Abstract
Background
Subjective memory is commonly considered to be a unidimensional measure. However, theories of performance-based memory suggest that subjective memory could be divided into more than one dimension.
Objective
To divide subjective memory into theoretically related components of memory and explore the relationship to disease.
Methods
In this study, various aspects of self-reported memory were studied with respect to demographics and diseases in the third wave of the HUNT epidemiological study in middle Norway. The study included all individuals 55 years of age or older, who responded to a nine-item questionnaire on subjective memory and questionnaires on health (n=18 633).
Results
A principle component analysis of the memory items resulted in two memory components; the criterion used was an eigenvalue above 1, which accounted for 54% of the total variance. The components were interpreted as long-term memory (LTM; the first component; 43% of the total variance) and short-term memory (STM; the second component; 11% of the total variance). Memory impairment was significantly related to all diseases (except Bechterew’s disease), most strongly to brain infarction, heart failure, diabetes, cancer, chronic obstructive pulmonary disease and whiplash. For most diseases, the STM component was more affected than the LTM component; however, in cancer, the opposite pattern was seen.
Conclusions
Subjective memory impairment as measured in HUNT contained two components, which were differentially associated with diseases.
Keywords: subjective memory, long-term memory, short-term memory, disease, health, HUNT
Strengths and limitations of this study.
The main strengths of this study are the population-based sample and the availability of data on a broad variety of essential health-related factors.
The main limitations are related to subjective reports and sampling bias.
Introduction
Memory complaints are commonly reported by elderly individuals, and the prevalence increases with age, according to population-based1–3 and community-based studies.4 5 However, estimates of the prevalence and incidence of subjective memory impairment (SMI) vary widely among studies, a fact that may be due to the lack of a standard definition of SMI6 as well as a lack of standard methods for assessing SMI.
Another problem with assessing SMI is that the relationship between self-reported memory impairment and performance-based measures of memory is far from clear, as both positive4 5 7–10 and negative associations have been obtained.11 However, there are many indications that SMI is related to depression9 12; personality traits such as neuroticism13 14; vascular factors such as heart disease and stroke15 16; brain changes such as white matter hyperintensities,17 brain metabolic dysfunction18 and structural changes19–21; and psychosocial stress.16 Research on the relationship between SMI and development of Alzheimer’s disease (AD) and dementia has demonstrated mixed results, both positive 22–24 and negative associations.25 Recently, the Subjective Cognitive Decline (SCD-I) Working Group has suggested that memory-related features increase the risk of developing preclinical AD.26 These features include subjective decline in memory, onset within last 5 years, age >60 years, concerns of cognitive decline, self-perceived worse performance compared with others and confirmation of decline by an informant. In summary, SMI seems to be related to many different diseases, directly or indirectly, but there is no clear understanding of the relationship between SMI and disease.
Subjective memory is commonly conceptualised as a unidimensional entity, although theories of performance-based memory and empirical findings from neurocognitive research and clinical praxis show that this is not the case.27–30 Rather, human memory consists of several functional systems encompassing short-term memory (STM; working memory) and long-term memory (LTM), which is usually divided into declarative and non-declarative memory. Declarative memory can be further divided into episodic and semantic memory and non-declarative memory can be divided into procedural and perceptual memory. In addition, three processes operate on these memory systems: encoding, maintaining/consolidating and retrieving. The same typology could be used to conceptualise subjective memory similarly to the standard model of human memory.
Research on subjective memory analysed in terms of current theories of memory, for instance, using the different components of memory, is sparse, although there are exceptions showing that subjective memory contains several dimensions.31 In particular, it is interesting to investigate the relationship between aspects of SMI and health and disease, since SMI in general has been found to be associated with many diseases (see above).
The purpose of this study was to differentiate SMI into memory components, following theories of memory, and to identify demographic and health-related factors associated with these components, using cross-sectional data from a population-based study of health in the Nord-Trøndelag county in middle Norway.
Methods
Participants
The Nord-Trøndelag region in Norway has about 130 000 inhabitants. The third wave of the health survey (HUNT3) was conducted from October 2006 to June 2008. All citizens aged ≥20 years (n=93 860) were invited to participate and 51 352 individuals accepted to participate (54.1%). Details of the HUNT3 survey have been reported previously.2 In this study, all individuals aged ≥55 years who completed all items in the Metamemory Questionnaire (MMQ) were investigated (n=18 633). The demographic characteristics of these individuals are presented in table 1.
Table 1.
Demographic characteristics
| Age (mean±SD), years | 66.8±8.4 |
| Range, years | 55–97 |
| Gender, frequency of females (%) | 9761 (52.4) |
| Education, number and frequency (%) | |
| Basic | 7029 (37.7) |
| High school | 6086 (32.7) |
| College/university | 3332 (17.8) |
| Missing | 2186 (11.7) |
Metamemory questionnaire
Nine items about memory were included in the HUNT3 MMQ (see APPENDIX in online supplementary material). These items were originally designed for a study on health and ageing in the four Nordic countries and were intended to capture memory performance in a single summed score.32 However, the items can also be evaluated as indexing different aspects of memory. Two items are concerned with memory performance in general (items 1 and 2); three are concerned with accessing previously acquired information (semantic memory; items 4, 5 and 8) and four are concerned with recent events or ongoing activity (working and episodic memory; items 3, 6, 7 and 9).
bmjopen-2016-013586supp001.doc (23KB, doc)
The HUNT3 health questionnaire
The HUNT3 survey also included questions on 20 diseases/syndromes covering 10 types of disease according to the manual of the International Classification of Diseases, 10th revision (ICD-10)33: neoplasms (cancer), blood and blood-forming (kidney), endocrine (diabetes), mental (depression), nervous (epilepsy), sensory (cataract, glaucoma, macula degeneration), circulatory (angina pectoris, heart infarction, heart failure, other heart diseases, brain infarction), respiratory (asthma, chronic obstructive pulmonary disease (COPD)), musculoskeletal (osteoarthritis, rheumatoid arthritis, fibromyalgia, Bechterew’s disease) and external causes (whiplash). Three types of disease were not included in the HUNT3 questionnaire: infectious, digestive and skin diseases. The questions were expressed as follows: Have you had a heart infarction? The responses were no (coded 0) or yes (coded 1). For a detailed description of the health questionnaire, see the website www.ntnu.edu/hunt/databank.
Depression in HUNT3
The Hospital Anxiety and Depression Scale (HADS) was used to assess the degree of depression in participants.34 This method has high sensitivity and specificity in relation to DSM-IV diagnosis.35 Depression was defined as a HADS depression score ≥8.
Statistical analysis
A principal component analysis (PCA) was performed on the nine SMI items using an eigenvalue >1 as the criterion to decide the number of relevant factors followed by varimax rotation to find a simple structure. The frequency of reported disease is presented as a percentage. The possible influence of demographic factors (seven age groups, gender and three levels of education) as independent variables was analysed by means of three-way multivariate analysis of variance with memory (two components; repeated measure) as the dependent variable. The relationships between the components and the 20 diseases were analysed by one-way MANOVA with memory (LTM and STM components) as the dependent variable and each disease as the independent variable. The z-scores for LTM and STM for each disease were used to visualise the profile of memory across diseases.
Results
Components of the HUNT3 memory questionnaire
PCA of the nine memory items resulted in two memory components. The first component accounted for 43% of the total variance and the second for 11.4%. On the basis of orthogonally rotated component matrix and the pattern of loadings between item and component, the first component was interpreted as LTM. The items with large loadings in this component concerned retrieval of previously acquired factual knowledge. The second component was interpreted as STM because items with large loadings were related to current and recently apprehended information (not previously acquired), see table 2. This structure indicates that self-reported memory was related to at least two aspects of memory. To check the robustness of the statistical model, a maximum likelihood (ML) analysis with oblique rotation was performed and the results were essentially equivalent to the result with PCA. ML analyses of subgroups based on age or education did not make a difference.
Table 2.
Matrix showing loadings between memory items and two orthogonally rotated components
| Item number | Component I | Component II |
| 5. Remember dates | 0.696 | 0.214 |
| 2. Changed memory | 0.692 | 0.192 |
| 1. Memory problems | 0.666 | 0.319 |
| 4. Remember names | 0.639 | 0.146 |
| 8. Events years ago | 0.572 | 0.205 |
| 3. Events minutes ago | 0.228 | 0.750 |
| 9. Keeping track | 0.123 | 0.736 |
| 6. Planned activities | 0.244 | 0.716 |
| 7. Events days ago | 0.378 | 0.684 |
Loadings >0.500 are marked as bold.
Figure 1 shows the frequency of complaints for the nine items. The pattern of complaints was different for the two components of SMI. The frequency was more pronounced for items associated with the LTM component (items 1, 2, 4, 5 and 8), that is, information that has been stored. In contrast, the frequency of complaints was relatively sparse for items associated with the STM component (items 3, 6, 7 and 9), that is, information that has recently been attended to or has recently existed in the mind.
Figure 1.
Frequency of memory problems across nine items divided into two subgroups of memory: long-term memory and short-term memory. No complaints refers to ‘no/never’ responses to the items in Metamemory Questionnaire.
Memory components and demographic characteristics
Regression-based scores were calculated for each component (LTM and STM). A three-way MANOVA was performed on the LTM and STM components as within factors, and demographic characteristics (age, gender and education) as between factors. Age was divided into seven groups (55–59, 60–64, 65–69, 70–74, 75–79, 80–84 and >85 years), and education was divided into three levels (basic: ≤9 years; high school: 10–12 years; and college/university: ≥13 years). The memory components were not significantly different (F<1.0, df=1/16330, p>0.1), whereas two demographic characteristics were significant: age (F=36.25, df=6/16330, p<0.001, η2=0.013) and gender (F=115.22, df=1/16330, p<0.001, η2=0.007). Education was marginally significant (F=2.94, df=2/16330, p=0.053, η2 <0.001).
All three two-way interactions including the memory components were significant: memory versus age (F=14.54, df=6/16330, p<0.001, η2=0.005), memory versus gender (F=13.72, df=1/16330, p<0.001, η2=0.001) and memory versus education (F=13.22, df=2/16330, p<0.001, η2=0.002). The memory versus age interaction was the result of a differential pattern of LTM versus STM results across the age groups as shown by a relatively higher score on STM for the oldest group. The mean values in the STM component were significantly poorer than the LTM component in the youngest age group (55–59: t=−5.61, df=4647, p<0.001) and the two oldest age groups (80–84: t=2.81, df=1124, p<0.01; and ≥85: t=3.97, df=389, p<0.001), whereas the pattern was the opposite (LTM was affected more than STM) in two of the intervening age groups (60–64: t=3.74, df=4541, p<0.001; and 65–69: t=5.21, df=3349, p<0.001). The difference between memory components in two of the age groups (70–74 and 75–79 years of age; p>0.1) was non-significant. The age versus memory interaction is presented in figure 2.
Figure 2.
The relationships between seven age groups and problems (component score) with long-term memory (LTM) and short-term memory (STM).
The significant effect of gender was due to the fact that, in general, females reported fewer complaints than males. The significant interaction between memory and gender was related to the finding that males reported more STM than LTM complaints (t=5.70, df=8843, p<0.001), whereas females reported more LTM than STM complaints (t=5.46, df=9697, p<0.001).
The interaction between memory problems and level of education demonstrated a differential pattern. Those with a basic education had more STM than LTM problems (t=4.66, df=7040, p<0.001) and those with a high level of education had more LTM than STM problems (t=6.52, df=2161, p<0.001).
Among the three two-way interactions with demographic characteristics, age versus gender was significant (F=3.12, df=6/16330, p<0.01, η2=0.001) due to increasing difference in memory problems between men and women with higher age. The age versus education was not significant (F<1.0, df=12/16330, p>0.1, η2 <0.001) nor gender versus education (F<1.0, df=2/16330, p>0.1, η2=0.001).
None of the four three-way and the single four-way interactions were significant (all p values >0.1).
Memory components and health
The 20 binary questions on diseases in the HUNT3 questionnaire showed that osteoarthritis, cataract and asthma were the most common diseases, followed by heart and brain diseases and depression, while epilepsy and Bechterew’s disease were least common. It should be noted that there were no questions on dementia, although some affected individuals may have participated in HUNT3. The frequency of the 20 diseases/syndromes is reported in table 3.
Table 3.
Frequency of diseases, the effect of disease on memory and the interaction of disease and memory
| ICD-10 code/disease | Frequency | Percent | p Value | ||
| Disease | Memory | Disease x Memory | |||
| Musculatory and skeletal | |||||
| Osteoarthritis | 4722 | 26.8 | <0.001 | ns | ns |
| Rheumatoid arthritis | 926 | 5.2 | <0.001 | ns | ns |
| Fibromyalgia | 918 | 5.2 | <0.001 | ns | ns |
| Bechterew’s disease | 363 | 1.6 | ns | ns | ns |
| Sensory | |||||
| Cataract | 2684 | 15.3 | <0.001 | ns | ns |
| Glaucoma | 945 | 5.6 | <0.01 | ns | ns |
| Macular degeneration | 731 | 4.3 | <0.001 | ns | ns |
| Respiratory | |||||
| Asthma | 2155 | 11.6 | =0.004 | ns | ns |
| COPD | 967 | 5.2 | <0.001 | =0.037 | =0.020 |
| Mental | |||||
| Depression | 1748 | 11.0 | <0.001 | ns | =0.028 |
| Neoplasm | |||||
| Cancer | 1757 | 9.4 | <0.001 | =0.014 | =0.002 |
| Endocrine | |||||
| Diabetes | 1360 | 7.3 | <0.001 | =0.002 | <0.001 |
| Circulatory | |||||
| Angina pectoris | 1387 | 7.4 | <0.001 | ns | ns |
| Heart infarction | 1180 | 6.3 | <0.001 | ns | ns |
| Other heart diseases | 1054 | 5.7 | <0.001 | ns | ns |
| Brain infarction | 880 | 4.7 | <0.001 | <0.001 | <0.001 |
| Heart failure | 419 | 2.2 | <0.001 | <0.001 | <0.001 |
| Blood and blood forming organs | |||||
| Kidney | 630 | 3.4 | <0.001 | ns | ns |
| External lesions whiplash | 789 | 4.6 | <0.001 | 0.050 | ns |
| Nervous epilepsy | 281 | 1.6 | =0.005 | ns | ns |
COPD, chronic obstructive pulmonary disease; ICD, International Classification of Diseases; ns, not significant.
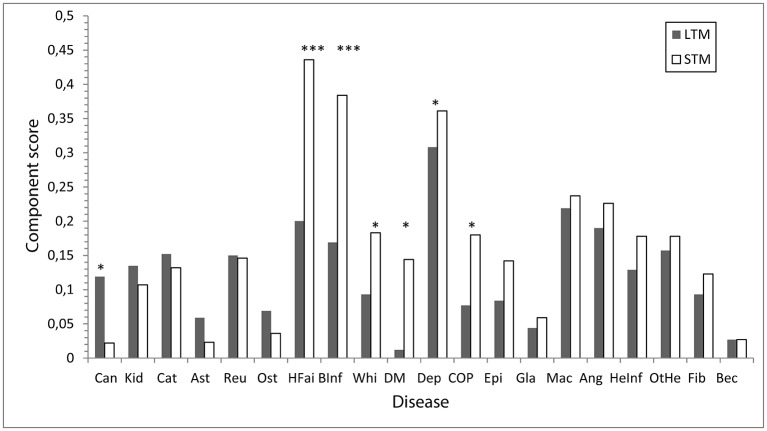
The effect of disease (yes or no) on memory components was analysed using one-way MANOVA with the two memory components as dependent variables and the disease as the independent variable. The outcomes of these analyses are presented in table 3. All diseases except Bechterew’s disease had a significant impact on memory (all ps<0.01 or<0.001), although the effect size was rather low (η2≤0.028). The memory components were significantly and differentially affected in six diseases: stroke (F=17.66, df=1/18629, p<0.008, η2=0.001), heart failure (F=10.90, df=1/18629, p<0.001, η2=0.005), diabetes (F=9.29, df=1/18629, p=0.001, η2 <0.001), cancer (F=6.05, df=1/18628, p=0.014, η2 <0.001), COPD (F=4.34, df=1/18624, p=0.037, η2 <0.001) and whiplash (F=3.84, df=1/17152, p<0.05, η2 <0.001). The disease and memory interaction was significant in the six diseases: depression (F=4.85, df=1/15280, p=0.028, η2 <0.001), stroke (F=21.52, df=1/18629, p<0.001, η2=0.001), heart failure (F=11.94, df=1/18629, p<0.001, η2 <0.001), diabetes (F=12.75, df=1/18627, p<0.001, η2 <0.001), cancer (F=9.19, df=1/18628, p=0.002, η2 <0.001) and COPD (F=5.40, df=1/18624, p=0.020, η2 <0.001). The significant interactions were associated with higher impairment in STM than in LTM for six diseases: heart failure, stroke, COPD, diabetes, whiplash and depression, see figure 3. The opposite pattern was seen with cancer; that is, there was more impairment in LTM than in STM. Thus, the memory components were differentially influenced by the six diseases. Self-reported memory was most affected by depression, followed by stroke and heart disease. The effect of depression was 10 times greater than that of the other diseases. For individuals without disease, there was no significant difference between the two memory components. Furthermore, the level of memory impairment was very close to z=0. In contrast, the level of impairment was marked and there was a clear difference between individuals with and without disease in both memory components, see figure 3.
Figure 3.
Degree of self-reported problems (mean component score) in long-term memory (LTM) and short-term memory (STM) components for individuals each of the 20 diseases. The six diseases with a significant effect on memory are indicated by symbols for p value. *p<0.05; **p<0.01; ***p<0.001. Ang, angina pectoris; Ast, asthma; Bec, Becheterw’s disease; BInf, brain infarction; Can, cancer; Cat, cataract; COP, chroniv obstructive pulmonary disease; Dep, depression; DM, diabetes mellitus; Epi, epilepsy; Fib, fribromyalgia; Gla, glaucoma; HeInf, heart infarction; HFai, heart failure; Kid, kidney; Mac, macular degeneration; Ost, osteoarthritis; OtHe, other heart diseases; Reu, rheumatoid arthritis; Whi, whip lash.
Discussion
Main findings in context
There were four main results from this study. First, the memory questionnaire in HUNT3 measured two independent components of self-reported memory problems. SMI for the LTM was associated with previously acquired information and seemed to be due to retrieval difficulties. SMI for the STM component was associated with information that was currently being attended to or information that had recently been attended to. The differentiation of subjective memory into two components has not been reported previously to our knowledge.
Although the finding that the memory questionnaire in HUNT3 has two components is new, it is in agreement with current conceptualisation of performance-based memory.27 29 36 The STM component may be thought of as related to attention and information that is in the mind at the moment or recently was in mind. The LTM component, on the other hand, may be conceived of as related to old knowledge or events that occurred long ago. The questionnaire used in the HUNT3 survey covered two of these memory systems: working memory and declarative memory. Consequently, the dimensionality of SMI should be investigated using methods other than the MMQ in future research. Methodological problems may otherwise be seen to be causing inconsistent results on the relationship between subjective and objective memory measures.10 31 37
Second, the pattern of impairment differed according to diseases. In depression, stroke, heart failure, diabetes and COPD, the impairment was greater in STM than in LTM, whereas the opposite pattern was seen in cancer. This difference in the pattern of associations between disease and components of subjective memory has not been reported previously to our knowledge.
A third finding was that the frequency of SMI increased with age for both LTM and STM. However, the pattern of increased memory problems was different. STM impairment was most pronounced in the oldest and next oldest age groups and was clearly more marked than LTM impairment in participants with specific diseases. LTM impairment was more pronounced than STM impairment in the earlier old age groups (60–80 years).
Finally, all diseases except Bechterew’s disease had a significant impact on memory, see figure 2. The three most pronounced effects were seen in association with depression, heart failure and brain infarction, in that order (i.e. in mental and cardiovascular diseases). The three smallest effects were seen in association with asthma, glaucoma and osteoarthritis, in that order. The disease affected both memory components, except in cancer, where the effect on LTM was greater than that on STM. It was not possible to discover why cancer has a different effect on memory than other diseases from our data, but it is possible that the treatment procedures for cancer result in more widespread cerebral consequences than for the other diseases studied, as cancer is commonly treated with a combination of chemotherapy, surgery and radiation for several months or years.38
Implications for public health and clinical practice
It is commonly observed that both depression and cardiovascular-related diseases are associated with poor subjective memory15 16 39 and objective memory impairment.40 41 Our study has shown that depression, brain infarction, heart failure and diabetes are linked with SMI and that this impairment is primarily associated with the STM component. This finding indicates that SMI is predominantly associated with attention and concentration, as indicated by the STM component, rather than with basic memory problems, as indicated by the LTM component. A clinical consequence of this finding and interpretation is that subjective memory problems have to be differentiated in primary care because the STM component and not the LTM component were associated with disease.
Memory impairment and age
Most diseases, both physical and mental, become more common with age, and the prevalence of memory impairment in general follows the same pattern. The typical finding in population-based and clinical-based studies has been that working memory seems particularly vulnerable to ageing and disease, while semantic memory seems to be better preserved with age. Another typical finding of population-based and clinical-based studies is that many diseases affect episodic memory and working memory, while semantic memory can remain functioning relatively well.42
Strengths and weaknesses of the study
The main strengths of this study are the number of subjects included, the population-based sample and the availability of data on a broad variety of essential health-related factors. These points support the external (generalisability to the general population) and internal (memory and disease relationship) validity of the study.
Another strength is that different methods of factor analysis resulted into a differentiation of the MMQ content in two similar components of subjective memory.
A weakness concerns the fact that the data were obtained from subjective reports, which are known to be of varying reliability and validity, although there are recent reports of a strong association between subjective and objective memory.43 There may have been sampling and/or survival biases in the available subjects: individuals with non-registered dementia may have participated, and awareness of one’s own condition is known to vary among people with objective memory impairment. In addition, some individuals with many diseases have died. These factors could have obscured the true prevalence and associations between the studied factors, thus increasing the uncertainty of the resulting generalisations.
Conclusions
SMI as measured by the MMQ was differentiated into two components related to LTM and STM. Moreover, the components were differentially associated with six diseases, particularly depression and cardiovascular diseases.
bmjopen-2016-013586supp002.docx (38KB, docx)
Supplementary Material
Acknowledgments
Nord-Trøndelag Health Study (The HUNT Study) is a collaborative study with the HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority and the Norwegian Institute of Public Health.
Footnotes
Contributors: OA, OB, IB and ES conceived the study. OA analysed the data and drafted the manuscript. All authors contributed to the final version.
Funding: This work was supported Nord-Trøndelag Health Trust, Norway.
Competing interests: None declared.
Patient consent: Next of kin consent obtained.
Ethics approval: The study was approved by the Regional Ethics Committee (REK Midt 2015/843).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data has to be requested from https://www.ntnu.edu/hunt/hunt-surveys.
References
- 1. Iliffe S, Pealing L. Subjective memory problems. BMJ 2010;340:c1425. 10.1136/bmj.c1425 [DOI] [PubMed] [Google Scholar]
- 2. Krokstad S, Langhammer A, Hveem K, et al. Cohort Profile: the HUNT study, Norway. Int J Epidemiol 2013;42:968–77. 10.1093/ije/dys095 [DOI] [PubMed] [Google Scholar]
- 3. Vestergren P, Nilsson LG. Perceived causes of everyday memory problems in a population-based sample aged 39-99. Appl Cogn Psychol 2010;24:1–10. [Google Scholar]
- 4. Gagnon M, Dartigues JF, Mazaux JM, et al. Self-reported memory complaints and memory performance in elderly french community residents: results of the PAQUID Research Program. Neuroepidemiology 1994;13:145–54. 10.1159/000110373 [DOI] [PubMed] [Google Scholar]
- 5. Jonker C, Launer LJ, Hooijer C, et al. Memory complaints and memory impairment in older individuals. J Am Geriatr Soc 1996;44:44–9. 10.1111/j.1532-5415.1996.tb05636.x [DOI] [PubMed] [Google Scholar]
- 6. Abdulrab K, Heun R, Impairment SM. Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry 2008;23:321–30. 10.1016/j.eurpsy.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 7. Beaudoin M, Desrichard O. Are memory self-efficacy and memory performance related? A meta-analysis. Psychol Bull 2011;137:211–41. 10.1037/a0022106 [DOI] [PubMed] [Google Scholar]
- 8. Genziani M, Stewart R, Béjot Y, et al. Subjective memory impairment, objective cognitive functioning and social activity in french older people: findings from the three cities study. Geriatr Gerontol Int 2013;13:1–7. 10.1111/j.1447-0594.2012.00873.x [DOI] [PubMed] [Google Scholar]
- 9. Hohman TJ, Beason-Held LL, Resnick SM. Cognitive complaints depressive symptoms and cognitive impairment: are they related? J Am Geriatr Soc 2011;59:1908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zandi T. Relationship between subjective memory complaints, objective memory performance, and depression among older adults. Am J Alzheimers Dis Other Demen 2004;19:353–60. 10.1177/153331750401900610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 2006;22:471–85. 10.1159/000096295 [DOI] [PubMed] [Google Scholar]
- 12. Stordal E, Bjartveit Krüger M, Dahl NH, et al. Depression in relation to age and gender in the general population: the Nord-Trøndelag Health Study (HUNT). Acta Psychiatr Scand 2001;104:210–6. 10.1034/j.1600-0447.2001.00130.x [DOI] [PubMed] [Google Scholar]
- 13. Ausén B, Edman G, Almkvist O, et al. Personality features in subjective cognitive impairment and mild cognitive impairment--early indicators of dementia? Dement Geriatr Cogn Disord 2009;28:528–35. 10.1159/000255104 [DOI] [PubMed] [Google Scholar]
- 14. Kliegel M, Zimprich D, Eschen A. What do subjective cognitive complaints in persons with aging-associated cognitive decline reflect? Int Psychogeriatr 2005;17:499–512. 10.1017/S1041610205001638 [DOI] [PubMed] [Google Scholar]
- 15. Langballe EM, Tambs K, Saltvedt I, et al. The association between vascular factors and subjective memory impairment in older people: the HUNT Study, Norway. Nor Epidemiol 2012;22:209–15. 10.5324/nje.v22i2.1568 [DOI] [Google Scholar]
- 16. Paradise MB, Glozier NS, Naismith SL, et al. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry 2011;11:108. 10.1186/1471-244X-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haley AP, Hoth KF, Gunstad J, et al. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am J Geriatr Psychiatry 2009;17:976–85. 10.1097/JGP.0b013e3181b208ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mosconi L, De Santi S, Brys M, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry 2008;63:609–18. 10.1016/j.biopsych.2007.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meiberth D, Scheef L, Wolfsgruber S, et al. Cortical thinning in individuals with subjective memory impairment. J Alzheimers Dis 2015;45:139–46. 10.3233/JAD-142322 [DOI] [PubMed] [Google Scholar]
- 20. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006;67:834–42. 10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol 2004;251:671–5. 10.1007/s00415-004-0390-7 [DOI] [PubMed] [Google Scholar]
- 22. Jessen F, Wiese B, Bachmann C, et al. German study on Aging, Cognition and Dementia in primary care patients Study Group. prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;67:414–22. [DOI] [PubMed] [Google Scholar]
- 23. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000;15:983–91. [DOI] [PubMed] [Google Scholar]
- 24. Kim JM, Stewart R, Kim SW, et al. A prospective study of changes in subjective memory complaints and onset of dementia in South Korea. Am J Geriatr Psychiatry 2006;14:949–56. 10.1097/01.JGP.0000214857.66638.ed [DOI] [PubMed] [Google Scholar]
- 25. Silva D, Guerreiro M, Faria C, et al. Significance of subjective memory complaints in the clinical setting. J Geriatr Psychiatry Neurol 2014;27:259–65. 10.1177/0891988714532018 [DOI] [PubMed] [Google Scholar]
- 26. Jessen F, Amariglio RE, van Boxtel M. Subjective cognitive decline Initiative (SCD-I) Working group. A conceptual framework for research on subjective cognitive decline in preclinical alzheimer’s disease. Alzheimer’s Dement 2014;10:844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baddeley AD. The psychology of memory Baddeley AD, The handbook of memory disorders. Chichester, UK: Wiley, 2002. [Google Scholar]
- 28. Giovanello KS, Verfaellie M. Memory systems of the brain: a cognitive neuropsychological analysis. Semin Speech Lang 2001;22:109–18. 10.1055/s-2001-13935 [DOI] [PubMed] [Google Scholar]
- 29. Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron 1998;20:445–68. 10.1016/S0896-6273(00)80987-3 [DOI] [PubMed] [Google Scholar]
- 30. Nadel L, Hardt O. Update on memory systems and processes. Neuropsychopharmacology 2011;36:251–73. 10.1038/npp.2010.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett-Levy J, Powell GE. The subjective memory questionnaire (SMQ). An investigation into the self-reporting of ‘real-life’ memory skills. British Journal of Social and Clinical Psychology 1980;19:177–88. 10.1111/j.2044-8260.1980.tb00946.x [DOI] [Google Scholar]
- 32. Fromholt P, Berg S. et al. Self-reported memory and cognitive performance among 75-year old people from three Nordic cities : Heikkinen E, Berg S, Schroll M, Steen B, Viidik A, ; Functional status, health and aging, the Nora study. Paris: Serdi publishing company, 1997:55–65. [Google Scholar]
- 33. International classification of diseases, edition 10 (ICD-10) WHO; 1989. [Google Scholar]
- 34. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 35. Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital anxiety and depression Scale. an updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 36. Brown SC, Craik FIM. Encoding and retrieval of information : Tulving E, Craik FIM, The Oxford handbook of memory. New York: Oxford University Press, 2000. [Google Scholar]
- 37. Barker A, Prior J, Jones R. Memory complaint in attenders at a self-referral memory clinic: the role of cognitive factors, affective symptoms and personality. Int J Geriatr Psychiatry 1995;10:777–81. 10.1002/gps.930100908 [DOI] [Google Scholar]
- 38. Janelsins MC, Kohli S, Mohile SG, et al. An update on Cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol 2011;38:431–8. 10.1053/j.seminoncol.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holmen J, Langballe EM, Midthjell K, et al. Gender differences in subjective memory impairment in a general population: the HUNT study, Norway. BMC Psychol 2013;1:19. 10.1186/2050-7283-1-19 [DOI] [Google Scholar]
- 40. Bergman I, Blomberg M, Almkvist O. The importance of impaired physical health and age in normal cognitive aging. Scand J Psychol 2007;48:115–25. 10.1111/j.1467-9450.2007.00594.x [DOI] [PubMed] [Google Scholar]
- 41. Reed NM. The relationship between subjective memory and objective cognition, depression and anxiety by dementia status. 2010, http://scholorworks.gsu.edu/gerontology_thesis/19
- 42. Glorioso C, Sibille E, destiny B. Between destiny and disease: genetics and molecular pathways of human central nervous system aging. Prog Neurobiol 2011;93:165–81. 10.1016/j.pneurobio.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Snitz BE, Small BJ, Wang T, et al. Do subjective memory complaints lead or follow objective cognitive change? A five-year population study of temporal influence. J Int Neuropsychol Soc 2015;21:732–42. 10.1017/S1355617715000922 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013586supp001.doc (23KB, doc)
bmjopen-2016-013586supp002.docx (38KB, docx)