Abstract
Vaccinia virus open reading frame (ORF) SalF7L has 31% amino acid identity to human 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD). Here we show that SalF7L encodes an active 3 beta-HSD, by the conversion of pregnenolone to the steroid hormone progesterone. The gene is transcribed early during infection into a 1.4 kb mRNA from an initiation site 12 bp upstream of the ORF. An antiserum raised against bacterially expressed SalF7L immunoprecipitated a 38 kDa polypeptide from infected cells, but not from mock infected cells or from cells infected with a mutant virus from which the SalF7L ORF had been removed. Deletion of the gene had no effect on virus replication in CV-1 cells in culture, yet the deletion mutant was attenuated when intranasally inoculated into mice. This steroid hormone synthesizing enzyme is a novel type of virus virulence factor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Wong-Staal F., Gallo R. C. Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol. 1984 Jul;133(1):273–276. [PubMed] [Google Scholar]
- Barton G. J., Sternberg M. J. A strategy for the rapid multiple alignment of protein sequences. Confidence levels from tertiary structure comparisons. J Mol Biol. 1987 Nov 20;198(2):327–337. doi: 10.1016/0022-2836(87)90316-0. [DOI] [PubMed] [Google Scholar]
- Bauer H. C., Bauer H. Micromethod for the determination of 3-beta-HSD activity in cultured cells. J Steroid Biochem. 1989 Oct;33(4A):643–646. doi: 10.1016/0022-4731(89)90054-x. [DOI] [PubMed] [Google Scholar]
- Beattie E., Tartaglia J., Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991 Jul;183(1):419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Berg J. M. DNA binding specificity of steroid receptors. Cell. 1989 Jun 30;57(7):1065–1068. doi: 10.1016/0092-8674(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Blasco R., Cole N. B., Moss B. Sequence analysis, expression, and deletion of a vaccinia virus gene encoding a homolog of profilin, a eukaryotic actin-binding protein. J Virol. 1991 Sep;65(9):4598–4608. doi: 10.1128/jvi.65.9.4598-4608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M. E., Foulds I. J., Campbell J. I., Binns M. M. Non-essential genes in the vaccinia virus HindIII K fragment: a gene related to serine protease inhibitors and a gene related to the 37K vaccinia virus major envelope antigen. J Gen Virol. 1988 Dec;69(Pt 12):2995–3003. doi: 10.1099/0022-1317-69-12-2995. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988 May 15;65(1):123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- Brown F., Schild G. C., Ada G. L. Recombinant vaccinia viruses as vaccines. Nature. 1986 Feb 13;319(6054):549–550. doi: 10.1038/319549a0. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Cooper J. A., Twardzik D. R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988 Mar;62(3):866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
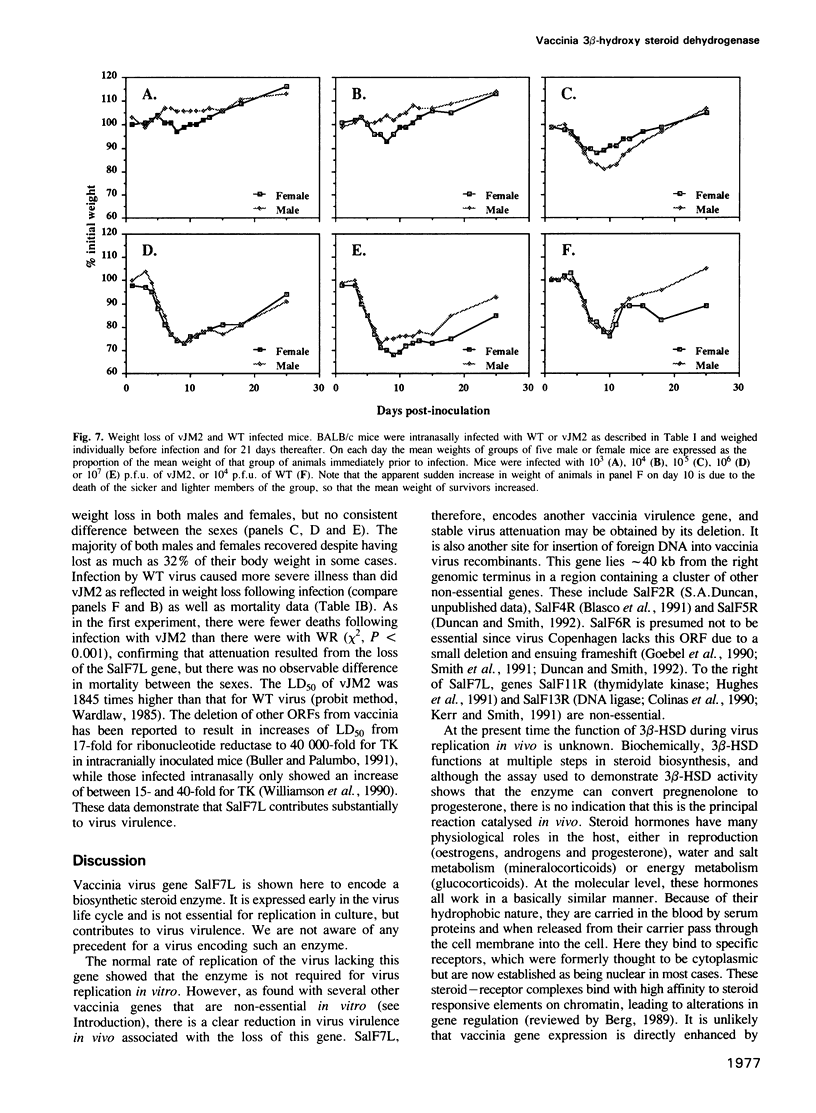
- Buller R. M., Palumbo G. J. Poxvirus pathogenesis. Microbiol Rev. 1991 Mar;55(1):80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Child S. J., Palumbo G. J., Buller R. M., Hruby D. E. Insertional inactivation of the large subunit of ribonucleotide reductase encoded by vaccinia virus is associated with reduced virulence in vivo. Virology. 1990 Feb;174(2):625–629. doi: 10.1016/0042-6822(90)90119-c. [DOI] [PubMed] [Google Scholar]
- Colinas R. J., Goebel S. J., Davis S. W., Johnson G. P., Norton E. K., Paoletti E. A DNA ligase gene in the Copenhagen strain of vaccinia virus is nonessential for viral replication and recombination. Virology. 1990 Nov;179(1):267–275. doi: 10.1016/0042-6822(90)90295-3. [DOI] [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Duncan S. A., Smith G. L. Vaccinia virus gene SalF5R is non-essential for virus replication in vitro and in vivo. J Gen Virol. 1992 May;73(Pt 5):1235–1242. doi: 10.1099/0022-1317-73-5-1235. [DOI] [PubMed] [Google Scholar]
- Edbauer C., Weinberg R., Taylor J., Rey-Senelonge A., Bouquet J. F., Desmettre P., Paoletti E. Protection of chickens with a recombinant fowlpox virus expressing the Newcastle disease virus hemagglutinin-neuraminidase gene. Virology. 1990 Dec;179(2):901–904. doi: 10.1016/0042-6822(90)90165-n. [DOI] [PubMed] [Google Scholar]
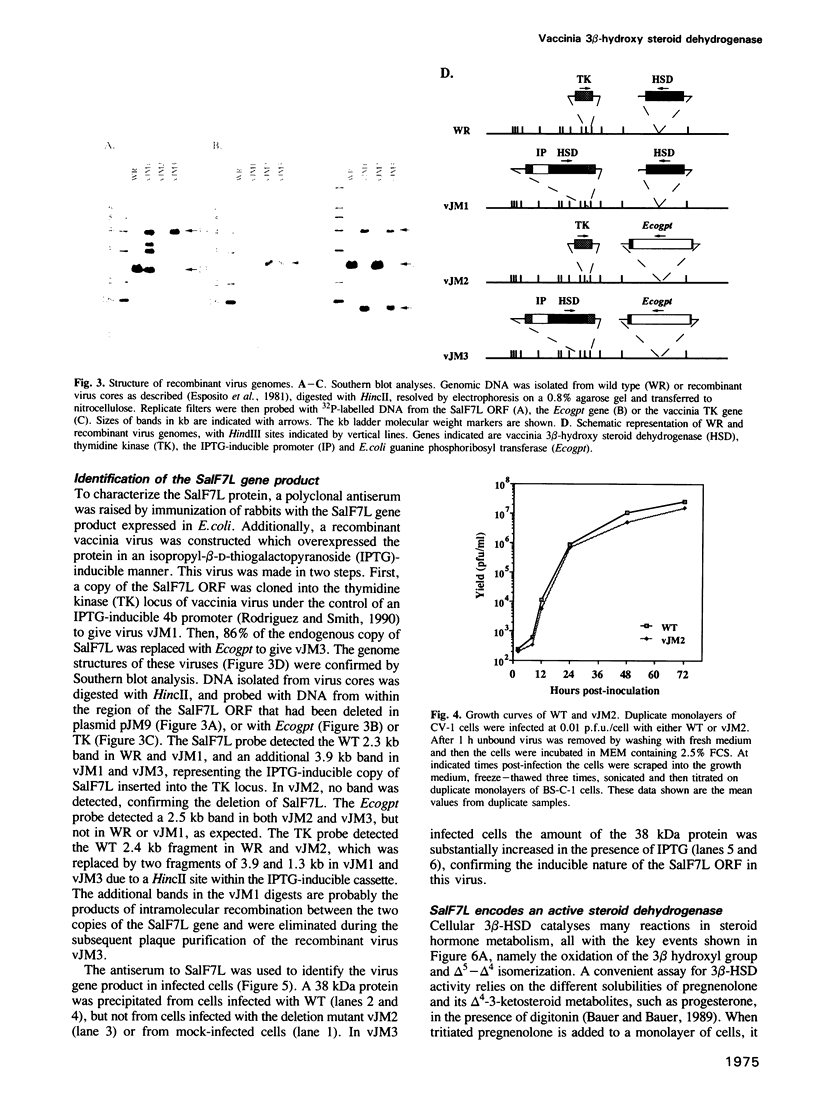
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987 Nov 19;330(6145):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Grippo J. F., Holmgren A., Pratt W. B. Proof that the endogenous, heat-stable glucocorticoid receptor-activating factor is thioredoxin. J Biol Chem. 1985 Jan 10;260(1):93–97. [PubMed] [Google Scholar]
- Howard S. T., Chan Y. S., Smith G. L. Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology. 1991 Feb;180(2):633–647. doi: 10.1016/0042-6822(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Johnston L. H., de Carlos A., Smith G. L. Vaccinia virus encodes an active thymidylate kinase that complements a cdc8 mutant of Saccharomyces cerevisiae. J Biol Chem. 1991 Oct 25;266(30):20103–20109. [PubMed] [Google Scholar]
- Johnson G. P., Goebel S. J., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. Vaccinia virus encodes a protein with similarity to glutaredoxins. Virology. 1991 Mar;181(1):378–381. doi: 10.1016/0042-6822(91)90508-9. [DOI] [PubMed] [Google Scholar]
- Kerr S. M., Johnston L. H., Odell M., Duncan S. A., Law K. M., Smith G. L. Vaccinia DNA ligase complements Saccharomyces cerevisiae cdc9, localizes in cytoplasmic factories and affects virulence and virus sensitivity to DNA damaging agents. EMBO J. 1991 Dec;10(13):4343–4350. doi: 10.1002/j.1460-2075.1991.tb05012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr S. M., Smith G. L. Vaccinia virus DNA ligase is nonessential for virus replication: recovery of plasmids from virus-infected cells. Virology. 1991 Feb;180(2):625–632. doi: 10.1016/0042-6822(91)90076-n. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Hügin A. W., Moss B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology. 1989 Aug;171(2):579–587. doi: 10.1016/0042-6822(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988 Sep 8;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes two proteins that are structurally related to members of the plasma serine protease inhibitor superfamily. J Virol. 1989 Feb;63(2):600–606. doi: 10.1128/jvi.63.2.600-606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. Complications of smallpox vaccination, 1968. N Engl J Med. 1969 Nov 27;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- Law K. M., Smith G. L. A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J Gen Virol. 1992 Mar;73(Pt 3):549–557. doi: 10.1099/0022-1317-73-3-549. [DOI] [PubMed] [Google Scholar]
- Luu The V., Lachance Y., Labrie C., Leblanc G., Thomas J. L., Strickler R. C., Labrie F. Full length cDNA structure and deduced amino acid sequence of human 3 beta-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol. 1989 Aug;3(8):1310–1312. doi: 10.1210/mend-3-8-1310. [DOI] [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Perkus M. E., Goebel S. J., Davis S. W., Johnson G. P., Norton E. K., Paoletti E. Deletion of 55 open reading frames from the termini of vaccinia virus. Virology. 1991 Jan;180(1):406–410. doi: 10.1016/0042-6822(91)90047-f. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Smith G. L. Inducible gene expression from vaccinia virus vectors. Virology. 1990 Jul;177(1):239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- Rosel J. L., Earl P. L., Weir J. P., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J Virol. 1986 Nov;60(2):436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Howard S. T. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J Gen Virol. 1991 Jun;72(Pt 6):1349–1376. doi: 10.1099/0022-1317-72-6-1349. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S. Two vaccinia virus proteins structurally related to the interleukin-1 receptor and the immunoglobulin superfamily. J Gen Virol. 1991 Mar;72(Pt 3):511–518. doi: 10.1099/0022-1317-72-3-511. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Howard S. T., Chan Y. S. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J Gen Virol. 1989 Sep;70(Pt 9):2333–2343. doi: 10.1099/0022-1317-70-9-2333. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bastin J., Gould K., Brownlee G., Andrew M., Coupar B., Boyle D., Chan S., Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988 Oct 1;168(4):1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. S. Respiratory infection of mice with vaccinia virus. J Gen Virol. 1967 Jul;1(3):399–402. doi: 10.1099/0022-1317-1-3-399. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Reith R. W., Jeffrey L. J., Arrand J. R., Mackett M. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J Gen Virol. 1990 Nov;71(Pt 11):2761–2767. doi: 10.1099/0022-1317-71-11-2761. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavagno G., Jaffe B., Esteban M. Role of prostaglandins and non-steroid anti-inflammatory drugs in the pathogenicity of vaccinia virus. J Gen Virol. 1987 Feb;68(Pt 2):593–600. doi: 10.1099/0022-1317-68-2-593. [DOI] [PubMed] [Google Scholar]
- Zhou J., Crawford L., McLean L., Sun X. Y., Stanley M., Almond N., Smith G. L. Increased antibody responses to human papillomavirus type 16 L1 protein expressed by recombinant vaccinia virus lacking serine protease inhibitor genes. J Gen Virol. 1990 Sep;71(Pt 9):2185–2190. doi: 10.1099/0022-1317-71-9-2185. [DOI] [PubMed] [Google Scholar]