Abstract
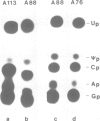
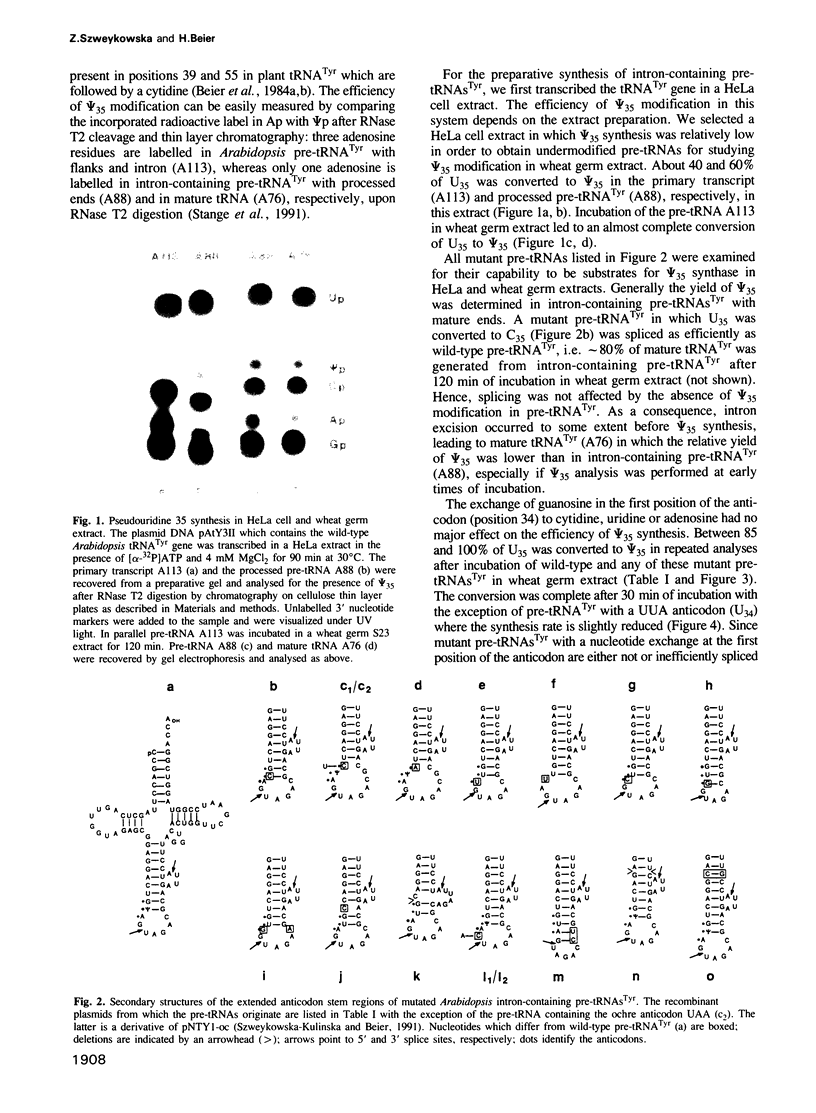
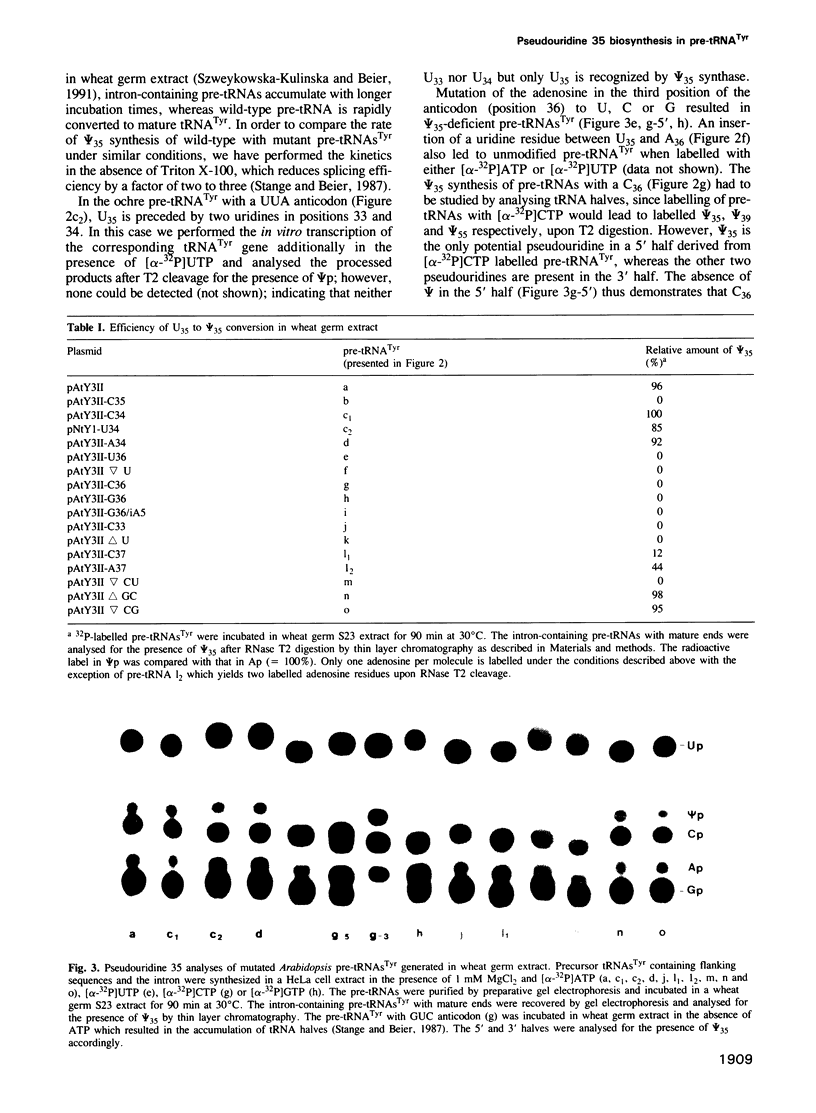
All eukaryotic cytoplasmic tRNAs(Tyr) contain pseudouridine in the centre of the anticodon (psi 35). Recently, it has been shown that the formation of psi 35 is dependent on the presence of introns in tRNA(Tyr) genes. Furthermore, we have investigated the structural and sequence requirements for the biosynthesis of psi 35. A number of mutant genes were constructed by oligonucleotide-directed mutagenesis of a cloned Arabidopsis tRNA(Tyr) gene. Nucleotide exchanges were produced in the first and third positions of the anticodon and at positions adjacent to the anticodon. Moreover, insertion and deletion mutations were made in the anticodon stem and in the intron. The mutant genes were transcribed in HeLa cell extract and the pre-tRNAs(Tyr) were used for studying psi 35 biosynthesis in HeLa cell and wheat germ extracts. We have made the following observations about the specificity of plant and vertebrate psi 35 syntheses: (i) insertion or deletion of one base pair in the anticodon stem does not influence the efficiency and accuracy of the psi 35 synthase; (ii) the presence of U35 in a stable double-stranded region prevents its modification to psi 35; and (iii) the consensus sequence U33N34U35A36Pu37 in the anticodon loop is an absolute requirement for psi 35 synthesis. Thus, psi 35 synthases recognize both tRNA tertiary structure and specific sequences surrounding the nucleotide to be modified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arena F., Ciliberto G., Ciampi S., Cortese R. Purification of pseudouridylate synthetase I from Salmonella typhimurium. Nucleic Acids Res. 1978 Dec;5(12):4523–4536. doi: 10.1093/nar/5.12.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier D., Stange N., Gross H. J., Beier H. Nuclear tRNA(Tyr) genes are highly amplified at a single chromosomal site in the genome of Arabidopsis thaliana. Mol Gen Genet. 1991 Jan;225(1):72–80. doi: 10.1007/BF00282644. [DOI] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Sickinger H. D. The molecular basis for the differential translation of TMV RNA in tobacco protoplasts and wheat germ extracts. EMBO J. 1984 May;3(5):1091–1096. doi: 10.1002/j.1460-2075.1984.tb01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Ericson J. U., Gustafsson C. E., Hagervall T. G., Jönsson Y. H., Wikström P. M. Transfer RNA modification. Annu Rev Biochem. 1987;56:263–287. doi: 10.1146/annurev.bi.56.070187.001403. [DOI] [PubMed] [Google Scholar]
- Carbon P., Haumont E., Fournier M., de Henau S., Grosjean H. Site-directed in vitro replacement of nucleosides in the anticodon loop of tRNA: application to the study of structural requirements for queuine insertase activity. EMBO J. 1983;2(7):1093–1097. doi: 10.1002/j.1460-2075.1983.tb01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choffat Y., Suter B., Behra R., Kubli E. Pseudouridine modification in the tRNA(Tyr) anticodon is dependent on the presence, but independent of the size and sequence, of the intron in eucaryotic tRNA(Tyr) genes. Mol Cell Biol. 1988 Aug;8(8):3332–3337. doi: 10.1128/mcb.8.8.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin H. J. Introduction of an intervening sequence into a human serine suppressor tRNA gene: effects on gene expression in vitro and in vivo. Nucleic Acids Res. 1988 Dec 23;16(24):11591–11606. doi: 10.1093/nar/16.24.11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans L., Grosjean H. Enzymatic conversion of guanosine 3' adjacent to the anticodon of yeast tRNAPhe to N1-methylguanosine and the wye nucleoside: dependence on the anticodon sequence. EMBO J. 1987 Feb;6(2):477–483. doi: 10.1002/j.1460-2075.1987.tb04778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans L., Haumont E., de Henau S., Grosjean H. Enzymatic 2'-O-methylation of the wobble nucleoside of eukaryotic tRNAPhe: specificity depends on structural elements outside the anticodon loop. EMBO J. 1986 May;5(5):1105–1109. doi: 10.1002/j.1460-2075.1986.tb04329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilloud E., Clarkson S. G. A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem. 1986 Jan 5;261(1):486–494. [PubMed] [Google Scholar]
- Green C. J., Kammen H. O., Penhoet E. E. Purification and properties of a mammalian tRNA pseudouridine synthase. J Biol Chem. 1982 Mar 25;257(6):3045–3052. [PubMed] [Google Scholar]
- Grosjean H., Nicoghosian K., Haumont E., Söll D., Cedergren R. Nucleotide sequences of two serine tRNAs with a GGA anticodon: the structure-function relationships in the serine family of E. coli tRNAs. Nucleic Acids Res. 1985 Aug 12;13(15):5697–5706. doi: 10.1093/nar/13.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Haumont E., Fournier M., de Henau S., Grosjean H. Enzymatic conversion of adenosine to inosine in the wobble position of yeast tRNAAsp: the dependence on the anticodon sequence. Nucleic Acids Res. 1984 Mar 26;12(6):2705–2715. doi: 10.1093/nar/12.6.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Abelson J. The yeast tRNATyr gene intron is essential for correct modification of its tRNA product. Nature. 1983 Apr 21;302(5910):681–687. doi: 10.1038/302681a0. [DOI] [PubMed] [Google Scholar]
- Kammen H. O., Marvel C. C., Hardy L., Penhoet E. E. Purification, structure, and properties of Escherichia coli tRNA pseudouridine synthase I. J Biol Chem. 1988 Feb 15;263(5):2255–2263. [PubMed] [Google Scholar]
- MacPherson J. M., Roy K. L. Two human tyrosine tRNA genes contain introns. Gene. 1986;42(1):101–106. doi: 10.1016/0378-1119(86)90155-1. [DOI] [PubMed] [Google Scholar]
- Madison J. T., Boguslawski S. J. Partial digestion of a yeast lysine transfer ribonucleic acid and reconstruction of the nucleotide sequence. Biochemistry. 1974 Jan 29;13(3):524–527. doi: 10.1021/bi00700a019. [DOI] [PubMed] [Google Scholar]
- Mattoccia E., Baldi I. M., Gandini-Attardi D., Ciafrè S., Tocchini-Valentini G. P. Site selection by the tRNA splicing endonuclease of Xenopus laevis. Cell. 1988 Nov 18;55(4):731–738. doi: 10.1016/0092-8674(88)90231-0. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Lee M. C., Knapp G. Transfer RNA splicing in Saccharomyces cerevisiae: defining the substrates. Nucleic Acids Res. 1984 Dec 21;12(24):9367–9382. doi: 10.1093/nar/12.24.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. M., Abelson J. Substrate recognition and splice site determination in yeast tRNA splicing. Cell. 1988 Nov 18;55(4):719–730. doi: 10.1016/0092-8674(88)90230-9. [DOI] [PubMed] [Google Scholar]
- Samuelsson T., Olsson M. Transfer RNA pseudouridine synthases in Saccharomyces cerevisiae. J Biol Chem. 1990 May 25;265(15):8782–8787. [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Beier D., Beier H. Expression of nuclear tRNA(Tyr) genes from Arabidopsis thaliana in HeLa cell and wheat germ extracts. Plant Mol Biol. 1991 May;16(5):865–875. doi: 10.1007/BF00015078. [DOI] [PubMed] [Google Scholar]
- Stange N., Beier H. A cell-free plant extract for accurate pre-tRNA processing, splicing and modification. EMBO J. 1987 Sep;6(9):2811–2818. doi: 10.1002/j.1460-2075.1987.tb02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange N., Beier H. A gene for the major cytoplasmic tRNATyr from Nicotiana rustica contains a 13 nucleotides long intron. Nucleic Acids Res. 1986 Nov 11;14(21):8691–8691. doi: 10.1093/nar/14.21.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szweykowska-Kulinska Z., Beier H. Nucleotide sequences of two nuclear tRNA(Tyr) genes from Triticum aestivum. Nucleic Acids Res. 1990 Apr 11;18(7):1894–1894. doi: 10.1093/nar/18.7.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szweykowska-Kulinska Z., Beier H. Plant nonsense suppressor tRNA(Tyr) genes are expressed at very low levels in vitro due to inefficient splicing of the intron-containing pre-tRNAs. Nucleic Acids Res. 1991 Feb 25;19(4):707–712. doi: 10.1093/nar/19.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T. H., Buck M., Ames B. N. Sequence specificity of tRNA-modifying enzymes. An analysis of 258 tRNA sequences. Biochim Biophys Acta. 1983 Nov 17;741(2):180–196. doi: 10.1016/0167-4781(83)90058-1. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- van Tol H., Beier H. All human tRNATyr genes contain introns as a prerequisite for pseudouridine biosynthesis in the anticodon. Nucleic Acids Res. 1988 Mar 25;16(5):1951–1966. doi: 10.1093/nar/16.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol H., Stange N., Gross H. J., Beier H. A human and a plant intron-containing tRNATyr gene are both transcribed in a HeLa cell extract but spliced along different pathways. EMBO J. 1987 Jan;6(1):35–41. doi: 10.1002/j.1460-2075.1987.tb04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]