Abstract
Beta‐hydroxy‐beta‐methylbutyrate (HMB) is a metabolite of the essential amino acid leucine that has been reported to have anabolic effects on protein metabolism. The aims of this article were to summarize the results of studies of the effects of HMB on skeletal muscle and to examine the evidence for the rationale to use HMB as a nutritional supplement to exert beneficial effects on muscle mass and function in various conditions of health and disease.
The data presented here indicate that the beneficial effects of HMB have been well characterized in strength‐power and endurance exercise. HMB attenuates exercise‐induced muscle damage and enhances muscle hypertrophy and strength, aerobic performance, resistance to fatigue, and regenerative capacity. HMB is particularly effective in untrained individuals who are exposed to strenuous exercise and in trained individuals who are exposed to periods of high physical stress. The low effectiveness of HMB in strength‐trained athletes could be due to the suppression of the proteolysis that is induced by the adaptation to training, which may blunt the effects of HMB. Studies performed with older people have demonstrated that HMB can attenuate the development of sarcopenia in elderly subjects and that the optimal effects of HMB on muscle growth and strength occur when it is combined with exercise. Studies performed under in vitro conditions and in various animal models suggest that HMB may be effective in treatment of muscle wasting in various forms of cachexia. However, there are few clinical reports of the effects of HMB on muscle wasting in cachexia; in addition, most of these studies evaluated the therapeutic potential of combinations of various agents. Therefore, it has not been possible to determine whether HMB was effective or if there was a synergistic effect. Although most of the endogenous HMB is produced in the liver, there are no reports regarding the levels and the effects of HMB supplementation in subjects with liver disease. Several studies have suggested that anabolic effects of HMB supplementation on skeletal muscle do not occur in healthy, non‐exercising subjects.
It is concluded that (i) HMB may be applied to enhance increases in the mass and strength of skeletal muscles in subjects who exercise and in the elderly and (ii) studies examining the effects of HMB administered alone are needed to obtain conclusions regarding the specific effectiveness in attenuating muscle wasting in various muscle‐wasting disorders.
Keywords: Cachexia, Sarcopenia, Leucine, Supplements, Exercise, HMB
Introduction
Exacerbated loss of skeletal muscle is a hallmark of cachexia, which occurs frequently in patients with chronic infections, cancer, liver cirrhosis, congestive heart failure, renal and pulmonary insufficiency, and other disorders. Slow but progressive loss of muscle mass and strength is common during ageing. Both cachexia‐related and ageing‐related sarcopenia decrease the ability to respond to illness or injury, worsen the prognosis of many diseases, and significantly enhance morbidity and mortality.1
Although knowledge of the aetiology and pathogenesis of the loss of muscle mass in cachexia and in elderly subjects is emerging, muscle wasting in these conditions is associated with a poor responsiveness to anabolic stimuli that makes conventional nutritional strategies ineffective. Furthermore, various therapeutic agents and nutritional supplements (e.g. growth hormone, branched‐chain amino acids, and glutamine) have not been shown to produce consistent effects on muscle or present adverse side effects.2, 3, 4, 5 Thus, addressing these conditions remains an open problem. Therefore, there is an ongoing intensive search for novel therapies that can attenuate the loss of muscle mass and strength in muscle‐wasting disorders and in the elderly.
Studies performed during the past 20 years, mostly in athletes, indicate that beta‐hydroxy‐beta‐methylbutyrate (HMB) is a promising agent that may be applied to enhance increases in the mass and strength of muscle, aerobic performance, and resistance to fatigue. The aims of the article are (i) to summarize the results of animal and human studies that have examined the effects of HMB on skeletal muscle and (ii) to examine the evidence for the rationale for the use of HMB as a nutritional supplement in various conditions of health and disease.
Synthesis and metabolism of HMB
The HMB is a metabolite of leucine, which is one of the three essential branched‐chain amino acids (BCAA; leucine, valine, and isoleucine). These amino acids share similar metabolic pathways. Leucine has a well‐known anabolic role in muscle by acting as a signalling molecule that stimulates protein synthesis.
The first reaction in the pathway of HMB synthesis in the body (Figure 1) is the reversible transamination of leucine to alpha‐ketoisocaproic acid (KIC) by BCAA aminotransferase. This reaction primarily occurs in skeletal muscle. Because the activities of the next enzymes in leucine catabolism [branched‐chain alpha‐keto acid dehydrogenase (BCKD) and KIC dioxygenase] are low in skeletal muscle, most of the KIC is released from the muscles into the blood and is further metabolized in various tissues.
Figure 1.
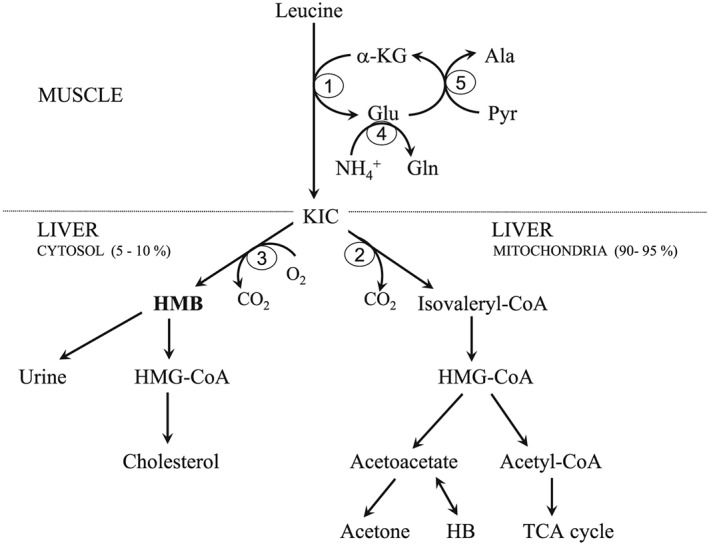
Pathways of HMB synthesis and catabolism HMB, beta‐hydroxy‐beta‐methylbutyrate; KIC, alpha‐ketoisocaproic acid; HB, beta‐hydroxybutyrate; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐CoA. 1, BCAA aminotransferase; 2, BCKA dehydrogenase; 3, KIC dioxygenase; 4, glutamine synthetase; 5, alanine aminotransferase.
The majority of KIC is decarboxylated by BCKD to isovaleryl‐CoA in the liver mitochondria where it is next gradually converted to acetyl‐CoA and ketone bodies. It is estimated that only 5–10% of the KIC is metabolized by the cytosolic enzyme KIC dioxygenase to produce HMB.6 Expression of KIC dioxygenase is also high in the kidneys but is low in the brain and skeletal muscle.7 On the basis of KIC dioxygenase activities were estimated that a 70 kg human would produce from 0.2 to 0.4 g HMB/day depending on the level of dietary leucine.8 The estimates have been confirmed recently in humans using bolus injection of isotopically labelled HMB. The rate of appearance for HMB was 0.66 μmol/kg fat‐free mass/hour that accounts for 0.66% of leucine turnover.9 The HMB levels in blood plasma are 2–5 μM both before and after feeding a mixed meal, and the levels increase after consumption of a meal containing leucine.
Isotopic data showed that the primary fate of HMB catabolism (Figure 1) is conversion to 3‐hydroxy‐3‐methyl‐glutaryl‐CoA (HMG‐CoA), which is a direct precursor of cholesterol.10 A small amount of HMB is excreted in the urine. Kidney excretion may account for the relatively short half‐life of HMB, which was found to be approximately 1 h in rats, 2 h in pigs, and 1–3 h in humans.10 Additional detailed information regarding HMB biosynthesis and metabolism is provided in other articles.10, 11
Effects of HMB
The HMB exerts a number of effects that may explain the potential benefits of its supplementation on muscle mass and performance (Figure 2).
Figure 2.
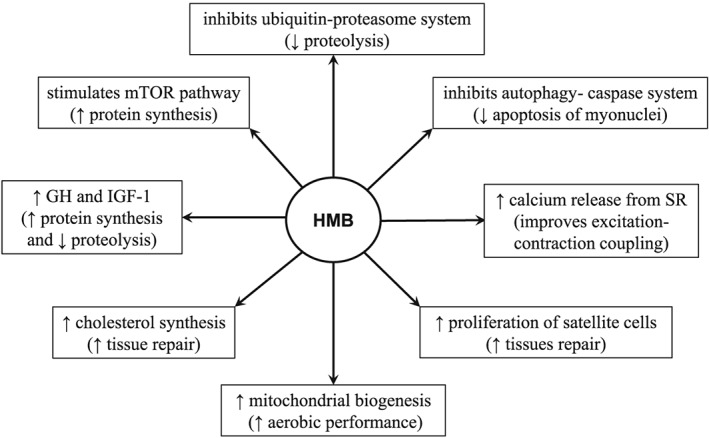
Suggested mechanisms for the favourable effects of HMB supplementation on skeletal muscle.
Effects on protein metabolism in skeletal muscle
The HMB has been shown to affect muscle protein turnover by stimulating protein synthesis via up‐regulation of anabolic signalling pathways and by decreasing proteolysis via down‐regulation of catabolic signalling pathways.
The HMB stimulates protein synthesis via mTOR, a protein kinase that has a central role in controlling mRNA translation efficiency.12 Recently, Girón et al. used rat L6 myotubes to show that HMB was much more effective than leucine in increasing protein synthesis through the mTOR system and that the effects of leucine on protein synthesis and the mTOR pathway were enhanced when the L6 cells were transfected with a plasmid that codes for KIC dioxygenase.7 Increases in the expression of pituitary growth hormone mRNA and the IGF‐1 levels in blood serum of rats after 1 month of HMB supplementation suggest that HMB may also stimulate protein synthesis through the growth hormone/IGF‐1 axis.13
Muscle protein breakdown is decreased by HMB via two major protein degradation pathways, the ubiquitin proteasome and the autophagy‐lysosome systems. HMB has been shown to decrease proteasome expression and proteasome enzyme activities, attenuate the up‐regulation of caspases, and reduce the apoptosis of myonuclei.14, 15, 16, 17 We found that HMB treatment of septic rats suppressed myofibrillar protein degradation more in the soleus muscle (which is composed mostly of red, slow‐twitch fibres) than in extensor digitorum longus muscle (which is composed mostly of white, fast‐twitch fibres).15
The aforementioned studies collectively indicate that HMB supplementation may restore the balance between protein synthesis and proteolysis in skeletal muscle. Kimura et al. suggested that beneficial effects of HMB include activation of the PI3K/Akt signalling pathway leading to FoxO1 and FoxO3a phosphorylation and attenuated MuRF‐1 expression.18 Other effects of HMB that could potentially affect muscle growth and performance include expression of the proliferation marker MyoD and differentiation‐specific markers MEF2 and stimulation of myogenic cell proliferation via the MAPK/ERK and PI3K/Akt pathways.19 Recent studies have demonstrated that HMB supplementation increases mitochondrial biogenesis and fat oxidation.20 Vallejo et al. demonstrated that dietary supplementation of mice with HMB enhances calcium release from the sarcoplasmic reticulum during repetitive bouts of activity, which suggests that HMB improves excitation‐contraction coupling in muscle cells.21
Effects on leucine metabolism
It can be hypothesized that the administration of HMB affects the metabolism of its precursors. An increase in the levels of leucine in the blood plasma, a decrease in leucine clearance, and no significant changes in leucine oxidation were observed in healthy rats treated with HMB.22 The increase in the level of leucine in the blood plasma was associated with decreased concentrations of alanine, glutamine, and glutamate, which indicated that the conversion of leucine to KIC by BCAA aminotransferase in the muscles decreased (please see Figure 1 for more clarification on this point). The data indicate that the HMB supplementation may decrease the dose of leucine required to promote its positive effects on protein balance or ameliorate its decreased levels as occurs in various muscle‐wasting disorders.
Leucine concentrations in blood plasma and muscles were not altered after oral HMB consumption in humans.12 We suppose that the difference between the results of animal and human studies is due to the dose, duration, and route of HMB administration. HMB was infused in a dose of 0.1 g/kg for 210 min in the animal study; 2.42 g (~ 0.04 g/kg) of HMB was given orally in a single dose in the human study.
Effects on cholesterol metabolism
The effects of HMB on the cholesterol levels in exercising humans were summarized by Nissen et al.11 Compared with the placebo, HMB supplementation (3 g HMB/d) resulted in a decrease in total cholesterol and LDL cholesterol and a decrease in systolic blood pressure in subjects whose average starting cholesterol was higher than 5.17 mmol/L. In contrast, no effect was observed in subjects with cholesterol values below 5.17 mmol/L. Nissen and Abumrad suggested that the cholesterol synthesized from HMB may be utilized for repair or regeneration of damaged cells and that some of the favourable effects of HMB may be related to its role in cholesterol metabolism.10 However, an increase in the blood cholesterol level was observed in healthy rats 24 h after HMB administration.22 We suggest that studies to examine the effects of HMB on blood lipids in various populations are needed.
HMB as a nutritional supplement
A major advantage of the use of HMB as a nutritional supplement is associated with its anti‐catabolic action in skeletal muscle. Here, we summarize reports of the effects of HMB supplementation on the muscles with respect to exercise, as well as in elderly subjects, those with muscle‐wasting disorders, and healthy subjects.
Exercise performance and effects of HMB supplementation
Data from animal and human studies suggest that during exercise protein synthesis in the muscles remains unchanged or decreases and that protein breakdown is unchanged or increases.23 After exercise, protein synthesis increases, and a net gain in muscle protein may be achieved by regular exercise. The adaptive increase in protein synthesis that leads to hypertrophy is likely mediated by activation of the Akt/mTOR/p70 S6 kinase pathway.24 However, if sufficient rest is not included in a training programme, prolonged exercise may lead to symptoms of poor performance, fatigue, depression, and impairment of immune functions. Muscle injury frequently occurs after unaccustomed exercise, notably if the exercise involves a large amount of eccentric (muscle lengthening) contractions. The initial mechanical trauma is believed to cause an inflammatory response that clears the debris from the injured area.25 The results are a decrease in force production, muscular soreness, and increases in inflammatory markers, for example, lactate dehydrogenase and creatine kinase in the blood.
Exercise greatly increases energy expenditure and promotes the oxidation of BCAA. Studies with 13C‐labelled leucine showed that the oxidation of BCAAs increases two‐fold to three‐fold during exercise.26, 27 Significant decreases in the levels of blood plasma leucine occur following aerobic (11 to 33%), anaerobic (5–8%), and strength‐training (30%) exercise sessions.28 There are no reports regarding the production of HMB and its levels in the blood plasma during or after exercise.
Through a Medline search, we found 18 human studies that reported various benefits of HMB in both strength‐power and endurance sports (Table 1). The reported benefits include positive effects on muscle hypertrophy, strength, reduction of muscle damage, aerobic performance, resistance to fatigue, and regenerative capacity. The conclusion of a meta‐analysis of studies, in which the duration of the training was at least 3 weeks and included resistance training two or more times a week was that HMB supplementation increases lean mass and strength.46 On the other hand, several studies, specifically those in strength‐trained athletes, do not support beneficial effects of HMB (Table 2).
Table 1.
Human studies that report beneficial effects of beta‐hydroxy‐beta‐methylbutyrate in exercise
| Study design | Benefits | Reference |
|---|---|---|
| Untrained individuals, HMB (1.5 or 3 g/day), resistance training for 3 or 7 weeks | ↑ muscle mass and strength, ↓ muscle damage | Nissen et al.8 |
| Untrained men, HMB (3 or 6 g/day), resistance training for 8 weeks | ↑ muscle mass and strength, ↓ muscle damage | Gallagher et al. 29 |
| Untrained individuals, HMB (3 g/day), resistance training for 8 weeks | ↑ muscle mass and strength, ↓ muscle damage | Jowko et al.30 |
| Recreationally resistance‐trained men, HMB (3 g) before lower body resistance exercise | Combination of HMB and cold water immersion after exercise improved performance recovery | Gonzalez et al. 31 |
| Strength‐trained and power‐trained individuals, HMB (3 g/day) and ATP (400 mg/day), resistance training for 8 weeks | ↑ LBM and strength | Lowery et al. 32 |
| Volleyball players, HMB (3 g/day) for 7 weeks | ↑ muscle mass and strength, ↑ anaerobic performance | Portal et al. 33 |
| Non‐resistance‐trained men, amino acid‐based formula containing HMB, heavy resistance training for 12 weeks | ↑ muscle mass and strength, ↓ muscle damage | Kraemer et al. 34 |
| Non‐resistance‐trained men, HMB (3 g/day) + KIC (0.3 g/day) for 14 days prior to a single bout of heavy resistance exercise | ↓ muscle damage | Van Someren et al. 35 |
| Volunteers running at least 48 km/week, HMB (3 g/day) for 6 weeks prior to a prolonged run (20 km) | ↓ muscle damage | Knitter et al. 36 |
| Cyclists, HMB (3 g/day) for 2 weeks | ↑ aerobic performance | Vukovich and Dreifort37 |
| Recreationally active subjects, HMB (3 g/day), ergometer tests over a 4 week period | ↑ aerobic performance | Robinson et al. 38 |
| Active college students, HMB (3 g/day), exercise for 5 weeks | ↑aerobic performance | Lamboley et al. 39 |
| Rowers, HMB (3 g/day) for 12 weeks | ↑ LBM and aerobic performance | Durkalec‐Michalski and Jeszka40 |
| Athletes practicing wrestling, judo, jiu‐jitsu, karate or rowing; HMB (3 g/day) for 12 weeks | ↑ LBM and aerobic performance | Durkalec‐Michalski and Jeszka41 |
| Trained and untrained individuals, HMB (3 g/day), resistance training for 4 weeks | ↑ LBM and muscle strength regardless of gender and training status. | Panton et al.42 |
| Resistance‐trained men, HMB (3 g/day), resistance training for 12 weeks | ↑ LBM and muscle strength | Wilson et al. 43 |
| Resistance‐trained men, HMB (3 g) before high‐volume resistance exercise | ↓ muscle damage and improved recovery | Wilson et al. 44 |
| Untrained individuals, HMB (3 g/day), high intensity training for 4 weeks | ↑ physical working capacity | Miramonti et al. 45 |
LMB, lean body mass.
Table 2.
Human studies not supporting beneficial effects of beta‐hydroxy‐beta‐methylbutyrate in exercise
| Study design | Result | Reference |
|---|---|---|
| Non‐resistance‐trained subjects, HMB (40 mg/kg /day) for 6 days before maximal isokinetic exercise of elbow flexors | No beneficial effect on muscle soreness, arm girth, and torque measures | Paddon‐Jones et al.47 |
| Resistance‐trained men, HMB (0.3 or 6 g/day), resistance training for 28 days | No beneficial effect on muscle strength and body composition | Kreider et al. 48 |
| Rugby players, HMB (3 g/day) for 6 weeks | No beneficial effect on aerobic and anaerobic ability | O'Connor and Crowe49 |
| Rugby players, HMB (3 g/day) for 6 weeks | No beneficial effect on muscle strength and endurance | O'Connor and Crowe50 |
| Resistance‐trained athletes, HMB (3 g/day) for 6 weeks | No beneficial effect on muscle strength, body composition, and markers of muscle damage | Slater et al.51 |
| Football players, HMB (3 g/day) for 4 weeks | No beneficial effect on muscle strength and body composition | Ransone et al. 52 |
| Resistance‐trained subjects, HMB (3 g/day) for 9 weeks | No beneficial effect on body composition | Thomson et al. 53 |
| Football players, HMB (3 g/day) for 10 days | No beneficial effect on anaerobic power and creatine kinase and myoglobin in blood | Hoffman et al. 54 |
| Recreational exercisers, HMB (3 g/day) + KIC (0.3 g/day) for 11 days before downhill running | No benefits on indices of muscle damage | Nunan et al. 55 |
Some researchers analysed conditions in which HMB was effective and/or ineffective and proposed that HMB is particularly effective in untrained individuals who are exposed to strenuous exercise and in trained individuals who are exposed to periods of high physical stress.56, 57, 58 The low effectiveness of HMB in strength‐trained athletes could be due to the suppression of the proteolysis that is induced by the adaptation to training, which may blunt the effects of HMB.51 Other studies suggest that a longer pre‐exercise supplementation period may be necessary.47 The position statement of the International Society of Sports Nutrition declared that HMB enhances recovery by attenuating exercise‐induced skeletal muscle damage, appears to be most effective when consumed for 2 weeks prior to an exercise bout, and enhances skeletal muscle hypertrophy, strength, and power when an appropriate exercise protocol is utilized.59
Elderly and effects of HMB supplementation
Sarcopenia, which is present in approximately 5 to 10% of persons over 65 years of age is associated with weakness, falls, and a decreased ability to respond to illness or injury.60 Muscle wasting may be exacerbated during a period of disuse during a prolonged bed rest and by decreased food intake, particularly during an illness.
A hallmark of sarcopenia in elderly subjects is a decreased ability to increase muscle protein synthesis in response to anabolic signals such as food intake and resistance exercise. In other words, this condition suppresses the stimulatory effect of food and other signals on mRNA translation, the rate‐controlling step for protein synthesis, which is primarily regulated by the mTOR signalling pathway. Such anabolic resistance of muscle to nutrients is probably due to oxidative stress and low‐grade inflammation.
There is growing evidence that the severe decreases in the skeletal muscle mass and function that occur with ageing may be mitigated by HMB supplementation (Table 3). Based on the meta‐analysis of seven randomized controlled trials, HMB supplementation can prevent the loss of lean body mass in older adults without causing a significant change in fat mass.72 The rationale for HMB supplementation in ageing subjects is strongly supported by recent findings of a negative correlation between the HMB levels in blood plasma with age and the lower levels of KIC dioxygenase in the livers of old rats than in young rats.73
Table 3.
Effects of beta‐hydroxy‐beta‐methylbutyrate on muscle in elderly
| Study design | Effects of HMB | Reference |
|---|---|---|
| Animal studies | ||
| Rats, 20 months of age, HMB and β‐alanine supplementation (equivalent to human doses of 3 and 2.4 g per day, respectively) for 8 weeks | No significant effect on muscle mass, force or fatigability; ↓ expression of MuRF1 | Russ et al. 61 |
| Mice, 19 months of age, HMB (514 mg/kg) or β‐alanine (411 mg/kg) supplementation for 8 weeks | ↓ decline in muscle function | Vallejo et al. 21 |
| Rats, 34 months of age, hindlimb suspension for 2 weeks and reload for 2 weeks, HMB (340 mg/kg/day) orally | ↓ fibre area (in plantaris and soleus muscles) | Hao et al. 17 |
| Rats, 34 months of age, hindlimb suspension for 2 weeks and reload for 2 weeks, HMB (340 mg/kg/day) orally for 35 days | ↑ muscle mass, fibre cross‐sectional area and proliferation of stem cells during the reloading period | Alway et al. 62 |
| Human studies | ||
| HMB/Arg/Lys mixture (2 g/5 g/1.5 g per day) for 1 year | ↑ lean tissue mass and protein turnover | Baier et al. 63 |
| Bed‐ridden subjects, HMB (2 g/day) for 2 or 4 weeks | ↓ urinary urea nitrogen excretion | Hsieh et al. 64 |
| HMB (3 g/day) and exercise for 8 weeks | ↑ body fat loss | Vukovich et al. 65 |
| HMB/Arg/Lys mixture (2/5/1.5 g per day) for 12 weeks | ↑ limb circumference, leg and handgrip strength; ↑ whole body protein synthesis | Flakoll et al. 66 |
| HMB/Arg/Lys mixture (2/5/1.5 g per day) for 1 year | ↑ muscle mass, ↑ muscle strength only when vitamin D status was adequate | Fuller et al. 67 |
| HMB/Arg/Gln mixture for 2 weeks | ↑ collagen synthesis in muscle | Williams et al. 68 |
| HMB (1.5 g/day) + mild fitness programme for 8 weeks | ↑ muscle strength, ↑ physical performance parameters | Berton et al. 69 |
| HMB (3 g/day), bed rest for 10 days followed by resistance training for 8 weeks | HMB supplementation preserves muscle mass during bed rest | Deutz et al. 70 |
| HMB (3 g/day) for 24 weeks, both exercise and non‐exercise groups | ↑ muscle strength, no difference between exercising and non‐exercising groups | Stout et al. 71 |
Apart from the benefits to muscle, there are other effects of HMB that may be beneficial to old people. HMB ameliorated the effects of ageing in the dendritic tree of the pyramidal neurons in the medial prefrontal cortex of both male and female rats and improved the working and cognitive flexibility in old‐age rats.74, 75 Studies are needed to examine whether HMB supplementation in elderly subjects also improves the anabolic response of muscle to a meal.
Cachexia and effects of HMB supplementation
There is scientific consensus that the loss of muscle protein in cachexia cannot be reversed by nutritional therapy. A major pathogenic process involves the activation of the ubiquitin‐proteasome system by several pathways, including cytokines, reactive oxygen species, and cyclooxygenases. In addition, the up‐regulation of autophagy and lysosomal genes has been documented at the transcript and protein levels in various catabolic conditions.76 There are reports demonstrating that muscles composed mostly by white fibres are more sensitive to catabolic stimuli, particularly to sepsis, when compared with muscles with high content of red fibres.77, 78 Also with advancing age, there is a preferential atrophy of white fibres.79 However, the explanation of origin of these clinically important differences in response of red and white fibres to signals causing the loss of muscle is not available.
In muscle‐wasting disorders, it increases the activity of the BCKD, the rate‐limiting enzyme in leucine oxidation, resulting in the use of leucine and the other two BCAAs as important sources of energy.80, 81, 82 It may be hypothesized that enhanced flux of KIC through BCKD decreases the metabolism of KIC via the KIC dioxygenase pathway and impairs HMB production. However, there are no reports regarding HMB production and its levels in cachectic illness.
The therapeutic potential of HMB in the treatment of cachexia was investigated using various experimental protocols. Through a Medline search, we found eight studies performed under in vitro conditions (Table 4), 11 under in vivo conditions using various animal models of muscle wasting (Table 5), and 13 in various forms of cachexia in humans (Table 6).
Table 4.
Effects of beta‐hydroxy‐beta‐methylbutyrate under in vitro conditions of skeletal muscle atrophy
| Model | Effects of HMB | Reference |
|---|---|---|
| Murine myotubes exposed to HMB (50 μM) and PIF | ↓ depression of protein synthesis | Eley et al.83 |
| Murine myotubes exposed to HMB (50 μM) and PIF | ↓ protein degradation and proteasome activity | Smith et al. 84 |
| Murine myotubes exposed to HMB (50 μM) and PIF, LPS, or angiotensin II | ↓ proteasome activity | Mirza et al. 85 |
| Murine myotubes exposed to HMB (50 μM) and LPS, TNF‐α, IFN‐γ, or angiotensin II | ↓ proteolysis, ROS formation and caspase activation | Eley et al. 16 |
| Murine myotubes exposed to HMB (50 μM) and LPS | ↓ protein degradation and caspase activation | Russell and Tisdale86 |
| Murine myotubes exposed to HMB (50 μM) and dexamethasone | ↓ protein degradation, ↓ expression of atrogin‐1 and MuRF1, ↓ reduction of myotube size | Aversa et al. 87 |
| Murine myotubes exposed to HMB (10 mM) and myostatin | ↓ fibre atrophy | Mobley et al. 88 |
| Rat L6 myotubes exposed to HMB (25 μM) and dexamethasone | ↓ lysosomal proteolysis induced by dexamethasone | Girón et al. 89 |
PIF, proteolysis‐inducing factor; LPS, lipopolysaccharides.
Table 5.
Effects of beta‐hydroxy‐beta‐methylbutyrate in animal models of muscle wasting
| Study design | Effects | Reference |
|---|---|---|
| Rats, AH‐130 ascites hepatoma, HMB‐enriched chow for 24 days | ↓ body weight and muscle loss | Aversa et al. 90 |
| Rats, Walker 256 tumour, HMB (320 mg/kg) by p.o. gavage | ↑ survival time | Caperuto et al.91 |
| Mice, MAC 16 tumour, HMB (0.25 g/kg) by p.o. gavage for 4 days | ↓ body weight loss | Mirza et al.85 |
| Rats, Walker 256 tumour, HMB (76 mg/kg) by p.o. gavage for 8 weeks | ↓ tumour weight, ↓ NF‐κB signalling, ↑ glycogen content in liver and muscle | Nunes et al. 92 |
| Mice, MAC 16 tumour, HMB (0.25 g/kg) or EPA (0.6 g/kg), or both by p.o. gavage for 8 days | ↓ muscle protein degradation and proteasome activity in all groups compared to controls, ↑ protein synthesis in HMB group | Smith et al. 14 |
| Rats, endotoxin (5 mg/kg i.p.), HMB (0.5 g/kg) via osmotic pump for 24 hours | ↓ proteolysis and proteasome activity in muscle | Kovarik et al. 15 |
| Rats, monolateral hindlimb immobilization or dexamethasone treatment, leucine (2.7 g/kg/day) or HMB (0.6 g/kg/day) orally for 1, 2, 3 or 7 days | No effect of on muscle mass and fibre cross‐sectional area in both models, ↓ expression of Mafbx/Atrogin after dexamethasone. Leucine had favourable effects on most of the parameters. | Baptista et al. 93 |
| Mice, Duchenne muscular dystrophy model (mdx mice), diet with added creatine, linoleic acid, alpha‐lipoic acid or HMB (individually and in combination) and exercise for 8 weeks | ↓ muscle loss and grip strength fatigue, ↑ grip strength | Payne et al. 94 |
| Rats, dexamethasone and co‐administration of HMB (320 mg/kg/day orally) for 21 days | ↓ the loss of body weight, lean mass and reduction of fibre cross‐sectional area | Girón et al. 89 |
| Rats, co‐administration of dexamethasone and HMB (150 or 600 mg/kg/day orally) for 5 days | ↓ muscle loss and damage and reduction in grip strength, ↓ MuRF1 expression | Noh et al. 95 |
| Mice, calorie restricted (−30%) and exercise, HMB (0.5 g/kg orally) for 6 weeks | Greater grip strength, gastrocnemius mass and fibre cross‐sectional area. No atrogin‐1 expression while elevation in controls. | Park et al.96 |
EPA, eicosapentaenoic acid.
Table 6.
Effects of beta‐hydroxy‐beta‐methylbutyrate in humans with muscle‐wasting disorder
| Origin of muscle loss | Study design | Effects | Reference |
|---|---|---|---|
| AIDS | HMB/Arg/Gln mixture (3/14/14 g per day) for 8 weeks | ↑ lean body mass and improved immune status | Clark et al. 97 |
| Cancer | HMB/Arg/Gln mixture (3/14/14 g per day) for 24 weeks | ↑ body weight and FFM | May et al.98 |
| Cancer | HMB/Arg/Gln mixture (3/14/14 g per day) for 8 weeks | Trend towards an increased body mass | Berk et al. 99 |
| AIDS or cancer | HMB/Arg/Gln mixture (3/14/14 g per day) for 8 weeks | Decreased feeling of weakness, increased RBC, haematocrit, lymphocytes, eosinophils, and urea | Rathmacher et al. 100 |
| Chronic obstructive pulmonary disease | HMB (3 g/day) for 7 days | Improved pulmonary function, ↓CRP | Hsieh et al. 101 |
| Chronic cardiac or pulmonary disease | Oral supplementation with proteins and HMB (1.5 g HMB/day) for 90 days | Decreased mortality, improved indices of nutritional status | Deutz et al. 102 |
| Chronic pulmonary disease | Oral supplementation with proteins and HMB (1.5 g HMB/day) for 12 weeks | Improved body composition, health‐related quality of life, and muscle strength | Olveira et al. 103 |
| Critically ill trauma patients, bed rest, enteral nutrition | HMB (3 g/day), HMB/Arg/Gln mixture or placebo via feeding tube for 28 days | Improvement in nitrogen balance | Kuhls et al. 104 |
| Total knee arthroplasty | HMB/Gln/Arg mixture (2.4/14/14 g per day) for 4 weeks | Prevention of reduction of maximal strength of quadriceps muscle | Nishizaki et al. 105 |
| Hip fracture | HMB (3 g)/vitamin D/protein combination for 30 days | Accelerated healing, shortening immobilization period, ↑ muscle strength | Ekinci et al.106 |
| Gastric bypass | HMB/Gln/Arg mixture (1.5/7/7 g per day) for 8 weeks | No benefits when compared with controls | Clements et al.107 |
| Renal failure | HMB (3 g/day) for 6 months | No benefits | Fitschen et al. 108 |
| Rheumatoid arthritis | HMB/Gln/Arg mixture (3/14/14 g per day) for 12 weeks | No benefits when compared with placebo | Marcora et al.109 |
Experimental studies performed using in vitro models (mainly murine myotubes) and animal models of muscle wasting indicate that HMB may be effective in a number of disorders, notably in conditions of enhanced proteolysis in sepsis, cancer, immobilization, and steroid medication. Most of the studies demonstrated that the action of HMB is mediated by attenuating the proteasome activity and protein breakdown and not by stimulating protein synthesis (Tables 4 and 5). In human studies, positive results were observed in chronic pulmonary disease, hip fracture, and in AIDS‐related and cancer‐related cachexia but not in rheumatoid cachexia, renal failure, and gastric bypass (Table 6). Unfortunately, these clinical studies frequently used mixed supplements that contained various components including glutamine, arginine, leucine, higher caloric or protein content, and vitamins. Therefore, it was not possible to determine which of the supplements was effective or if there was a synergistic effect.
It should be noted that sarcopenia and/or cachexia is the most common complication of cirrhosis and adversely affects survival, quality of life, and the development of other complications.110 Considering that most of the HMB is synthesized from leucine in the liver and that the activation of leucine oxidation in skeletal muscle causes a decrease in the leucine level in the blood plasma of subjects with liver cirrhosis,111, 112 impaired HMB synthesis and its deficiency may be expected to develop in liver disease. However, to date, no animal or human studies of HMB supplementation in liver disease have been performed.
Healthy subjects and effects of HMB consumption
The HMB is one of the most frequently used supplements not only among athletes but also by people aiming to losses fat mass and prevent the age‐related loss of skeletal muscle. However, some studies indicate that the beneficial effects of HMB on skeletal muscle observed in subjects who exercise, elderly subjects and/or in muscle‐wasting conditions do not occur in healthy, non‐exercising subjects. Nissen and Abumrad reported in a study of non‐exercising women who received supplements of 3 g of HMB/day for 4 weeks that there were no changes in the body composition whereas in a similar study in which the women were subjected to resistance exercise, an increase in lean tissue and a decrease in fat mass were observed.10 In our study, the HMB counteracted the changes in the muscles of septic rats but had no effect in healthy animals.15
The explanation of the lack of favourable effects of HMB on muscle in healthy individuals who do not engage in a regular physical activity is not available. It seems that HMB modulates the balance between protein synthesis and proteolysis in a favour of anabolic reactions and that its requirement increases in conditions of accelerated turnover of muscle proteins that occurs in exercise and muscle‐wasting conditions. In this context, it should be noted that impaired insulin sensitivity has been demonstrated in healthy sedentary rats supplemented with HMB for 4 weeks.113
Dosage and drug forms
A dose of 3 g of HMB per day is routinely recommended by manufacturer to maintain or improve muscle mass and function. This dose corresponds to intake of approximately 60 g of leucine. Consumption of such an amount of leucine should enhance the activity of the BCKD (the rate‐limiting enzyme of BCAA catabolism) and the oxidation of all the three BCAA, which would cause depletion of valine and isoleucine in body fluids. Such an imbalance in concentrations of the BCAA could exert adverse effects on protein metabolism in various tissues, and therefore, HMB supplementation cannot be replaced by leucine.114
Commercially, HMB is available as the calcium salt. Recently, administration of HMB in a free‐acid form of HMB in a gel has been investigated. Fuller et al. showed that administration of the free form of HMB resulted in more rapid and higher plasma concentrations and improved clearance of the HMB when compared with the calcium salt form and suggested that administration of HMB as free acid could improve HMB availability and efficacy to tissues.115 However, another study demonstrated higher bioavailability of HMB after administration of the calcium salt of HMB when compared with free‐acid form.116 Further studies are needed to determine the pros and cons of these two forms of HMB.
Toxicity and adverse effects
Several studies have demonstrated that supplemental HMB is well tolerated and has no toxic effects.11, 100, 117 However, although HMB is made naturally by the body, it is possible that the positive effects of HMB on the protein balance in muscle may exert some adverse effects in other tissues. Stimulation of protein synthesis and suppression of proteolysis by HMB decreases the release of various amino acids from muscles to the blood and may impair their availability in visceral tissues. Glutamine is of particular interest on the basis that it acts as an essential substrate for enterocytes and immune cells and its deficiency decreases protein synthesis in skeletal muscle.118, 119 The plasma glutamine concentration is already low in many patients with critical illness, and a decreased glutamine level has been reported after HMB treatment.22 Therefore, studies are needed to examine whether the positive effects of HMB on muscle mass in cachexia are associated with glutamine depletion and adverse effects in other tissues.
Conclusions
The reports summarized here indicate that HMB provides a number of benefits to subjects involved in strength‐power and endurance sports. The effects on muscle mass and strength, particularly during resistance training, are likely related to the suppression of proteolysis and a positive effect on protein synthesis. Its benefits in aerobic performance are probably more associated with improved mitochondrial biogenesis and fat oxidation. Favourable effects on the recovery from exercise‐induced damage may be related to the role of HMB as a precursor of cholesterol, which modulates membrane fluidity and affects ion channels, and membrane excitability.
Studies have demonstrated that HMB can prevent the development of sarcopenia in elderly subjects and that the optimal action of HMB on muscle growth and strength occurs when it is combined with exercise. Unfortunately, exercise is performed only by a small percentage of elderly subjects. Several studies suggest that HMB supplementation is ineffective in healthy sedentary subjects.
Studies performed under in vitro conditions and animal studies suggest that HMB may be effective as a treatment for muscle wasting in various forms of cachexia. However, clinical reports are rare, and most of them examined the therapeutic potential of combinations of various agents. It was therefore not possible to determine, which of the supplements was effective. Further studies examining the effects of HMB administered alone are needed to reach a conclusion regarding the specific effectiveness of HMB in attenuating muscle wasting in a range of catabolic conditions. Although most of the endogenous HMB is produced in the liver and impaired HMB production may be assumed to occur in liver disease, there are no reports regarding the metabolism of HMB and the effects of its supplementation in subjects with liver disease.
Conflict of interest
None declared.
Acknowledgements
The author was supported in part by the programmes PRVOUK P37/02 and PROGRES Q40/02. The author of this manuscript certifies that he complies with ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.120
Holeček, M. (2017) Beta‐hydroxy‐beta‐methylbutyrate supplementation and skeletal muscle in healthy and muscle‐wasting conditions. Journal of Cachexia, Sarcopenia and Muscle, 8: 529–541. doi: 10.1002/jcsm.12208.
References
- 1. Kalantar‐Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle 2013;4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma 1987;27:262–266. [DOI] [PubMed] [Google Scholar]
- 3. Anker SD, Coats AJ, Morley JE. Evidence for partial pharmaceutical reversal of the cancer anorexia‐cachexia syndrome: the case of anamorelin. J Cachexia Sarcopenia Muscle 2015;6:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Konishi M, Ishida J, von Haehling S, Anker SD, Springer J. Nutrition in cachexia: from bench to bedside. J Cachexia Sarcopenia Muscle 2016;7:107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 1999;341:785–792. [DOI] [PubMed] [Google Scholar]
- 6. Van Koevering M, Nissen S. Oxidation of leucine and alpha‐ketoisocaproate to beta‐hydroxybeta‐methylbutyrate in vivo. Am J Phys 1992;262:E27–E31. [DOI] [PubMed] [Google Scholar]
- 7. Girón MD, Vílchez JD, Salto R, Manzano M, Sevillano N, Campos N, et al. Conversion of leucine to β‐hydroxy‐β‐methylbutyrate by α‐keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in L6 rat myotubes. J Cachexia Sarcopenia Muscle 2016;7:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC, et al. Effect of leucine metabolite beta‐hydroxy‐beta‐methylbutyrate on muscle metabolism during resistance‐exercise training. J Appl Physiol 1996;81:2095–2104. [DOI] [PubMed] [Google Scholar]
- 9. Walker DK, Thaden JJ, Wierzchowska‐McNew A, Engelen MP, Deutz NE. Determination of β‐hydroxy‐β‐methylbutyrate concentration and enrichment in human plasma using chemical ionization gas chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2017;1040:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite β‐hydroxy‐β–methylbutyrate (HMB) . J Nutr Biochem 1997;8:300–311. [Google Scholar]
- 11. Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC. beta‐Hydroxy‐beta‐methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 2000;130:1937–1945. [DOI] [PubMed] [Google Scholar]
- 12. Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, et al. Effects of leucine and its metabolite beta‐hydroxy‐beta‐methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013;591:2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerlinger‐Romero F, Guimarães‐Ferreira L, Giannocco G, Nunes MT. Chronic supplementation of beta‐hydroxy‐beta methylbutyrate (HMβ) increases the activity of the GH/IGF‐I axis and induces hyperinsulinemia in rats. Growth Horm IGF Res 2011;21:57–62. [DOI] [PubMed] [Google Scholar]
- 14. Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome‐induced proteolysis in skeletal muscle by {beta}‐hydroxy‐{beta}‐methylbutyrate in cancer‐induced muscle loss. Cancer Res 2005;65:277–283. [PubMed] [Google Scholar]
- 15. Kovarik M, Muthny T, Sispera L, Holecek M. Effects of β‐hydroxy‐β‐methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J Physiol Biochem 2010;66:311–319. [DOI] [PubMed] [Google Scholar]
- 16. Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta‐hydroxy‐beta‐methylbutyrate. Am J Physiol Endocrinol Metab 2008;295:E1409–E1416. [DOI] [PubMed] [Google Scholar]
- 17. Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. β‐Hydroxy‐β‐methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension‐induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol 2011;301:R701–R715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimura K, Cheng XW, Inoue A, Hu L, Koike T, Kuzuya M. β‐Hydroxy‐β‐methylbutyrate facilitates PI3K/Akt‐dependent mammalian target of rapamycin and FoxO1/3a phosphorylations and alleviates tumor necrosis factor α/interferon γ‐induced MuRF‐1 expression in C2C12 cells. Nutr Res 2014;34:368–374. [DOI] [PubMed] [Google Scholar]
- 19. Kornasio R, Riederer I, Butler‐Browne G, Mouly V, Uni Z, Halevy O. Beta‐hydroxy‐beta‐methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 1793;2009:755–763. [DOI] [PubMed] [Google Scholar]
- 20. He X, Duan Y, Yao K, Li F, Hou Y, Wu G. Yin Y β‐Hydroxy‐β‐methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016;48:653–664. [DOI] [PubMed] [Google Scholar]
- 21. Vallejo J, Spence M, Cheng AL, Brotto L, Edens NK, Garvey SM, Brotto M. Cellular and physiological effects of dietary supplementation with β‐hydroxy‐β‐methylbutyrate (HMB) and β‐alanine in late middle‐aged mice. PLoS One 2016;11:e0150066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holecek M, Muthny T, Kovarik M, Sispera L. Effect of beta‐hydroxy‐beta‐methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol 2009;47:255–259. [DOI] [PubMed] [Google Scholar]
- 23. Poortmans JR, Carpentier A, Pereira‐Lancha LO, Lancha A. Protein turnover, amino acid requirements and recommendations for athletes and active populations. Braz J Med Biol Res 2012;45:875–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, et al. Immediate response of mammalian target of rapamycin (mTOR)‐mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 2003;553:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clarkson PM, Hubal MJ. Exercise‐induced muscle damage in humans. Am J Phys Med Rehabil 2002;81:S52–S69. [DOI] [PubMed] [Google Scholar]
- 26. Wolfe RR, Goodenough RD, Wolfe MH, Royle GT, Nadel ER. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol Respir Environ Exerc Physiol 1982;52:458–466. [DOI] [PubMed] [Google Scholar]
- 27. Knapik J, Meredith C, Jones B, Fielding R, Young V, Evans W. Leucine metabolism during fasting and exercise. J Appl Physiol (1985) 1991;70:43–47. [DOI] [PubMed] [Google Scholar]
- 28. Mero A. Leucine supplementation and intensive training. Sports Med 1999;27:347–358. [DOI] [PubMed] [Google Scholar]
- 29. Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta‐hydroxy‐beta‐methylbutyrate ingestion, part I: effects on strength and fat free mass. Med Sci Sports Exerc 2000;32:2109–2115. [DOI] [PubMed] [Google Scholar]
- 30. Jówko E, Ostaszewski P, Jank M, Sacharuk J, Zieniewicz A, Wilczak J, et al. Creatine and beta‐hydroxy‐beta‐methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight‐training program. Nutrition 2001;17:558–566. [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez AM, Stout JR, Jajtner AR, Townsend JR, Wells AJ, Beyer KS, et al. Effects of β‐hydroxy‐β‐methylbutyrate free acid and cold water immersion on post‐exercise markers of muscle damage. Amino Acids 2014;46:1501–1511. [DOI] [PubMed] [Google Scholar]
- 32. Lowery RP, Joy JM, Rathmacher JA, Baier SM, Fuller JC, Shelley MC, et al. Interaction of beta‐hydroxy‐beta‐methylbutyrate free acid and adenosine triphosphate on muscle mass, strength, and power in resistance trained individuals. J Strength Cond Res 2016;30:1843–1854. [DOI] [PubMed] [Google Scholar]
- 33. Portal S, Zadik Z, Rabinowitz J, Pilz‐Burstein R, Adler‐Portal D, Meckel Y, et al. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: a prospective randomized, double‐blind, placebo‐controlled study. Eur J Appl Physiol 2011;111:2261–2269. [DOI] [PubMed] [Google Scholar]
- 34. Kraemer WJ, Hatfield DL, Volek JS, Fragala MS, Vingren JL, Anderson JM, et al. Effects of amino acids supplement on physiological adaptations to resistance training. Med Sci Sports Exerc 2009;41:1111–1121. [DOI] [PubMed] [Google Scholar]
- 35. van Someren KA, Edwards AJ, Howatson G. Supplementation with beta‐hydroxy‐beta‐methylbutyrate (HMB) and alpha‐ketoisocaproic acid (KIC) reduces signs and symptoms of exercise‐induced muscle damage in man. Int J Sport Nutr Exerc Metab 2005;15:413–424. [DOI] [PubMed] [Google Scholar]
- 36. Knitter AE, Panton L, Rathmacher JA, Petersen A, Sharp R. Effects of beta‐hydroxy‐beta‐methylbutyrate on muscle damage after a prolonged run. J Appl Physiol 2000;89:1340–1344. [DOI] [PubMed] [Google Scholar]
- 37. Vukovich MD, Dreifort GD. Effect of beta‐hydroxy beta‐methylbutyrate on the onset of blood lactate accumulation and V(O)(2) peak in endurance‐trained cyclists. J Strength Cond Res 2001;15:491–497. [PubMed] [Google Scholar]
- 38. Robinson EH, Stout JR, Miramonti AA, Fukuda DH, Wang R, Townsend JR, et al. High‐intensity interval training and β‐hydroxy‐β‐methylbutyric free acid improves aerobic power and metabolic thresholds. J Int Soc Sports Nutr 2014;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamboley CR, Royer D, Dionne IJ. Effects of beta‐hydroxy‐beta‐methylbutyrate on aerobic‐performance components and body composition in college students. Int J Sport Nutr Exerc Metab 2007;17:56–69. [DOI] [PubMed] [Google Scholar]
- 40. Durkalec‐Michalski K, Jeszka J. The efficacy of a β‐hydroxy‐β‐methylbutyrate supplementation on physical capacity, body composition and biochemical markers in elite rowers: a randomised, double‐blind, placebo‐controlled crossover study. J Int Soc Sports Nutr 2015;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Durkalec‐Michalski K, Jeszka J. The effect of β‐hydroxy‐β‐methylbutyrate on aerobic capacity and body composition in trained athletes. J Strength Cond Res 2016;30:2617–2626. [DOI] [PubMed] [Google Scholar]
- 42. Panton LB, Rathmacher JA, Baier S, Nissen S. Nutritional supplementation of the leucine metabolite beta‐hydroxy‐beta‐methylbutyrate (hmb) during resistance training. Nutrition 2000;16:734–739. [DOI] [PubMed] [Google Scholar]
- 43. Wilson JM, Lowery RP, Joy JM, Andersen JC, Wilson SM, Stout JR, et al. The effects of 12 weeks of beta‐hydroxy‐beta‐methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance‐trained individuals: a randomized, double‐blind, placebo‐controlled study. Eur J Appl Physiol 2014;114:1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilson JM, Lowery RP, Joy JM, Walters JA, Baier SM, Fuller JC, et al. β‐Hydroxy‐β‐methylbutyrate free acid reduces markers of exercise‐induced muscle damage and improves recovery in resistance‐trained men. Br J Nutr 2013;110:538–544. [DOI] [PubMed] [Google Scholar]
- 45. Miramonti AA, Stout JR, Fukuda DH, Robinson EH, Wang R, La Monica MB, et al. Effects of 4 weeks of high‐intensity interval training and β‐hydroxy‐β‐methylbutyric free acid supplementation on the onset of neuromuscular fatigue. J Strength Cond Res 2016;30:626–634. [DOI] [PubMed] [Google Scholar]
- 46. Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta‐analysis. J Appl Physiol (1985) 2003;94:651–659. [DOI] [PubMed] [Google Scholar]
- 47. Paddon‐Jones D, Keech A, Jenkins D. Short‐term beta‐hydroxy‐beta‐methylbutyrate supplementation does not reduce symptoms of eccentric muscle damage. Int J Sport Nutr Exerc Metab 2001;11:442–450. [DOI] [PubMed] [Google Scholar]
- 48. Kreider RB, Ferreira M, Wilson M, Almada AL. Effects of calcium beta‐hydroxy‐beta‐methylbutyrate (HMB) supplementation during resistance‐training on markers of catabolism, body composition and strength. Int J Sports Med 1999;20:503–509. [DOI] [PubMed] [Google Scholar]
- 49. O'Connor DM, Crowe MJ. Effects of beta‐hydroxy‐beta‐methylbutyrate and creatine monohydrate supplementation on the aerobic and anaerobic capacity of highly trained athletes. J Sports Med Phys Fitness 2003;43:64–68. [PubMed] [Google Scholar]
- 50. O'Connor DM, Crowe MJ. Effects of six weeks of beta‐hydroxy‐beta‐methylbutyrate (HMB) and HMB/creatine supplementation on strength, power, and anthropometry of highly trained athletes. J Strength Cond Res 2007;21:419–423. [DOI] [PubMed] [Google Scholar]
- 51. Slater G, Jenkins D, Logan P, Lee H, Vukovich M, Rathmacher JA, et al. Beta‐hydroxy‐betamethylbutyrate (HMB) supplementation does not affect changes in strength or body composition during resistance training in trained men. Int J Sport Nutr Exerc Metab 2001;11:384–396. [DOI] [PubMed] [Google Scholar]
- 52. Ransone J, Neighbors K, Lefavi R, Chromiak J. The effect of beta‐hydroxy beta‐methylbutyrate on muscular strength and body composition in collegiate football players. J Strength Cond Res 2003;17:34–39. [DOI] [PubMed] [Google Scholar]
- 53. Thomson JS, Watson PE, Rowlands DS. Effects of nine weeks of beta‐hydroxy‐beta‐methylbutyrate supplementation on strength and body composition in resistance trained men. J Strength Cond Res 2009;23:827–835. [DOI] [PubMed] [Google Scholar]
- 54. Hoffman JR, Cooper J, Wendell M, Im J, Kang J. Effects of beta‐hydroxy beta‐methylbutyrate on power performance and indices of muscle damage and stress during high‐intensity training. J Strength Cond Res 2004;18:747–752. [DOI] [PubMed] [Google Scholar]
- 55. Nunan D, Howatson G, van Someren KA. Exercise‐induced muscle damage is not attenuated by beta‐hydroxy‐beta‐methylbutyrate and alpha‐ketoisocaproic acid supplementation. J Strength Cond Res 2010;24:531–537. [DOI] [PubMed] [Google Scholar]
- 56. Albert FJ, Morente‐Sánchez J, Ortega FB, Castillo MJ, Gutiérrez Á. Usefulness of β‐hydroxy‐β‐methylbutyrate (HMB) supplementation in different sports: an update and practical implications. Nutr Hosp 2015;32:20–33. [DOI] [PubMed] [Google Scholar]
- 57. Pasiakos SM, Carbone JW. Assessment of skeletal muscle proteolysis and the regulatory response to nutrition and exercise. IUBMB Life 2014;66:478–484. [DOI] [PubMed] [Google Scholar]
- 58. Rowlands DS, Thomson JS. Effects of beta‐hydroxy‐beta‐methylbutyrate supplementation during resistance training on strength, body composition, and muscle damage in trained and untrained young men: a meta‐analysis. J Strength Cond Res 2009;23:836–846. [DOI] [PubMed] [Google Scholar]
- 59. Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, et al. International society of sports nutrition position stand: beta‐hydroxy‐beta‐methylbutyrate (HMB). J Int Soc Sports Nutr 2013;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology‐update 2014. J Cachexia Sarcopenia Muscle 2014;5:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Russ DW, Acksel C, Boyd IM, Maynard J, McCorkle KW, Edens NK, et al. Dietary HMB and β‐alanine co‐supplementation does not improve in situ muscle function in sedentary, aged male rats. Appl Physiol Nutr Metab 2015;40:1294–1301. [DOI] [PubMed] [Google Scholar]
- 62. Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT. beta‐Hydroxy‐beta‐methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol 2013;48:973–984. [DOI] [PubMed] [Google Scholar]
- 63. Baier S, Johannsen D, Abumrad N, Rathmacher JA, Nissen S, Flakoll P. Year‐long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta‐hydroxy‐beta‐methylbutyrate (HMB), L‐arginine, and L‐lysine. JPEN J Parenter Enteral Nutr 2009;33:71–82. [DOI] [PubMed] [Google Scholar]
- 64. Hsieh LC, Chow CJ, Chang WC, Liu TH, Chang CK. Effect of beta‐hydroxy‐beta‐methylbutyrate on protein metabolism in bed‐ridden elderly receiving tube feeding. Asia Pac J Clin Nutr 2010;19:200–208. [PubMed] [Google Scholar]
- 65. Vukovich MD, Stubbs NB, Bohlken RM. Body composition in 70‐year‐old adults responds to dietary beta‐hydroxy‐beta‐methylbutyrate similarly to that of young adults. J Nutr 2001;131:2049–2052. [DOI] [PubMed] [Google Scholar]
- 66. Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta‐hydroxy‐betamethylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004;20:445–451. [DOI] [PubMed] [Google Scholar]
- 67. Fuller JC, Baier S, Flakoll P, Nissen SL, Abumrad NN, Rathmacher JA. Vitamin D status affects strength gains in older adults supplemented with a combination of β‐hydroxy‐β‐methylbutyrate, arginine, and lysine: a cohort study. JPEN J Parenter Enteral Nutr 2011;35:757–762. [DOI] [PubMed] [Google Scholar]
- 68. Williams JZ, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg 2002;236:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Berton L, Bano G, Carraro S, Veronese N, Pizzato S, Bolzetta F, et al. Effect of oral beta‐hydroxy‐beta‐methylbutyrate (HMB) supplementation on physical performance in healthy old women over 65 years: an open label randomized controlled trial. PLoS One 2015;10:e0141757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, et al. Effect of beta‐hydroxy beta‐methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 2013;32:704–712. [DOI] [PubMed] [Google Scholar]
- 71. Stout JR, Smith‐Ryan AE, Fukuda DH, Kendall KL, Moon JR, Hoffman JR, et al. Effect of calcium beta‐hydroxy‐beta‐methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double‐blind pilot trial. Exp Gerontol 2013;48:1303–1310. [DOI] [PubMed] [Google Scholar]
- 72. Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, et al. Effect of beta‐hydroxy‐beta‐methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta‐analysis. Arch Gerontol Geriatr 2015;61:168–175. [DOI] [PubMed] [Google Scholar]
- 73. Shreeram S, Ramesh S, Puthan JK, Balakrishnan G, Subramanian R, Reddy MT, et al. Age associated decline in the conversion of leucine to β‐hydroxy‐β‐methylbutyrate in rats. Exp Gerontol 2016;80:6–11. [DOI] [PubMed] [Google Scholar]
- 74. Kougias DG, Nolan SO, Koss WA, Kim T, Hankosky ER, Gulley JM, et al. Beta‐hydroxy‐beta‐methylbutyrate ameliorates aging effects in the dendritic tree of pyramidal neurons in the medial prefrontal cortex of both male and female rats. Neurobiol Aging 2016;40:78–85. [DOI] [PubMed] [Google Scholar]
- 75. Hankosky ER, Sherrill LK, Ruvola LA, Haake RM, Kim T, Hammerslag LR, et al. Effects of β‐hydroxy‐β‐methyl butyrate on working memory and cognitive flexibility in an animal model of aging. Nutr Neurosci 2016; https://doi.org/10.1080/1028415X.2016.1145376. [DOI] [PubMed] [Google Scholar]
- 76. Sandri M. Protein breakdown in muscle wasting: role of autophagy‐lysosome and ubiquitin‐proteasome. Int J Biochem Cell Biol 2013;45:2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kadlcikova J, Holecek M, Safranek R, Tilser I, Kessler BM. Effects of proteasome inhibitors MG132, ZL3VS and AdaAhx3L3VS on protein metabolism in septic rats. Int J Exp Pathol 2004;85:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Muthny T, Kovarik M, Sispera L, Tilser I, Holecek M. Protein metabolism in slow‐ and fast‐twitch skeletal muscle during turpentine‐induced inflammation. Int J Exp Pathol 2008;89:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 2010;91:1123S–1127S. [DOI] [PubMed] [Google Scholar]
- 80. Holecek M. Leucine metabolism in fasted and tumor necrosis factor‐treated rats. Clin Nutr 1996;15:91–93. [DOI] [PubMed] [Google Scholar]
- 81. Holecek M, Sprongl L, Skopec F, Andrýs C, Pecka M. Leucine metabolism in TNF‐alpha‐ and endotoxin‐treated rats: contribution of hepatic tissue. Am J Phys 1997;273:E1052–E1058. [DOI] [PubMed] [Google Scholar]
- 82. Holecek M, Sprongl L, Tichý M, Pecka M. Leucine metabolism in rat liver after a bolus injection of endotoxin. Metabolism 1998;47:681–685. [DOI] [PubMed] [Google Scholar]
- 83. Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by beta‐hydroxy‐beta‐methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab 2007;293:E923–E931. [DOI] [PubMed] [Google Scholar]
- 84. Smith HJ, Wyke SM, Tisdale MJ. Mechanism of the attenuation of proteolysis‐inducing factor stimulated protein degradation in muscle by beta‐hydroxy‐beta‐methylbutyrate. Cancer Res 2004;64:8731–8735. [DOI] [PubMed] [Google Scholar]
- 85. Mirza KA, Pereira SL, Voss AC, Tisdale MJ. Comparison of the anticatabolic effects of leucine and Ca‐β‐hydroxy‐β‐methylbutyrate in experimental models of cancer cachexia. Nutrition 2014;30:807–813. [DOI] [PubMed] [Google Scholar]
- 86. Russell ST, Tisdale MJ. Mechanism of attenuation by beta‐hydroxy‐beta‐methylbutyrate of muscleprotein degradation induced by lipopolysaccharide. Mol Cell Biochem 2009;330:171–179. [DOI] [PubMed] [Google Scholar]
- 87. Aversa Z, Alamdari N, Castillero E, Muscaritoli M, Rossi Fanelli F, Hasselgren PO. β‐Hydroxy‐β‐methylbutyrate (HMB) prevents dexamethasone‐induced myotube atrophy. Biochem Biophys Res Commun 2012;423:739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mobley CB, Fox CD, Ferguson BS, Amin RH, Dalbo VJ, Baier S, et al. L‐leucine, beta‐hydroxy‐beta‐methylbutyric acid (HMB) and creatine monohydrate prevent myostatin‐induced Akirin‐1/Mighty mRNA down‐regulation and myotube atrophy. J Int Soc Sports Nutr 2014;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Girón MD, Vílchez JD, Shreeram S, Salto R, Manzano M, Cabrera E, et al. β‐Hydroxy‐β‐methylbutyrate (HMB) normalizes dexamethasone‐induced autophagy‐lysosomal pathway in skeletal muscle. PLoS One 2015;10:e0117520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aversa Z, Bonetto A, Costelli P, Minero VG, Penna F, Baccino FM, et al. beta‐hydroxy‐betamethylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Oncol 2011;38:713–720. [DOI] [PubMed] [Google Scholar]
- 91. Caperuto EC, Tomatieli RV, Colquhoun A, Seelaender MC, Costa Rosa LF. Beta‐hydoxy‐beta‐methylbutyrate supplementation affects Walker 256 tumor‐bearing rats in a time‐dependent manner. Clin Nutr 2007;26:117–122. [DOI] [PubMed] [Google Scholar]
- 92. Nunes EA, Kuczera D, Brito GA, Bonatto SJ, Yamazaki RK, Tanhoffer RA, et al. Beta‐hydroxy‐beta‐methylbutyrate supplementation reduces tumor growth and tumor cell proliferation ex vivo and prevents cachexia in Walker 256 tumor‐bearing rats by modifying nuclear factor‐kappaB expression. Nutr Res 2008;28:487–493. [DOI] [PubMed] [Google Scholar]
- 93. Baptista IL, Silva WJ, Artioli GG, Guilherme JP, Leal ML, Aoki MS, et al. Leucine and HMB differentially modulate proteasome system in skeletal muscle under different sarcopenic conditions. PLoS One 2013;8:e76752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Payne ET, Yasuda N, Bourgeois JM, Devries MC, Rodriguez MC, Yousuf J, Tarnopolsky MA. Nutritional therapy improves function and complements corticosteroid intervention in mdx mice. Muscle Nerve 2006;33:66–77. [DOI] [PubMed] [Google Scholar]
- 95. Noh KK, Chung KW, Choi YJ, Park MH, Jang EJ, Park CH, et al. β‐Hydroxy β‐methylbutyrate improves dexamethasone‐induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS One 2014;9:e102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Park BS, Henning PC, Grant SC, Lee WJ, Lee SR, Arjmandi BH, et al. HMB attenuates muscle loss during sustained energy deficit induced by calorie restriction and endurance exercise. Metabolism 2013;62:1718–1729. [DOI] [PubMed] [Google Scholar]
- 97. Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, et al. Nutritional treatment for acquired immunodeficiency virus‐associated wasting using beta‐hydroxy beta‐methylbutyrate, glutamine, and arginine: a randomized, double‐blind, placebo‐controlled study. JPEN J Parenter Enteral Nutr 2000;24:133–139. [DOI] [PubMed] [Google Scholar]
- 98. May PE, Barber A, D'Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer‐related wasting using oral supplementation with a combination of beta‐hydroxybetamethylbutyrate, arginine, and glutamine. Am J Surg 2002;183:471–479. [DOI] [PubMed] [Google Scholar]
- 99. Berk L, James J, Schwartz A, Hug E, Mahadevan A, Samuels M, et al. A randomized, double‐blind, placebo‐controlled trial of a beta‐hydroxyl beta‐methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer 2008;16:1179–1188. [DOI] [PubMed] [Google Scholar]
- 100. Rathmacher JA, Nissen S, Panton L, Clark RH, Eubanks May P, Barber AE, et al. Supplementation with a combination of beta‐hydroxy‐beta‐methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J Parenter Enteral Nutr 2004;28:65–75. [DOI] [PubMed] [Google Scholar]
- 101. Hsieh LC, Chien SL, Huang MS, Tseng HF, Chang CK. Anti‐inflammatory and anticatabolic effects of short‐term beta‐hydroxy‐beta‐methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr 2006;15:544–550. [PubMed] [Google Scholar]
- 102. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr 2016;35:18–26. [DOI] [PubMed] [Google Scholar]
- 103. Olveira G, Olveira C, Doña E, Palenque FJ, Porras N, Dorado A, et al. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (prospective, randomised study). Clin Nutr 2016;35:1015–2102. [DOI] [PubMed] [Google Scholar]
- 104. Kuhls DA, Rathmacher JA, Musngi MD, Frisch DA, Nielson J, Barber A, et al. Beta‐hydroxy‐beta‐methylbutyrate supplementation in critically ill trauma patients. J Trauma 2007;62:125–131. [DOI] [PubMed] [Google Scholar]
- 105. Nishizaki K, Ikegami H, Tanaka Y, Imai R, Matsumura H. Effects of supplementation with a combination of β‐hydroxy‐β‐methyl butyrate, L‐arginine, and L‐glutamine on postoperative recovery of quadriceps muscle strength after total knee arthroplasty. Asia Pac J Clin Nutr 2015;24:412–420. [DOI] [PubMed] [Google Scholar]
- 106. Ekinci O, Yanık S, Terzioğlu Bebitoğlu B, Yılmaz Akyüz E, Dokuyucu A, Erdem Ş. Effect of calcium β‐hydroxy‐β‐methylbutyrate (CaHMB), vitamin D, and protein supplementation on postoperative immobilization in malnourished older adult patients with hip fracture: a randomized controlled study. Nutr Clin Pract 2016;31:829–835. [DOI] [PubMed] [Google Scholar]
- 107. Clements RH, Saraf N, Kakade M, Yellumahanthi K, White M, Hackett JA. Nutritional effect of oral supplement enriched in beta‐hydroxy‐beta‐methylbutyrate, glutamine and arginine on resting metabolic rate after laparoscopic gastric bypass. Surg Endosc 2011;25:1376–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fitschen PJ, Biruete A, Jeong J, Wilund KR. Efficacy of beta‐hydroxy‐beta‐methylbutyrate supplementation in maintenance hemodialysis patients. Hemodial Int 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 109. Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta‐hydroxy‐betamethylbutyrate, glutamine and arginine: a randomized controlled trial. Clin Nutr 2005;24:442–454. [DOI] [PubMed] [Google Scholar]
- 110. Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle 2012;3:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Holecek M, Tilser I, Skopec F, Sprongl L. Leucine metabolism in rats with cirrhosis. J Hepatol 1996;24:209–216. [DOI] [PubMed] [Google Scholar]
- 112. Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched‐chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids 2011;40:575–584. [DOI] [PubMed] [Google Scholar]
- 113. Yonamine CY, Teixeira SS, Campello RS, Gerlinger‐Romero F, Rodrigues CF Jr, Guimarães‐Ferreira L, et al. Beta hydroxy beta methylbutyrate supplementation impairs peripheral insulin sensitivity in healthy sedentary Wistar rats. Acta Physiol (Oxf) 2014;212:62–74. [DOI] [PubMed] [Google Scholar]
- 114. Holecek M, Siman P, Vodenicarovova M, Kandar R. Alterations in protein and amino acid metabolism in rats fed a branched‐chain amino acid‐ or leucine‐enriched diet during postprandial and postabsorptive states. Nutr Metab (Lond) 2016;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fuller JC, Sharp RL, Angus HF, Baier SM, Rathmacher JA. Free acid gel form of β‐hydroxy‐β‐methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br J Nutr 2011;105:367–372. [DOI] [PubMed] [Google Scholar]
- 116. Shreeram S, Johns PW, Subramaniam S, Ramesh S, Vaidyanathan V, Puthan JK, et al. The relative bioavailability of the calcium salt of β‐hydroxy‐β‐methylbutyrate is greater than that of the free fatty acid form in rats. J Nutr 2014;144:1549–1555. [DOI] [PubMed] [Google Scholar]
- 117. Baxter JH, Carlos JL, Thurmond J, Rehani RN, Bultman J, Frost F. Dietary toxicity of calcium–hydroxy–methylbutyrate (CaHMB). Food Chem Toxicol 2005;43:1731–1741. [DOI] [PubMed] [Google Scholar]
- 118. Buchman AL. Glutamine for the gut: mystical properties or an ordinary amino acid? Curr Gastroenterol Rep 1999;1:417–423. [DOI] [PubMed] [Google Scholar]
- 119. Holecek M, Sispera L. Glutamine deficiency in extracellular fluid exerts adverse effects on protein and amino acid metabolism in skeletal muscle of healthy, laparotomized, and septic rats. Amino Acids 2014;46:1377–1384. [DOI] [PubMed] [Google Scholar]
- 120. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


