Abstract
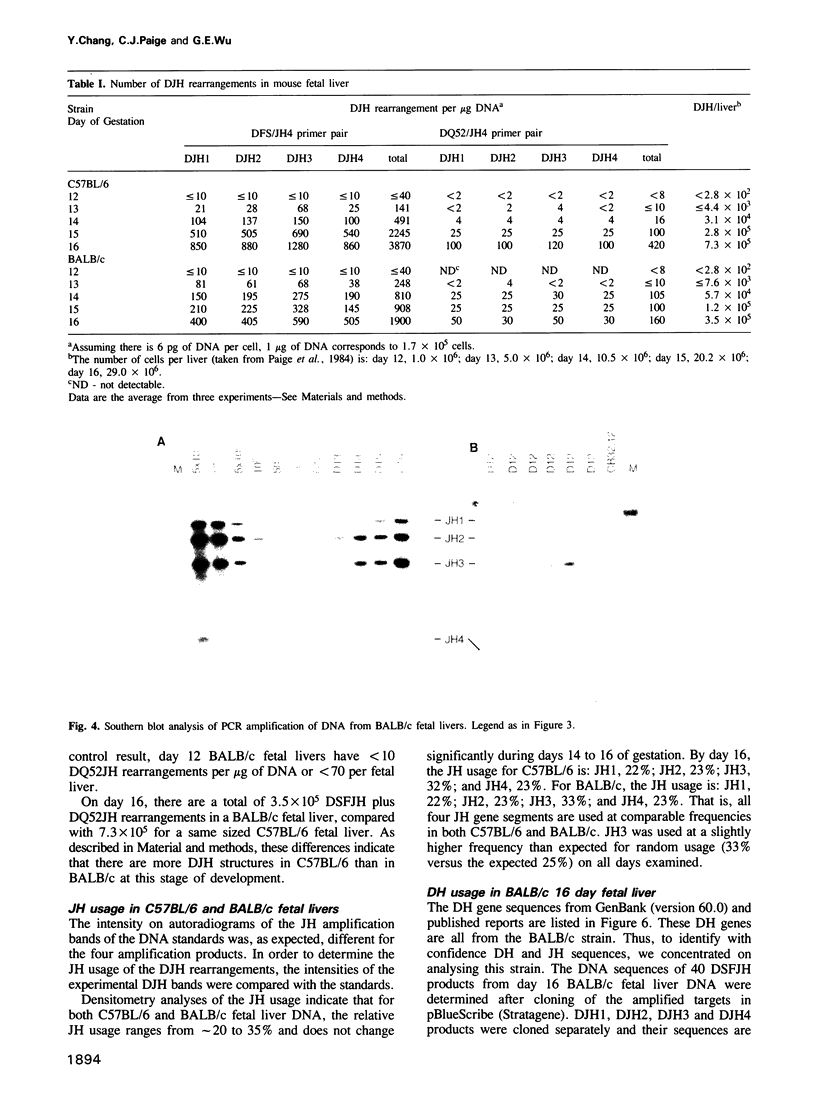
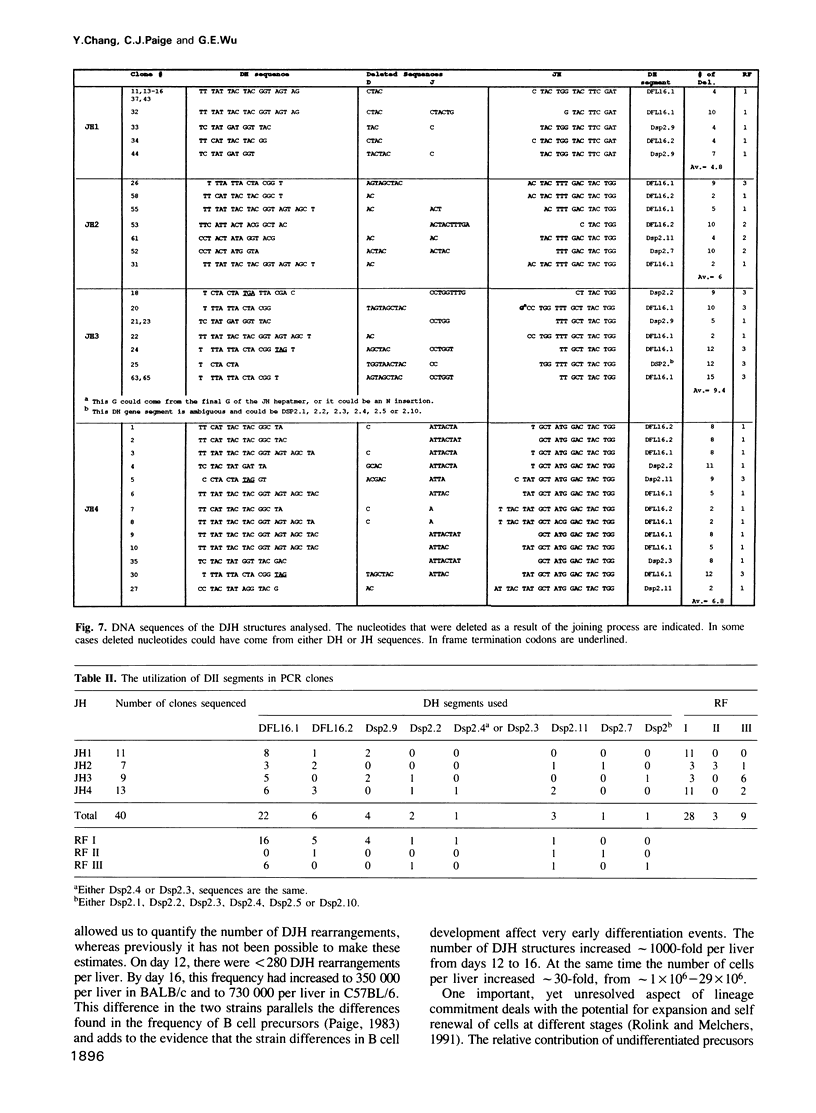
The primary immunoglobulin (Ig) repertoire in the mouse develops during fetal life in the liver. The first Ig gene rearrangement--the joining of a DH to a JH gene segment--contributes largely to the diversity found in CDR3, as well as potentially encoding the D mu protein which is believed to function in the development of a B cell. In this report, the number of DJH joins in two mouse strains, C57BL/6 and BALB/c, were enumerated from days 12 to 16 of fetal development. It was found that the number of DJH structures increased from less than 300 per liver on day 12 to greater than 700,000 (C57BL/6) and 300,000 (BALB/c) on day 16. Each JH gene segment was used approximately equally on each day examined. When the DJH structures were examined by cloning and sequencing it was found that the DJH reading frame (RF) usage (with respect to JH) was not random--RF1 was used 70% of the time. Moreover, a single D gene segment, DFL16.1, was used in greater than 50% of all joins reinforcing the notion that the fetal repertoire is restricted in its antigen binding potential.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Atkinson M. J., Michnick D. A., Paige C. J., Wu G. E. Ig gene rearrangements on individual alleles of Abelson murine leukemia cell lines from (C57BL/6 x BALB/c) F1 fetal livers. J Immunol. 1991 Apr 15;146(8):2805–2812. [PubMed] [Google Scholar]
- Cohn M., Langman R. E. The protecton: the unit of humoral immunity selected by evolution. Immunol Rev. 1990 Jun;115:11–147. doi: 10.1111/j.1600-065x.1990.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Craig N. L. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Kitamura D., Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991 Apr 5;65(1):47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
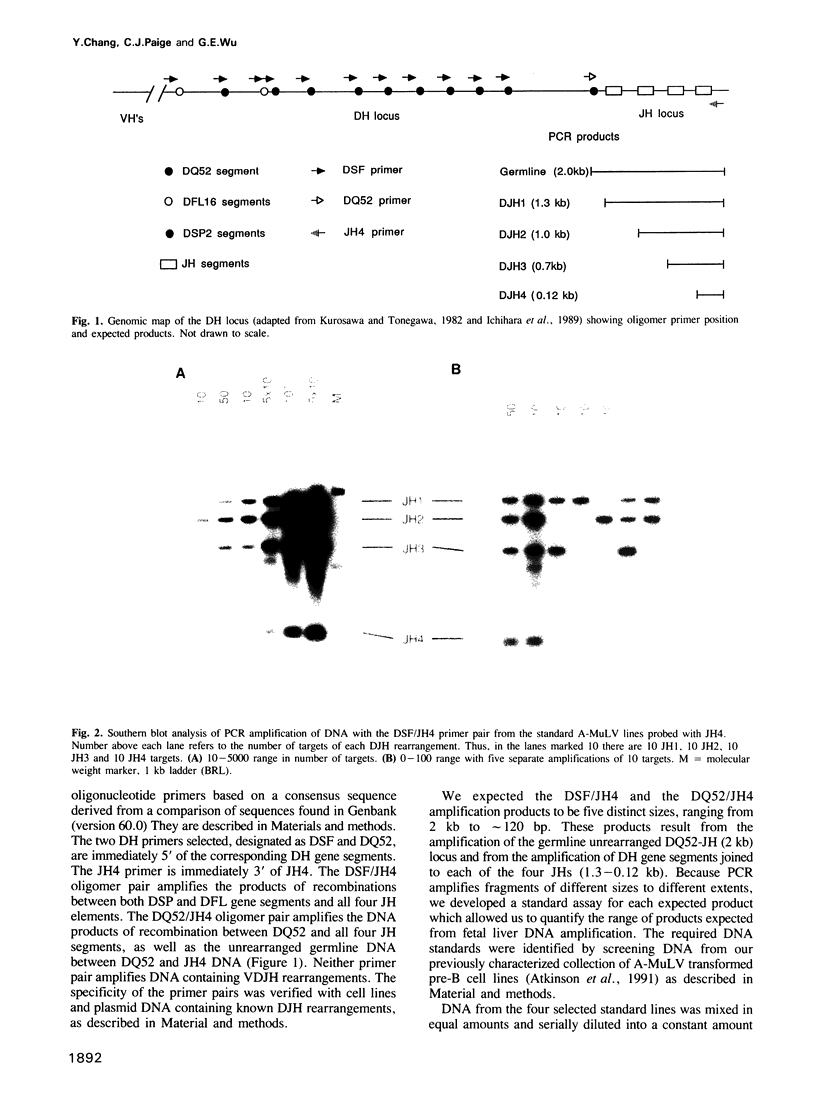
- Ichihara Y., Hayashida H., Miyazawa S., Kurosawa Y. Only DFL16, DSP2, and DQ52 gene families exist in mouse immunoglobulin heavy chain diversity gene loci, of which DFL16 and DSP2 originate from the same primordial DH gene. Eur J Immunol. 1989 Oct;19(10):1849–1854. doi: 10.1002/eji.1830191014. [DOI] [PubMed] [Google Scholar]
- Kincade P. W. Experimental models for understanding B lymphocyte formation. Adv Immunol. 1987;41:181–267. doi: 10.1016/s0065-2776(08)60032-2. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler A. M., Lin P. S., Gearhart P. J. Adult B-cell repertoire is biased toward two heavy-chain variable-region genes that rearrange frequently in fetal pre-B cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2454–2458. doi: 10.1073/pnas.84.8.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. M., Hesse J. E., Mizuuchi K., Gellert M. Novel strand exchanges in V(D)J recombination. Cell. 1988 Dec 23;55(6):1099–1107. doi: 10.1016/0092-8674(88)90254-1. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Maeda T., Sugiyama H., Tani Y., Kishimoto S. The DJH complex remains active in recombination to VH segments after the loss of mu-chain expression in mu-positive pre-B cells. J Immunol. 1989 May 15;142(10):3652–3656. [PubMed] [Google Scholar]
- Meek K. D., Hasemann C. A., Capra J. D. Novel rearrangements at the immunoglobulin D locus. Inversions and fusions add to IgH somatic diversity. J Exp Med. 1989 Jul 1;170(1):39–57. doi: 10.1084/jem.170.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K. Analysis of junctional diversity during B lymphocyte development. Science. 1990 Nov 9;250(4982):820–823. doi: 10.1126/science.2237433. [DOI] [PubMed] [Google Scholar]
- Melchers F., Strasser A., Bauer S. R., Kudo A., Thalmann P., Rolink A. Cellular stages and molecular steps of murine B-cell development. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):183–189. doi: 10.1101/sqb.1989.054.01.023. [DOI] [PubMed] [Google Scholar]
- Misener V., Downey G. P., Jongstra J. The immunoglobulin light chain related protein lambda 5 is expressed on the surface of mouse pre-B cell lines and can function as a signal transducing molecule. Int Immunol. 1991 Nov;3(11):1129–1136. doi: 10.1093/intimm/3.11.1129. [DOI] [PubMed] [Google Scholar]
- Misener V., Jongstra-Bilen J., Young A. J., Atkinson M. J., Wu G. E., Jongstra J. Association of Ig L chain-like protein lambda 5 with a 16-kilodalton protein in mouse pre-B cell lines is not dependent on the presence of Ig H chain protein. J Immunol. 1990 Aug 1;145(3):905–909. [PubMed] [Google Scholar]
- Paige C. J., Gisler R. H., McKearn J. P., Iscove N. N. Differentiation of murine B cell precursors in agar culture. Frequency, surface marker analysis and requirements for growth of clonable pre-B cells. Eur J Immunol. 1984 Nov;14(11):979–987. doi: 10.1002/eji.1830141104. [DOI] [PubMed] [Google Scholar]
- Paige C. J. Surface immunoglobulin-negative B-cell precursors detected by formation of antibody-secreting colonies in agar. Nature. 1983 Apr 21;302(5910):711–713. doi: 10.1038/302711a0. [DOI] [PubMed] [Google Scholar]
- Ramsden D. A., Wu G. E. Mouse kappa light-chain recombination signal sequences mediate recombination more frequently than do those of lambda light chain. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Jackson S., Alt F. W. VHDJH formation and DJH replacement during pre-B differentiation: non-random usage of gene segments. EMBO J. 1986 Sep;5(9):2131–2138. doi: 10.1002/j.1460-2075.1986.tb04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Kudo A., Karasuyama H., Kikuchi Y., Melchers F. Long-term proliferating early pre B cell lines and clones with the potential to develop to surface Ig-positive, mitogen reactive B cells in vitro and in vivo. EMBO J. 1991 Feb;10(2):327–336. doi: 10.1002/j.1460-2075.1991.tb07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Sadowski P. Site-specific recombinases: changing partners and doing the twist. J Bacteriol. 1986 Feb;165(2):341–347. doi: 10.1128/jb.165.2.341-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Strasser A., Rolink A., Melchers F. One synchronous wave of B cell development in mouse fetal liver changes at day 16 of gestation from dependence to independence of a stromal cell environment. J Exp Med. 1989 Dec 1;170(6):1973–1986. doi: 10.1084/jem.170.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Abe M., Nishikawa S., Nakayama E., Shiku H. Preferential usage of JH2 in D-J joinings with DQ52 in murine lymphocytes. Int Immunol. 1989;1(6):643–646. doi: 10.1093/intimm/1.6.643. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Tsubata R., Reth M. Cell surface expression of the short immunoglobulin mu chain (D mu protein) in murine pre-B cells is differently regulated from that of the intact mu chain. Eur J Immunol. 1991 Jun;21(6):1359–1363. doi: 10.1002/eji.1830210605. [DOI] [PubMed] [Google Scholar]
- Tsukada S., Sugiyama H., Oka Y., Kishimoto S. Estimation of D segment usage in initial D to JH joinings in a murine immature B cell line. Preferential usage of DFL16.1, the most 5' D segment and DQ52, the most JH-proximal D segment. J Immunol. 1990 May 15;144(10):4053–4059. [PubMed] [Google Scholar]
- Wood C., Tonegawa S. Diversity and joining segments of mouse immunoglobulin heavy chain genes are closely linked and in the same orientation: implications for the joining mechanism. Proc Natl Acad Sci U S A. 1983 May;80(10):3030–3034. doi: 10.1073/pnas.80.10.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. E., Atkinson M. J., Ramsden D. A., Paige C. J. VH gene repertoire. Semin Immunol. 1990 May;2(3):207–216. [PubMed] [Google Scholar]