Abstract
Objective
This paper aims to study if vaginal breech delivery is associated with increased risk for neonatal mortality (NNM) or cerebral palsy (CP) in Norway where vaginal delivery accounts for 1/3 of all breech deliveries.
Design
Cohort study using information from the national Medical BirthRegister and Cerebral Palsy Register.
Setting
Births in Norway 1999–2009.
Participants
520 047 term-born singletons without congenital malformations.
Main outcome measures
NNM, CP and a composite outcome of these and death during birth.
Results
Compared with cephalic births, breech births had substantially increased risk for NNM but not for CP. Vaginal delivery was planned for 7917 of 16 700 fetuses in breech, while 5561 actually delivered vaginally. Among these, NNM was 0.9 per 1000 compared with 0.3 per 1000 in vaginal cephalic delivery, and 0.8 per 1000 in those actually born by caesarean delivery (CD) in breech. Compared with planned cephalic delivery, planned vaginal delivery was associated with excess risk for NNM (OR 2.4; 95% CI 1.2 to 4.9), while the OR associated with planned breech CD was 1.6 (95% CI 0.7 to 3.7). These risks were attenuated when NNM was substituted by the composite outcome. Vaginal breech delivery was not associated with excess risk for CP compared with vaginal cephalic delivery.
Conclusion
Vaginal breech delivery, regardless of whether planned or actual, and actual breech CD were associated with excess risk for NNM compared with vaginal cephalic delivery, but not with CP. The risk for NNM and CP in planned breech CD did not differ significantly from planned vaginal cephalic delivery. However, the absolute risk for these outcomes was low, and taking into consideration potential long-term adverse consequences of CD for the child and later deliveries, we therefore conclude that vaginal breech delivery may be recommended, provided competent obstetric care and strict criteria for selection to vaginal delivery.
Keywords: breech delivery, epidemiology, cerebral palsy, perinatal mortality
Strengths and limitations of this study.
More than 500 000 births included in the study.
Prospectively recording of the data in the two registers
Restriction of the analyses to singletons at term without congenital malformation
The number of infants with adverse outcomes in breech were low.
Register-based data have limited ability to address explanatory factors.
Introduction
The delivery of a fetus in breech position is a controversial issue.1 The Term Breech Trial (TBT)2 reported lower perinatal mortality and morbidity of fetuses in breech position following planned caesarean delivery compared with planned vaginal delivery. The study had great impact, changing clinical practice in a number of countries.3–6 However, the conclusion of the TBT was criticised by several experts.7–9 The Norwegian Board of Health invited a group of national experts to review the evidence underlying these recommendations. The expert group reviewed the literature published between 1980 and 2001. Taking into account the much lower perinatal mortality in Norway than that reported in TBT, they concluded that vaginal breech delivery would still be safe, provided careful selection of mothers, qualified clinicians and adequate fetal assessment.10 Therefore, approximately 1/3 of fetuses in breech position in Norway are still delivered vaginally.6 In a prospective study in France and Belgium, Goffinet et al compared vaginal delivery with planned caesarean delivery in breech. They concluded, in line with the Norwegian recommendations, that vaginal delivery is a safe option when strict selection criteria are followed.11 The controversies of mode of delivery have also been reflected in studies of the long-term outcome of infants born in breech position. Several studies reported that infants born in breech had increased risk for cerebral palsy (CP).12–15 Although it was unclear whether the mode of delivery affected this increased risk,16–18 it has been suggested that planned caesarean delivery may prevent some cases of CP.12 13
In the vast majority of previous studies on adverse outcome of vaginal breech delivery, the comparison group has been caesarean breech delivery. However, the main results of these studies do not take into account the risk for complications of caesarean delivery in later pregnancies both for the mother and for the child. There is also an increased awareness of later health problems in children born by caesarean suggested in recent reports.19 Therefore, to assess if vaginal breech delivery as currently practised in Norway can be characterised as safe, the appropriate comparison group of breech deliveries should be vaginal cephalic birth; which is the natural way of giving birth. In line with this, a recent systematic review and meta-analysis of breech deliveries recommended that comparative studies of vaginal breech with vaginal cephalic deliveries should be undertaken.20
The aim of this study was therefore to explore if singletons without congenital malformations born vaginally at term have higher risk for stillbirth, neonatal mortality (NNM) and CP if they are born in breech position compared with cephalic position.
Methods
In this population-based study, perinatal data of all children born in Norway from 1999 to 2009 were retrieved from the Medical Birth Registry of Norway (MBRN), and combined with information recorded in the Cerebral Palsy Register of Norway (CPRN). The 11-digit personal identification number unique for every Norwegian citizen was used to link information from the two registers. The MBRN records demographic variables, as well as information on maternal health before and during pregnancy, interventions and complications during delivery and neonatal outcomes. Registration in this register has been compulsory since 1967 ensuring prospective recording of this information at birth.21
The CPRN is an informed consent-based national quality register established in 2006, and aims to record detailed information on all children with CP born in Norway since 1996.22 Information is reported at diagnosis at 5 years and at 15–17 years of age. Neuropaediatric habilitation centres in Norway provide summary and detailed data about the children. A validation study indicated that 80% of children with CP in Norway born in 1999–2009 have detailed information in the CPRN.23
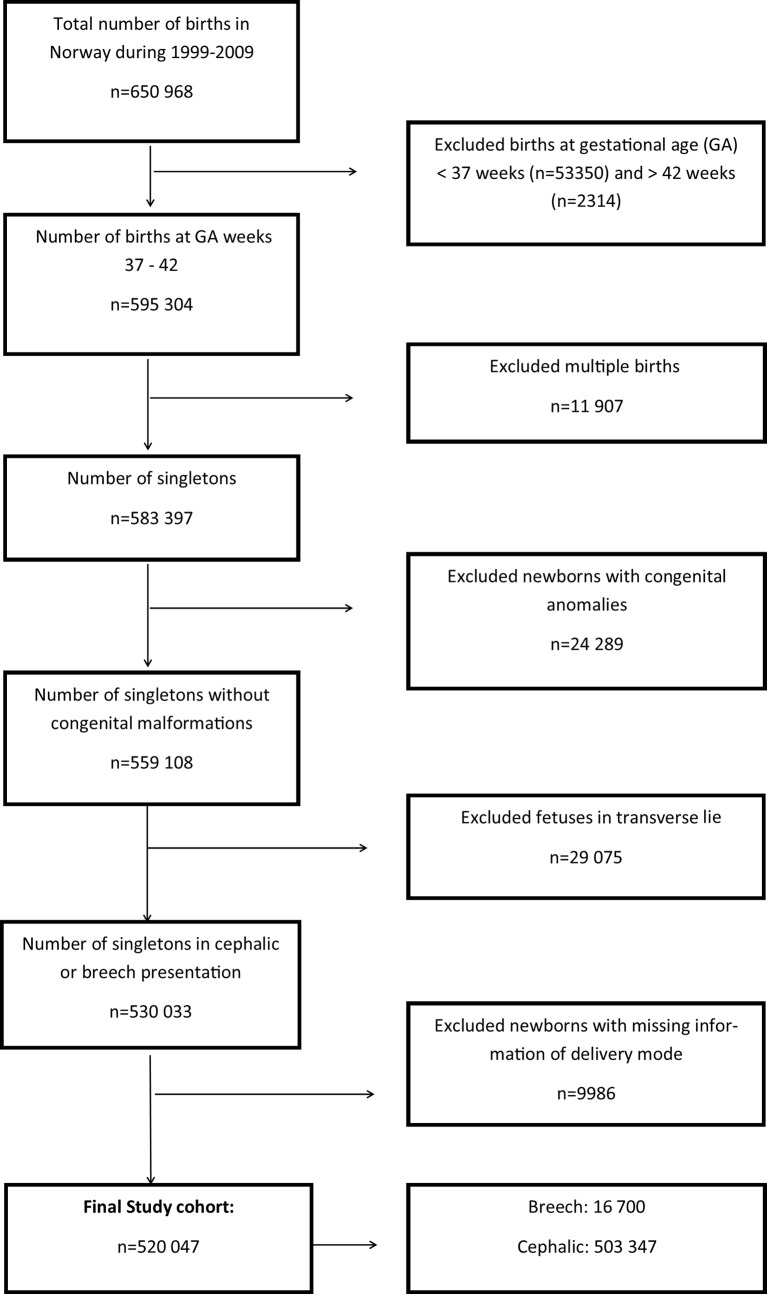
We excluded children born preterm (before week 37), multiple births, children with congenital malformations, children in transverse lie and those with lacking information on mode of delivery (figure 1).
Figure 1.
Flow chart of the study population.
Study variables
The predefined main outcome measures were stillbirth, NNM and CP. Stillbirth and NNM were defined according to the WHO.24 Stillbirth was further divided into those who were dead before birth (antepartum) and during birth (intrapartum). CP was diagnosed and confirmed at 5 years of age according to the definition and classification proposed by the Surveillance of Cerebral Palsy in Europe.25 Paediatricians at the neurohabilitation centres in Norway completed the information of each child on a standardised form. Since fetuses who die antepartum usually are delivered vaginally, stillbirth was not included in the analyses of risk associated with mode of delivery. However, since intrapartum death, NNM and CP may share the same causes we, as a secondary outcome, also calculated a composite adverse outcome variable comprising the sum of intrapartum death, NNM and CP.
Information on maternal age, parity, gestational age, mode of delivery, the child’s sex, birth weight and Apgar scores were collected from the MBRN. Newborns with a birth weight below −2 SD of the population mean weight26 for gestational age, adjusted for sex were defined as small-for-gestational age (SGA).
Analytical approach
First, we assessed the risks for stillbirth, NNM, CP and the composite outcome for children born in breech compared with cephalic position, independent of mode of delivery.
Second, we explored the risks for NNM, CP and the composite outcome according to ‘actual mode of delivery’ by comparing vaginal or caesarean breech delivery with vaginal cephalic delivery.
According to the Norwegian Society for Gynecology and Obstetrics, vaginal breech delivery can be recommended if gestational age is at least 34 weeks, estimated birth weight is between 2000 and 4000 g and no maternal and fetal contraindications for vaginal delivery exist. An essential premise of this recommendation is that the obstetric department is capable to perform immediate caesarean delivery and that trained paediatric personnel are available. Thus, some of the planned vaginal breech deliveries will be converted to a caesarean delivery during the birth process. The analysis of actual mode of delivery will therefore not evaluate these recommendations of vaginal births correctly, since the caesarean group will be a mixture of both planned and emergency caesarean delivery, and the vaginal group will comprise only those not changed to a caesarean delivery during birth.
Third, we therefore repeated the analyses, but now we compared the outcome of planned mode of breech delivery at admission to the obstetric department with planned vaginal cephalic delivery. We divided cephalic and breech births into the two categories originally planned vaginal and caesarean deliveries, based on the initial handling of the birth, using information on how the birth started (spontaneous, induced or by caesarean delivery) and how caesarean delivery was recorded (as elective, emergency or planned). Births that did not satisfy these criteria were categorised as planned vaginal delivery.
The three outcomes, NNM, CP and the composite adverse outcome variables, were then assessed related to the four exposure groups: cephalic position and planned vaginal births (reference group), cephalic position and planned caesarean delivery, breech position and planned vaginal births and breech position and planned caesarean delivery.
Finally, we explored if the risk for NNM, CP and the composite outcome differed between children born by vaginal delivery and caesarean delivery or between planned vaginal delivery and planned caesarean delivery within the group of children who were born in breech.
Statistical analyses
IBM SPSS software for Windows V.22 was used for data analyses. Differences in proportions between groups were analysed using the χ2 test and prevalence rates with 95% CIs were calculated according to Newcombe and Altman.27 In the estimates of the prevalence of NNM, stillbirths were excluded and in the estimates of the prevalence of CP, stillbirths and children with postneonatal CP were excluded. We used logistic regression to estimate ORs with 95% CIs for adverse outcome of children in breech position at birth, using cephalic presentation as the reference. Moreover, we explored the roles of potential confounders including maternal age, parity, gestational age, child sex and SGA status in multivariable logistic regression analyses based on a priori knowledge and directed acyclic graphs methodology.28
Patient involvement
No patients were involved in setting the research question or the outcome measures nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Results
A total of 650 968 children were born in Norway during the study period. The study population of singleton children born at a gestational age of at least 37 weeks in either cephalic or breech position and with no congenital anomalies comprised 520 047 children (figure 1). A total of 841 (2 per 1000) of these were stillborn. Of the live born, 239 (0.5 per 1000) died in the neonatal period, and 552 children were diagnosed with CP. Of the latter, 32 had a postneonatal cause of their CP, resulting in 520 with congenital CP (1 per 1000 live born).
Among the 520 047 included children, 16 700 (3%) were in breech and 503 347 (97%) in cephalic position (figure 1). More mothers in the breech group were nullipara, and higher proportions of their infants were female, were born SGA and had low Apgar scores (table 1). The mean gestational age of children born in breech was 39.1 weeks compared with 39.7 weeks for children born in cephalic position. Of the 16 700 women with a fetus in breech 7917 (47%) were planned for vaginal delivery, while 5561 (33%) actually delivered vaginally. The corresponding figures for planned caesarean delivery were 8783 (53%), while 11 139 (67%) actually delivered by caesarean. For women with fetuses in cephalic position, 94% were planned to vaginal delivery while 90% delivered vaginally; 6% were planned to caesarean delivery and 10% delivered by caesarean.
Table 1.
Maternal and infants characteristics in pregnancies where the child was born in breech or in cephalic position
| Breech position | Cephalic position | |||
| n 16 700 |
(%) (100) |
n 503 347 |
(%) (100) |
|
| Maternal age* | ||||
| ≤19 years | 290 | (2) | 11 889 | (2) |
| 20–34 years | 13 412 | (80) | 409 401 | (81) |
| ≥35 years | 2 998 | (18) | 82 031 | (17) |
| Parity | ||||
| Nullipara | 9 280 | (56) | 199 822 | (40) |
| Primipara | 4 599 | (27) | 184 068 | (36) |
| >1 para | 2 822 | (17) | 119 457 | (24) |
| Sex† | ||||
| Male | 7 540 | (45) | 257 128 | (51) |
| Female | 9 160 | (55) | 246 216 | (49) |
| Small-for-gestational age‡ | 424 | (2.5) | 7 130 | (1.4) |
| Apgar score at 5 min§ | ||||
| 0–3 | 93 | (0.6) | 1 477 | (0.3) |
| 4–6 | 427 | (2.4) | 7 913 | (1.7) |
| 7–10 | 16 139 | (97) | 492 858 | (98) |
*Information on maternal age were missing in 26 children in cephalic.
†Information on sex were missing in one child in cephalic.
‡Information on small-for-gestational age were missing in 12 children in breech and 361 in cephalic.
§Information on Apgar at 5 min were missing in 41 children in breech and 1099 in cephalic.
Children born in breech had increased risk for stillbirth, NNM, CP and the composite outcome compared with children born in cephalic position (table 2). Sixty-eight of the stillborn children (7 in breech and 61 in cephalic position) died during delivery.
Table 2.
All births: prevalence and unadjusted ORs with 95% CIs for various adverse outcomes among singletons born at term, without congenital anomalies in cephalic and breech positions
| Number of infants with adverse outcome | Total number of infants* | Prevalence per 1000 (CI) | OR (CI) | |
| Stillbirths | ||||
| Cephalic | 794 | 503 347 | 1.6 (1.5 to 1.7) | 1.0 (Reference) |
| Breech | 47 | 16 700 | 2.8 (2.1 to 3.7) | 1.8 (1.3 to 2.4) |
| Stillbirth antepartum | ||||
| Cephalic | 733 | 503 286 | 1.5 (1.4 to 1.6) | 1.0 (Reference) |
| Breech | 40 | 16 693 | 2.4 (1.8 to 3.3) | 1.6 (1.2 to 2.3) |
| Stillbirth intrapartum | ||||
| Cephalic | 61 | 502 614 | 0.1 (0.1 to 0.2) | 1.0 (Reference) |
| Breech | 7 | 16 600 | 0.4 (0.2 to 0.9) | 3.5 (1.6 to 7.6) |
| NNM* | ||||
| Cephalic | 225 | 502 553 | 0.5 (0.4 to 0.5) | 1.0 (Reference) |
| Breech | 14 | 16 653 | 0.8 (0.5 to 1.4) | 1.9 (1.1 to 3.2) |
| CP† | ||||
| Cephalic | 498 | 502 524 | 1.0 (0.9 to 1.1) | 1.0 (Reference) |
| Breech | 22 | 16 650 | 1.3 (0.9 to 2.0) | 1.3 (0.9 to 2.1) |
| Composite outcome‡ | ||||
| Cephalic | 784 | 502 585 | 1.6 (1.5 to 1.7) | 1.0 (Reference) |
| Breech | 43 | 16 657 | 2.6 (1.9 to 3.5) | 1.7 (1.2 to 2.3) |
*Removed stillbirths from the denominator in the analyses of NNM and CP.
†Removed postneonatal CP from the denominator in the analyses of CP as outcome.
‡Comprising intrapartum death, NNM and CP.
NNM, neonatal mortality; CP, cerebral palsy.
According to actual mode of delivery, children in breech, regardless of whether they were born vaginally or by caesarean delivery, had a nearly threefold increased OR for NNM compared with children born vaginally in cephalic, while the OR for CP was 1.7 (CI 1.0 to 2.8) if the child was delivered by caesarean delivery (table 3). As expected, children delivered by caesarean in cephalic position had higher prevalence of NNM, CP and the composite outcome compared with vaginal cephalic delivery, reflecting that caesarean delivery in this group is mainly done in high-risk births (table 3).
Table 3.
Actual mode of delivery: prevalence and unadjusted ORs with 95% CIs for various adverse outcomes among singletons born at term, without congenital anomalies according to actual mode of delivery
| Number of infants with adverse outcome | Total number of infants* | Prevalence per 1000 (CI) | OR (CI) | |
| NNM* | ||||
| Cephalic | ||||
| Vaginal delivery | 137 | 451 064 | 0.3 (0.3 to 0.4) | 1.0 (Reference) |
| Caesarean delivery | 88 | 51 489 | 1.7 (1.4 to 2.1) | 5.6 (4.3 to 7.4) |
| Breech | ||||
| Vaginal delivery | 5 | 5 518 | 0.9 (0.4 to 2.1) | 3.0 (1.2 to 7.3) |
| Caesarean delivery | 9 | 11 135 | 0.8 (0.4 to 1.5) | 2.7 (1.4 to 5.2) |
| CP † | ||||
| Cephalic | ||||
| Vaginal delivery | 388 | 451 042 | 0.9 (0.8 to 1.0) | 1.0 (Reference) |
| Caesarean delivery | 110 | 51 482 | 2.1 (1.8 to 2.6) | 2.5 (2.0 to 3.1) |
| Breech | ||||
| Vaginal delivery | 6 | 5 517 | 1.1 (0.5 to 2.4) | 1.3 (0.6 to 2.8) |
| Caesarean delivery | 16 | 11 133 | 1.4 (0.9 to 2.3) | 1.7 (1.0 to 2.8) |
| Composite outcome‡ | ||||
| Cephalic | ||||
| Vaginal delivery | 553 | 451 070 | 1.2 (1.1 to 1.3) | 1.0 (Reference) |
| Caesarean delivery | 231 | 51 515 | 4.5 (3.9 to 5.1) | 3.7 (3.1 to 4.3) |
| Breech | ||||
| Vaginal delivery | 17 | 5 523 | 3.1 (1.9 to 4.9) | 2.5 (1.5 to 4.1) |
| Caesarean delivery | 26 | 11 134 | 2.3 (1.6 to 3.4) | 1.9 (1.3 to 2.8) |
*Removed stillbirths from the denominator in the analyses of NNM and CP.
†Removed postneonatal CP from the denominator in the analyses of CP as outcome.
‡Comprising intrapartum death, NNM and CP.
NNM, neonatal mortality; CP, cerebral palsy.
According to planned mode of delivery, vaginal breech delivery had an estimated 2.4 (CI 1.2 to 4.9) times increased risk of NNM and a 2.1 (CI 1.4 to 3.1) times increased risk of the composite outcome. Planned caesarean breech delivery had an estimated 1.6 (CI 0.7 to 3.7) increased risk of NNM and a 1.5 (CI 0.9 to 2.3) times increased risk of the composite outcome, both compared with planned vaginal cephalic delivery (table 4). The prevalence of CP in planned vaginal breech and in planned caesarean breech delivery did not differ significantly from planned vaginal cephalic delivery (table 4). Among children born in the cephalic position, the prevalence of NNM, CP and the composite outcome was higher among those born by caesarean than among those born by vaginal delivery.
Table 4.
Planned mode of delivery: prevalence and unadjusted ORs with 95% CIs for various adverse outcomes among singletons born at term, without congenital anomalies according to planned mode of delivery
| Number of infants with adverse outcome | Total number of infants* | Prevalence per 1000 (CI) | OR (CI) | |
| NNM* | ||||
| Cephalic | ||||
| Planned vaginal delivery | 198 | 474 223 | 0.4 (0.4 to 0.5) | 1.0 (Reference) |
| Planned caesarean delivery | 27 | 28 330 | 1.0 (0.7 to 1.4) | 2.3 (1.5 to 3.4) |
| Breech | ||||
| Planned vaginal delivery | 8 | 7 873 | 1.0 (0.5 to 2.0) | 2.4 (1.2 to 4.9) |
| Planned caesarean delivery | 6 | 8 780 | 0.7 (0.3 to 1.5) | 1.6 (0.7 to 3.7) |
| CP† | ||||
| Cephalic | ||||
| Planned vaginal delivery | 453 | 474 198 | 1.0 (0.9 to 1.1) | 1.0 (Reference) |
| Planned caesarean delivery | 45 | 28 326 | 1.6 (1.2 to 2.1) | 1.7 (1.2 to 2.3) |
| Breech | ||||
| Planned vaginal delivery | 10 | 7 872 | 1.3 (0.7 to 2.3) | 1.3 (0.7 to 2.5) |
| Planned caesarean delivery | 12 | 8 778 | 1.4 (0.8 to 2.4) | 1.4 (0.8 to 2.5) |
| Composite outcome‡ | ||||
| Cephalic | ||||
| Planned vaginal delivery | 705 | 474 252 | 1.5 (1.4 to 1.6) | 1.0 (Reference) |
| Planned caesarean delivery | 79 | 28 333 | 2.8 (2.2 to 3.5) | 1.9 (1.5 to 2.4) |
| Breech | ||||
| Planned vaginal delivery | 24 | 7 878 | 3.0 (2.1 to 4.5) | 2.1 (1.4 to 3.1) |
| Planned caesarean delivery | 19 | 8 779 | 2.2 (1.4 to 3.4) | 1.5 (0.9 to 2.3) |
*Removed stillbirths from the denominator in the analyses of NNM and CP.
† Removed postneonatal CP from the denominator in the analyses of CP as outcome.
‡Comprising intrapartum death, NNM and CP.
NNM, neonatal mortality; CP, cerebral palsy.
In analyses restricted to the 16 700 children in breech position, the risk for NNM was not increased among infants actually born by vaginal delivery compared with caesarean delivery, while the OR for NNM in the group where vaginal delivery was planned was 1.5 (CI 0.5 to 4.3) compared with planned caesarean delivery (table 5). The risk for CP was not increased for children born by vaginal delivery compared with caesarean delivery regardless of actual or planned mode of delivery (table 5).
Table 5.
Restricted to breech deliveries: prevalence and unadjusted ORs with 95% CIs for various adverse outcomes among singletons in breech position born at term, without congenital anomalies according to actual and planned mode of delivery
| Number of infants with adverse outcome | Total number of infants* | Prevalence per 1000 (CI) | OR (CI) | |
| NNM* | ||||
| Actual mode of delivery | ||||
| Caesarean delivery | 9 | 11 135 | 0.8 (0.4 to 1.5) | 1.0 (Reference) |
| Vaginal delivery | 5 | 5 518 | 0.9 (0.4 to 2.1) | 1.1 (0.4 to 3.3) |
| Planned mode of delivery | ||||
| Caesarean delivery | 6 | 8 780 | 0.7 (0.3 to 1.5) | 1.0 (Reference) |
| Vaginal delivery | 8 | 7 873 | 1.0 (0.5 to 2.0) | 1.5 (0.5 to 4.3) |
| CP† | ||||
| Actual mode of delivery | ||||
| Caesarean delivery | 16 | 11 133 | 1.4 (0.9 to 2.3) | 1.0 (Reference) |
| Vaginal delivery | 6 | 5 517 | 1.1 (0.5 to 2.4) | 0.8 (0.3 to 1.9) |
| Planned mode of delivery | ||||
| Caesarean delivery | 12 | 8 778 | 1.4 (0.8 to 2.4) | 1.0 (Reference) |
| Vaginal delivery | 10 | 7 872 | 1.3 (0.7 to 2.3) | 0.9 (0.4 to 2.2) |
| Composite outcome‡ | ||||
| Actual mode of delivery | ||||
| Caesarean delivery | 26 | 11 134 | 2.3 (1.6 to 3.4) | 1.0 (Reference) |
| Vaginal delivery | 17 | 5 523 | 3.1 (1.9 to 4.9) | 1.3 (0.7 to 2.4) |
| Planned mode of delivery | ||||
| Caesarean delivery | 19 | 8 779 | 2.2 (1.4 to 3.4) | 1.0 (Reference) |
| Vaginal delivery | 24 | 7 878 | 3.0 (2.1 to 4.5) | 1.4 (0.8 to 2.6) |
*Removed stillbirths from the denominator in the analyses of NNM and CP.
†Removed postneonatal CP from the denominator in the analyses of CP as outcome.
‡Comprising intrapartum death, NNM and CP.
NNM, neonatal mortality; CP, cerebral palsy.
Multivariable analyses adjusting for gestational age, parity, maternal age, sex and SGA did not substantially affect any of the associations described above (data not shown).
Discussion
In this national cohort study of term singletons without congenital malformations, we found that vaginal breech delivery, regardless of whether it was planned or not, was associated with an excess risk for NNM and with a composite outcome of intrapartum death, NNM and CP, compared with cephalic vaginal delivery. However, also children who had actually been delivered by caesarean had excess risk for NNM and the composite outcome compared with those who were actually born vaginally in the cephalic position, whereas a 60% increased risk for NNM, and a 50% increased risk for the composite outcome among those born in breech by planned caesarean delivery did not reach statistical significance, compared with planned vaginal cephalic delivery. A slightly higher prevalence of CP among children in breech was not statistically significantly different from children born in cephalic position regardless of mode of delivery. The risk for the composite outcome of intrapartum death, NNM and CP associated with the breech position was attenuated compared with the risk for NNM
Regardless of mode of delivery, the absolute risks for the adverse outcomes of breech births were low, ranging between 0.7 and 1.0 per 1000 live born for NNM, and it may be noteworthy that the prevalence rates for all adverse outcomes associated with vaginal breech delivery and with caesarean breech delivery were of similar magnitude.
Strength and limitations
Strengths of the present study are the large number of births and the prospectively recording of the data in the two registers. Nonetheless, among children in breech position, the number of children with the adverse outcomes NNM and CP were low, and the results of the analyses restricted to children in breech should therefore be interpreted with caution.
We restricted the analyses to singletons born at term and without congenital malformations, limiting the possibility of confounding by these factors. Multivariable analyses suggested that maternal age, parity, the child’s sex, gestational age and SGA did not confound the associations between breech position and adverse outcome.
Analysis of the association between mode of delivery and adverse outcome after breech delivery is challenging. Selection to vaginal delivery is recommended on strict criteria and is therefore expected to identify pregnancies with low risk for adverse outcome compared with those selected for caesarean delivery. Furthermore, some of the planned vaginal deliveries will be converted to an emergency caesarean delivery intrapartum, increasing the risk for adverse outcome in the caesarean group. A comparison of adverse outcome between vaginal and caesarean deliveries would therefore be expected to favour the vaginal delivered group. While this was the case for children born in cephalic position (tables 3 and 4), the ORs for NNM were similar or even higher in the vaginal compared with the caesarean delivery group for children born in breech. Thus, caution is needed in the interpretation of the lack of difference between vaginal and caesarean delivery.
We categorised according to actual mode of delivery and planned mode of delivery, which is essential to evaluate the national recommendations. Although the risk for NNM and the composite outcome was higher for actual than for planned caesarean delivery, as would be expected if the classification was correct, we cannot rule out some errors in this classification. Since the forms of the MBRN are completed immediately after birth, it is possible that some deliveries originally planned as caesarean delivery may have been misclassified as emergency caesarean delivery. The latter is expected to be associated with higher risk for adverse outcome, and such misclassification would therefore erroneously reduce the risk associated with planned caesarean delivery. This misclassification would be expected to have most impact on the risk associated with planned breech caesarean delivery. A similar misclassification is also possible for planned vaginal cephalic deliveries, but in this case, the effect is negligible considering the large number of vaginal cephalic births and the low proportion of cephalic caesarean delivery. Thus, the lack of statistical significance of adverse outcome between planned breech caesarean delivery and planned vaginal cephalic delivery should be interpreted with caution.
Finally, the use of register-based data has limited ability to address explanatory factors, as suggested by Goffinet et al.11 In their prospective study of breech deliveries, they found that 33 (26%) of 129 cases with severe neonatal complications had non-lethal major or minor malformations that sometimes explained the neonatal complications.11 We cannot rule out that some undiagnosed or unrecorded malformations may have contributed to the higher proportions of children with adverse outcomes among those born in breech than among those born in cephalic position in our study.
Comparison with other studies
Our findings regarding excess risk for stillbirth29 and NNM associated with breech presentation are consistent with earlier findings,30–32 and an excess risk for NNM was also reported in recent studies including children born after the TBT2 in Denmark4 and in Norway.6
We found a slightly higher risk for NNM in planned vaginal than in planned caesarean delivery, and this could be considered to be consistent with the results of the TBT. On the other hand, the overall interpretation of our findings is that the risk for NNM was largely independent of mode of delivery, and this interpretation is not consistent with the results of the TBT. First, the different designs of the two studies may explain the different findings. The TBT was a randomised controlled trial considered to be the gold standard, while our study is an observational study. Nonetheless, the much lower perinatal mortality in Norway compared with the TBT may also explain some of the diverging results in the two studies. Moreover, to be eligible to participate in the TBT, women had to have a singleton live fetus at term (≥37 weeks gestation) in breech without any known lethal fetal congenital anomaly. Women were excluded if there was evidence of fetopelvic disproportion, or if the fetus was judged to be clinically large or to have an estimated fetal weight of 4000 g or more, hyperextension of the fetal head or other fetal anomaly or condition that might cause a mechanical problem at delivery. Women with contraindication for labour or vaginal delivery such as placenta praevia were also excluded.2 These criteria are similar to the criteria for vaginal breech delivery recommended by the Norwegian Society for Gynecology and Obstetrics. However, in the TBT, a higher proportion of women (43%) selected for vaginal delivery needed caesarean delivery compared with our study population where only 30% of those selected for planned vaginal delivery needed caesarean delivery. One may therefore speculate that the probability for adverse outcome in the planned vaginal group in the TBT was higher than in our study. Instead antenatal acquired vulnerability may have played a larger role in our population.
The lack of excess risk for CP associated with breech position at birth is consistent with four studies published before the TBT but inconsistent with four other studies. We are not aware of studies addressing the association between breech presentation at birth and CP in populations born after the TBT.2 A follow-up study of 923 children included in that trial did not have the statistical power to address this severe, neurodevelopmental outcome.33 Two earlier studies, including one from our own group, also found some evidence that the risk was associated with vaginal delivery.12 15 The lower risk for CP in the present study, compared with our previous Norwegian study12 could be explained by the larger sample size, better quality of the data in the MBRN21 and better ascertainment of cases in the CPRN in the present study.23 Nonetheless, it is also possible that changes in the delivery of breech births in Norway including an increasing proportion of fetuses born by planned caesarean delivery6 may have improved outcome and may reflect better selection of mothers for vaginal delivery.
Interpretation
The overall higher risk for stillbirth and the higher proportion of infants born SGA among children born in breech than in cephalic position may suggest that fetuses with antenatal acquired risk factors for adverse outcomes are more likely to present in breech than in cephalic position at birth. On the other hand, the slightly higher ORs for NNM and for the composite outcome among children born vaginally than by caesarean delivery both when restricted to the breech group as well as when compared with vaginal cephalic delivery may suggest that fetuses in breech are more likely to experience complications during birth if they are born vaginally than if delivered by caesarean. This interpretation may be further supported by the fact that women selected for vaginal breech delivery would be expected to have a particular low risk for complications during birth. Thus, a combination of antenatal acquired risk factors for neonatal death with increased vulnerability to the birth process is probably the most likely explanation of our findings.
Regarding CP, antenatal factors are considered to be involved in 90% of the cases with CP,34 and one might have expected an excess risk for CP in breech births, similar to that of NNM. The much lower risk for CP among children in breech, not statistically different from cephalic position could therefore suggest that antenatal factors increasing the risk for NNM are different or at least not completely overlapping with antenatal risk factors involved in the causal pathway leading to CP.
Implications
Taking into consideration the very low absolute risk for NNM and CP, the increasing evidence for acute and long-term maternal complications35 and for later health problems among children following caesarean delivery,36 37 our results suggest that vaginal delivery in selected cases may be an option for women with a fetus in breech position. This option requires that strict criteria are followed including access to competent obstetric care. In addition, a secondary advantage of having a certain volume of vaginal breech deliveries is that obstetricians retain their competence for unexpected vaginal breech deliveries. In the discussion with the pregnant mother and her partner regarding choice of delivery mode of a fetus in breech, the relative risk for NNM should be explained but related to the very low absolute risk. Moreover, it may be appropriate to emphasise that adverse outcome probably to a large degree is caused by antenatal acquired insults and that there are potential advantages of vaginal birth over caesarean delivery for long-term health of the child and the mother. Regarding obstetric care, awareness of the excess risk for fetal death should be emphasised, and studies are warranted to optimise antenatal follow-up of mothers with a fetus in breech.
Caution is needed if results of observational studies are included in the development of clinical guidelines, and more studies are needed to support our results. On the other hand, a new randomised controlled trial in our part of the world is unrealistic as it would require the participation of 20 000 women with a fetus in breech to document a difference in NNM between mothers selected for planned vaginal and planned caesarean delivery.10
Nonetheless, the higher risk of NNM among planned vaginal deliveries than for planned caesarean delivery compared with cephalic delivery warrants further studies, including perinatal audits and prospective studies as suggested by Goffinet et al.11
Conclusion
Vaginal breech delivery, regardless of whether planned or actual and actual caesarean breech delivery were associated with excess risk for NNM and for a composite outcome of intrapartum death, NNM and CP, compared with vaginal cephalic delivery. The risk for adverse outcome in planned caesarean breech delivery did not differ significantly from planned vaginal cephalic delivery. However, the absolute risk for these outcomes was low. Taking into consideration potential long-term adverse consequences of caesarean delivery for the child, the mother and for later deliveries we therefore conclude that vaginal delivery may be offered to women with a fetus in breech, provided competent obstetric care and strict criteria for selection to vaginal delivery. Our findings did not support the notion that some cases of CP may be prevented if all fetuses in breech are delivered by caesarean delivery.
Supplementary Material
Acknowledgments
The authors thank the Cerebral Palsy Register of Norway (CPRN) and the Medical Birth Registry of Norway (MBRN) for the data. A special thanks to all the children and parents participating in the CPRN.
Footnotes
Contributors: SB analysed the data, contributed to study design and data interpretation and wrote the first draft of the manuscript. GA was principally responsible for the data from the CPRN and revised the manuscript. MM contributed to the data interpretation and revised the manuscript. PR contributed to the research hypotheses, study design and revised the manuscript. SH contributed to the data interpretation and revised the manuscript. DM contributed to study design, the data analyses, data interpretation and revised the manuscript. TV proposed the research questions and contributed in the interpretation of the data and the revision of the manuscript. He is the guarantor of the study and accepts full responsibility for the work and the conduct of the study. All authors approved the final version of the submitted manuscript.
Funding: Supported by a grant from The Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmj.org/cio_disclosure.pdf (available on request from the corresponding author) and declare: no financial support from any organisation for the submitted work; no financial relationship with any companies that might have an interest in the submitted work in the previous 3 years and have no non-financial interests or relationships that may be relevant to the submitted work.
Ethics approval: The study was approved by the Regional Ethical Committee for Medical Research in Mid-Norway (ref. 2011/754).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The protocol is available on request from the corresponding author at: sbjellmo@hotmail.com.
References
- 1. van Roosmalen J, Meguid T. The dilemma of vaginal breech delivery worldwide. Lancet 2014;383:1863–4. 10.1016/S0140-6736(14)60618-8 [DOI] [PubMed] [Google Scholar]
- 2. Hannah ME, Hannah WJ, Hewson SA, et al. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet 2000;356:1375–83. 10.1016/S0140-6736(00)02840-3 [DOI] [PubMed] [Google Scholar]
- 3. Rietberg CC, Elferink-Stinkens PM, Visser GH. The effect of the Term Breech Trial on medical intervention behaviour and neonatal outcome in the Netherlands: an analysis of 35,453 term breech infants. BJOG 2005;112:205–9. 10.1111/j.1471-0528.2004.00317.x [DOI] [PubMed] [Google Scholar]
- 4. Hartnack Tharin JE, Rasmussen S, Krebs L. Consequences of the Term Breech Trial in Denmark. Acta Obstet Gynecol Scand 2011;90:767–71. 10.1111/j.1600-0412.2011.01143.x [DOI] [PubMed] [Google Scholar]
- 5. Hehir MP, O’Connor HD, Kent EM, et al. Changes in vaginal breech delivery rates in a single large metropolitan area. Am J Obstet Gynecol 2012;206:1–98. 10.1016/j.ajog.2012.03.029 [DOI] [PubMed] [Google Scholar]
- 6. Vistad I, Klungsøyr K, Albrechtsen S, et al. Neonatal outcome of singleton term breech deliveries in Norway from 1991 to 2011. Acta Obstet Gynecol Scand 2015;94:997–1004. 10.1111/aogs.12684 [DOI] [PubMed] [Google Scholar]
- 7. Glezerman M. Five years to the term breech trial: the rise and fall of a randomized controlled trial. Am J Obstet Gynecol 2006;194:20–5. 10.1016/j.ajog.2005.08.039 [DOI] [PubMed] [Google Scholar]
- 8. van Roosmalen J, Rosendaal F. There is still room for disagreement about vaginal delivery of breech infants at term. BJOG 2002;109:967–9. 10.1111/j.1471-0528.2002.01005.x [DOI] [PubMed] [Google Scholar]
- 9. Keirse MJ. Evidence-based childbirth only for breech babies? Birth 2002;29:55–9. 10.1046/j.1523-536X.2002.00157.x [DOI] [PubMed] [Google Scholar]
- 10. Håheim LL, Albrechtsen S, Berge LN, et al. Breech birth at term: vaginal delivery or elective cesarean section? A systematic review of the literature by a Norwegian review team. Acta Obstet Gynecol Scand 2004;83:126–30. 10.1111/j.0001-6349.2004.00349.x [DOI] [PubMed] [Google Scholar]
- 11. Goffinet F, Carayol M, Foidart JM, et al. Is planned vaginal delivery for breech presentation at term still an option? Results of an observational prospective survey in France and Belgium. Am J Obstet Gynecol 2006;194:1002–11. 10.1016/j.ajog.2005.10.817 [DOI] [PubMed] [Google Scholar]
- 12. Andersen GL, Irgens LM, Skranes J, et al. Is breech presentation a risk factor for cerebral palsy? A Norwegian birth cohort study. Dev Med Child Neurol 2009;51:860–5. 10.1111/j.1469-8749.2009.03338.x [DOI] [PubMed] [Google Scholar]
- 13. McIntyre S, Taitz D, Keogh J, et al. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol 2013;55:499–508. 10.1111/dmcn.12017 [DOI] [PubMed] [Google Scholar]
- 14. Jonas O, Stern LM, Macharper T. A South Australian study of pregnancy and birth risk factors associated with cerebral palsy. Int J Rehabil Res 1989;12:159–66. 10.1097/00004356-198906000-00004 [DOI] [PubMed] [Google Scholar]
- 15. Thorngren-Jerneck K, Herbst A. Perinatal factors associated with cerebral palsy in children born in Sweden. Obstet Gynecol 2006;108:1499–505. 10.1097/01.AOG.0000247174.27979.6b [DOI] [PubMed] [Google Scholar]
- 16. Krebs L, Topp M, Langhoff-Roos J. The relation of breech presentation at term to cerebral palsy. Br J Obstet Gynaecol 1999;106:943–7. 10.1111/j.1471-0528.1999.tb08434.x [DOI] [PubMed] [Google Scholar]
- 17. Morken NH, Albrechtsen S, Backe B, et al. Caesarean section does not prevent cerebral palsy in singleton term breech infants. Dev Med Child Neurol 2010;52:684–5. author reply 685–6 10.1111/j.1469-8749.2010.03656.x [DOI] [PubMed] [Google Scholar]
- 18. Westgren LM, Ingemarsson I. Breech delivery and mental handicap. Baillieres Clin Obstet Gynaecol 1988;2:187–94. 10.1016/S0950-3552(88)80071-3 [DOI] [PubMed] [Google Scholar]
- 19. Yuan C, Gaskins AJ, Blaine AI, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr 2016;170:e162385 10.1001/jamapediatrics.2016.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berhan Y, Haileamlak A. The risks of planned vaginal breech delivery versus planned caesarean section for term breech birth: a meta-analysis including observational studies. BJOG 2016;123 10.1111/1471-0528.13524 [DOI] [PubMed] [Google Scholar]
- 21. Norwegian Institute of Public Health. Med birth Registry of Norway. 2015. https://www.fhi.no/en/hn/health-registries/medical-birth-registry-of-norway/.
- 22. Andersen GL, Irgens LM, Haagaas I, et al. Cerebral palsy in Norway: prevalence, subtypes and severity. Eur J Paediatr Neurol 2008;12:4–13. 10.1016/j.ejpn.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 23. Cerebral pareseregsiteret i Norge (The Cerebral Palsy Register of Norway). 2014.. https://helsedirektoratet.no/Lists/Publikasjoner/Attachments/777/Dokumentkontroll-av-pasientjournaler-med-icd-10-kode-for-cerebral-parese-IS-2267.pdf
- 24. World Health Organization. Neonatal and perinatal mortality. 2006.. http://apps.who.int/iris/bitstream/10665/43444/1/9241563206_eng.pdf.
- 25. Cans C. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurology 2000;42:816–24. 10.1111/j.1469-8749.2000.tb00695.x [DOI] [PubMed] [Google Scholar]
- 26. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand 2000;79:440–9. 10.1034/j.1600-0412.2000.079006440.x [DOI] [PubMed] [Google Scholar]
- 27. Newcombe RG, Altman DG , et al. Proportions and their differences : Altman DG, Machin D, Bryant TN, Statistics with confidence. 2nd edn London: BMJ books, 2000:45–56. [Google Scholar]
- 28. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 29. Goldenberg RL, Harrison MS, McClure EM. Stillbirths: The hidden birth asphyxia - US and global perspectives. Clin Perinatol 2016;43:439–53. 10.1016/j.clp.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 30. Herbst A. Term breech delivery in Sweden: mortality relative to fetal presentation and planned mode of delivery. Acta Obstet Gynecol Scand 2005;84:593–601. 10.1111/j.0001-6349.2005.00852.x [DOI] [PubMed] [Google Scholar]
- 31. Gilbert WM, Hicks SM, Boe NM, et al. Vaginal versus cesarean delivery for breech presentation in California: a population-based study. Obstet Gynecol 2003;102:911–7. [DOI] [PubMed] [Google Scholar]
- 32. Krebs L, Langhoff-Roos J, Weber T. Breech at term--mode of delivery? A register-based study. Acta Obstet Gynecol Scand 1995;74:702–6. 10.3109/00016349509021178 [DOI] [PubMed] [Google Scholar]
- 33. Whyte H, Hannah ME, Saigal S, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. Am J Obstet Gynecol 2004;191:864–71. 10.1016/j.ajog.2004.06.056 [DOI] [PubMed] [Google Scholar]
- 34. Stoknes M, Andersen GL, Dahlseng MO, et al. Cerebral palsy and neonatal death in term singletons born small for gestational age. Pediatrics 2012;130:e1629–e1635. 10.1542/peds.2012-0152 [DOI] [PubMed] [Google Scholar]
- 35. Klar M, Michels KB. Cesarean section and placental disorders in subsequent pregnancies--a meta-analysis. J Perinat Med 2014;42:571–83. 10.1515/jpm-2013-0199 [DOI] [PubMed] [Google Scholar]
- 36. Black M, Bhattacharya S, Philip S, et al. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA 2015;314:2271–9. 10.1001/jama.2015.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blustein J, Liu J. Time to consider the risks of caesarean delivery for long term child health. BMJ 2015;350:2410. 10.1136/bmj.h2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.