risk factors of sarcopenia have not yet been fully investigated, and some risk factors shown in our study are consistent with those from previous studies. Castillo et al. identified both current smoking and physical inactivity as modifiable risk factors for sarcopenia.22 Although the sex-specific prevalence of sarcopenia varies by country, many observational studies, especially for the Korean population, found a higher prevalence of sarcopenia in men.1,23,24 Some inconsistent findings existed regarding the relationship between alcohol and sarcopenia. For instance, a number of previous studies suggested that alcohol decreased muscle protein synthesis.25 However, a meta-analysis established that there was no significant association between alcohol consumption and sarcopenia (OR [95% CI] for sarcopenia in the drinking group = 0.77 [0.67–0.88]).26 Our study also showed that nonalcohol drinkers had a higher OR (OR [95% CI] = 1.417 [1.107–1.813]). Further research is needed to confirm the effects of alcohol consumption on sarcopenia. Aging and low BMI were significant risk factors for sarcopenia.27 However, in our study, the middle-aged population had higher ORs of sarcopenia. Although the reason for this discrepancy is unclear, many external factors could affect the relationship between sleep duration and sarcopenia in older people.
In subgroup analysis by BMI group, long sleep duration was significantly associated with sarcopenia in thin and normal weight groups after adjusting for the subjects' age, sex, BMI, and household income, as well as current smoking, regular exercise, alcohol drinking, and hypertension statuses. However, after further adjustment of employment status, statistical significance was not present in thin patient groups. The small sample size could have caused sampling bias and affected the statistical significance.
The results of the current study are in line with a previous study suggesting that sleep duration was associated with sarcopenia.28 Chien et al. demonstrated a U-shape association between sleep duration and sarcopenia. Specifically, the authors emphasized that those who had ≥ 8 hours of sleep tended to exhibit sarcopenia (OR [95% CI] = 1.89 [1.01–3.54]). Sex differences were also shown in their study. In the sex-specific analyses, only women showed the association. The current study had some different results; men with a long sleep duration (≥ 9 hours) were more likely to exhibit sarcopenia (OR [95% CI] = 1.574 [1.118–2.215]). In women, a U-shaped association was present, without any statistical significance. Although the reason for this difference in the association between sleep duration, sarcopenia, and sex is unclear, there are several discrepancies between the current study and Chien et al.'s study. In the current study, sleep duration was categorized into 5 groups (≤ 5, 6, 7, 8 and ≥ 9 hours) whereas participants were grouped into only 3 groups (< 6, 6–8, and ≥ 8 hours) in the study by Chien et al. Furthermore, the current study population included all Korean adults, not just older adults.
Although the underlying mechanisms of the relationship between sleep duration and sarcopenia remain unclear, there are some possible explanations. Some longitudinal studies have proposed a bidirectional relationship between sleep duration and body composition.29 We also found that sleep duration was related to sarcopenia by using 2 different analyses. Sleep duration and sarcopenia shared common risk factors, and both are important elements related to good health. Lifestyle factors including low physical activity, sedentary behavior, and smoking have been reported to accelerate the progression of sarcopenia.30 As for sleep duration itself, it can be affected by various lifestyle factors. Physical inactivity, sedentary lifestyle, and low social economic status are all closely linked to sleep duration.31,32 One possible link between sleep duration and sarcopenia could be insulin resistance. A growing body of evidence supports that inappropriate amount of sleep duration leads to unfavorable health effects. In two meta-analyses, all-cause mortality was higher in those with long sleep duration.10,33 Also, Pyykkonen et al. reported that long sleep duration (≥ 9 hours) was closely related to increased insulin resistance after adjusting for confounding factors.14 Also, Brocato et al. documented that long sleepers were more likely to have metabolic syndrome components.34 Despite these observations, the exact mechanism and causality remain unclear, and some authors have suggested that insulin resistance and sarcopenia share a common cause.4 Rasmussen et al. proposed that resistance to insulin contributed to a decline in the synthesis of skeletal mass protein and resulted in sarcopenia in older people.35 In addition, circadian rhythm disruption and hormonal changes with long sleep duration might be a possible underlying mechanism to explain the relationship between sleep duration and sarcopenia.36 Also, another link between sleep duration and sarcopenia could be chronic inflammation.37 Patel et al. suggested that increased sleep duration was related to a proinflammatory marker such as C-reactive protein and interleukin-6.37 These inflammatory mediators play an important role in sarcopenia by provoking muscle proteolysis.38 Finally, Stranges et al. proposed that low socioeconomic status and un-disclosed general medical conditions are both associated with long sleep duration,39 and Coppin et al. found low socioeconomic status is closely related to physiological impairment.40 Together, these recent studies have shown habitual long sleep duration to be associated with unhealthy outcomes. However, there are no studies to date that demonstrate an exact mechanism of the relationship between long sleep duration and adverse health effects.
Although our study did not find significant association between short sleep duration and sarcopenia, sleep deprivation remains a major problem against healthy lifestyle. Many studies have demonstrated that short sleep duration leads to coronary heart disease and diabetes, which are closely linked to chronic inflammation and insulin resistance.41 Other studies have reported that both short and long sleep durations are related to sarcopenia in older females.28 Further prospective studies are required to clarify the relationship between sleep duration and sarcopenia.
This study had some limitations. First, participant data such as sleep duration, smoking habits, alcohol intake, exercise habits, employment status, and work schedule were all obtained via self-reported questionnaires, rather than by objective measures. Second, despite the significant relationship between long sleep duration and sarcopenia found in the current study, we cannot conclude whether long sleep duration is a risk factor for sarcopenia or just an epiphenomenon. Because of this study's cross-sectional design, our results should be cautiously interpreted, and prospective longitudinal studies are warranted. Third, we did not consider the sleep quality and sleep-wake phase of the participants, both of which were not part of the 2008–2011 KNHANES. Thus, we were not able to assess possible association between circadian disruption and sarcopenia. Fourth, the question regarding the sleep duration had not been distinguished for the weekend and weekdays. Therefore, the actual sleep duration could have been longer. Fifth, the proportions of sarcopenia varied largely according to BMI. Previous studies have reported that underweight and low BMI were significant risk factors for sarcopenia.27 Therefore, low BMI could attenuate the association between sleep duration and sarcopenia. Additionally, although dual-energy x-ray absorptiometry is a useful tool for estimating muscle mass, inhomogeneous adipose tissue may have caused some accuracy errors in soft tissue; therefore, our results should be interpreted with caution.42 Further studies using either computed tomography or magnetic resonance imaging are needed, and the effect of lean body mass on sarcopenia also needs more investigation. Finally, there may be other residual factors that we did not adequately take into account.
Nevertheless, our study has several strengths. First, this is the first observational study to assess the relationship between sleep duration and sarcopenia in a large sample population. Second, we used stratified, multistage probability sampling data and applied sample weights for the analyses. Therefore, our findings are more generalizable to the Korean adult population. In conclusion, we found that long sleep duration was independently associated with sarcopenia in Korean adults after adjusting for age, sex, BMI, household income, hypertension, exercise habits, smoking, alcohol consumption, employment status, and work schedule using a nationally representative dataset. However, future studies are needed to demonstrate this causal relationship and elucidate the biological mechanisms that underlie this association.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The Korean name of corresponding author (John A. Linton) is Yo-Han Ihn. The authors are grateful to Prof. Yong-Jae Lee (Department of Family Medicine, Yonsei Medical School). This study was conducted using raw data from the KNHANES performed by Korean Centers for Disease Control and Prevention (KCDC).
ABBREVIATIONS
- ASM
appendicular skeletal mass
- BMI
body mass index
- KCDC
Korea Centers for Disease Control and Prevention
- KNHANES
Korean National Health Examination and Nutrition Survey
REFERENCES
- 1.Kim YS, Lee Y, Chung YS, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci. 2012;67(10):1107–1113. doi: 10.1093/gerona/gls071. [DOI] [PubMed] [Google Scholar]
- 2.Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7(4):405–410. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BC, Kim MK, Han K, et al. Low muscle mass is associated with metabolic syndrome only in nonobese young adults: the Korea National Health and Nutrition Examination Survey 2008-2010. Nutr Res. 2015;35(12):1070–1078. doi: 10.1016/j.nutres.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229(2):R67–R81. doi: 10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Han BD, Han K, et al. Optimal cutoffs for low skeletal muscle mass related to cardiovascular risk in adults: The Korea National Health and Nutrition Examination Survey 2009-2010. Endocrine. 2015;50(2):424–433. doi: 10.1007/s12020-015-0577-y. [DOI] [PubMed] [Google Scholar]
- 6.Umegaki H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr Gerontol Int. 2016;16(3):293–299. doi: 10.1111/ggi.12688. [DOI] [PubMed] [Google Scholar]
- 7.Derbre F, Gratas-Delamarche A, Gomez-Cabrera MC, Vina J. Inactivity-induced oxidative stress: a central role in age-related sarcopenia? Eur J Sport Sci. 2014;14(Suppl 1):S98–S108. doi: 10.1080/17461391.2011.654268. [DOI] [PubMed] [Google Scholar]
- 8.Vitale G, Cesari M, Mari D. Aging of the endocrine system and its potential impact on sarcopenia. Eur J Intern Med. 2016;35:10–15. [Google Scholar]
- 9.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikehara S, Iso H, Date C, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32(3):295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 13.Niijima S, Nagai M, Hoshide S, Takahashi M, Shimpo M, Kario K. Long sleep duration: a nonconventional indicator of arterial stiffness in Japanese at high risk of cardiovascular disease: the J-HOP study. J Am Soc Hypertens. 2016;10(5):429–437. doi: 10.1016/j.jash.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Pyykkonen AJ, Isomaa B, Pesonen AK, et al. Sleep duration and insulin resistance in individuals without type 2 diabetes: the PPP-Botnia study. Ann Med. 2014;46(5):324–329. doi: 10.3109/07853890.2014.902226. [DOI] [PubMed] [Google Scholar]
- 15.Ju SY, Choi WS. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutr Diabetes. 2013;3:e65. doi: 10.1038/nutd.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43(1):69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2016;17(8):767.e1–e7. doi: 10.1016/j.jamda.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Jeon YK, Shin MJ, Kim MH, et al. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2011. Osteoporos Int. 2015;26(10):2423–2429. doi: 10.1007/s00198-015-3152-8. [DOI] [PubMed] [Google Scholar]
- 20.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35(6):561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 22.Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25(3):226–231. doi: 10.1016/s0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 23.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57(12):M772–M777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 24.Wu IC, Lin CC, Hsiung CA, et al. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: a pooled analysis for a broader adoption of sarcopenia assessments. Geriatr Gerontol Int. 2014;14(Suppl 1):52–60. doi: 10.1111/ggi.12193. [DOI] [PubMed] [Google Scholar]
- 25.Rom O, Kaisari S, Aizenbud D, Reznick AZ. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med J. 2012;3(4):e0024. doi: 10.5041/RMMJ.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffl M, Bohannon RW, Petr M, Kohlikova E, Holmerova I. Alcohol consumption as a risk factor for sarcopenia - a meta-analysis. BMC Geriatr. 2016;16:99. doi: 10.1186/s12877-016-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau EM, Lynn HS, Woo JW, Kwok TC, Melton LJ., 3rd Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005;60(2):213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 28.Chien MY, Wang LY, Chen HC. The relationship of sleep duration with obesity and sarcopenia in community-dwelling older adults. Gerontology. 2015;61(5):399–406. doi: 10.1159/000371847. [DOI] [PubMed] [Google Scholar]
- 29.López-García E, Faubel R, León-Muñoz L, Zuluaga MC, Banegas JR, Rodríguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87(2):310–316. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 30.Rom O, Kaisari S, Aizenbud D, Reznick AZ. Lifestyle and sarcopenia—etiology, prevention, and treatment. Rambam Maimonides Med J. 2012;3(4):e0024. doi: 10.5041/RMMJ.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29(7):881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51(4):285–293. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Liu TZ, Xu C, Rota M, et al. Sleep duration and risk of all-cause mortality: a flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. 2016;32:28–36. doi: 10.1016/j.smrv.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Brocato J, Wu F, Chen Y, et al. Association between sleeping hours and cardiometabolic risk factors for metabolic syndrome in a Saudi Arabian population. BMJ Open. 2015;5(11):e008590. doi: 10.1136/bmjopen-2015-008590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20(6):768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piovezan RD, Abucham J, Dos Santos RV, Mello MT, Tufik S, Poyares D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res Rev. 2015;23(Pt B):210–220. doi: 10.1016/j.arr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–204. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2012;67(6):671–676. doi: 10.1093/gerona/glr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stranges S, Dorn JM, Shipley MJ, et al. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. Am J Epidemiol. 2008;168(12):1353–1364. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppin AK, Ferrucci L, Lauretani F, et al. Low socioeconomic status and disability in old age: evidence from the InChianti study for the mediating role of physiological impairments. J Gerontol A Biol Sci Med Sci. 2006;61(1):86–91. doi: 10.1093/gerona/61.1.86. [DOI] [PubMed] [Google Scholar]
- 41.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 42.Blake GM, Fogelman I. How important are BMD accuracy errors for the clinical interpretation of DXA scans? J Bone Miner Res. 2008;23(4):457–462. doi: 10.1359/jbmr.071119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


