Abstract
Background
Cancer‐related fatigue (CRF) reduces quality of life and the activity level of patients with cancer. Cancer related fatigue can be reduced by exercise interventions that may concurrently increase muscle mass. We hypothesized that low muscle mass is directly related to higher CRF.
Methods
A total of 233 patients with advanced cancer starting palliative chemotherapy for lung, colorectal, breast, or prostate cancer were studied. The skeletal muscle index (SMI) was calculated as the patient's muscle mass on level L3 or T4 of a computed tomography scan, adjusted for height. Fatigue was assessed with the Functional Assessment of Chronic Illness Therapy‐fatigue questionnaire (cut‐off for fatigue <34). Multiple linear regression analyses were conducted to study the association between SMI and CRF adjusting for relevant confounders.
Results
In this group of patients with advanced cancer, the median fatigue score was 36 (interquartile range 26–44). A higher SMI on level L3 was significantly associated with less CRF for men (B 0.447, P 0.004) but not for women (B − 0.401, P 0.090). No association between SMI on level T4 and the Functional Assessment of Chronic Illness Therapy‐fatigue score was found (n = 82).
Conclusions
The association between SMI and CRF may lead to the suggestion that male patients may be able to reduce fatigue by exercise interventions aiming at an increased muscle mass. In women with advanced cancer, CRF is more influenced by other causes, because it is not significantly related to muscle mass. To further reduce CRF in both men and women with cancer, multifactorial assessments need to be performed in order to develop effective treatment strategies.
Keywords: Fatigue, Muscle, Neoplasms, Cancer, Exercise
Introduction
Cancer‐related fatigue (CRF) is characterized by significant physical, emotional, and/or cognitive exhaustion, which is disproportionate to the activity level and interferes with usual functioning in patients with cancer.1 Cancer‐related fatigue is one of the most prevalent symptoms experienced by patients with cancer, both during and after treatment.2, 3 Although advances are being made, the aetiology of CRF is not yet fully understood, and treatment is mostly symptomatic.4, 5 The pathophysiology of CRF is determined by central and peripheral aspects of fatigue.4, 6, 7 Pro‐inflammatory effects of cancer result in direct changes in the muscle metabolism, leading to reduced adenosine triphosphate levels, reduced protein synthesis, and localized electrolyte imbalances causing peripheral CRF.6 Central effects of elevated cytokine levels (among which interleukin‐2, interleukin‐6, and tumour necrosis factor alpha) cause changes in activity of the hypothalamus‐pituitary‐adrenal axis, leading to a sensation of reduced capacity to perform physical work, but also to a reduced androgen expression.6, 8 Both pathways could result in a loss of muscle mass, but whether a decreased muscle mass itself affects CRF is unclear.4, 9
Promising results for the treatment of CRF are seen with exercise interventions.10, 11, 12 Exercise interventions can improve muscle mass, strength, and functioning, depending on the type and intensity of exercise. Which type of exercise (e.g. aerobic, resistance), and at which intensity level, contributes most to the positive effect on CRF is not yet fully known4, 12, 13 and neither is the pathophysiological mechanism of this effect. Several studies have been performed investigating the effect of exercise interventions on CRF in patients with limited disease, and their results indicate improvement of CRF and increased muscle mass, but the effect sizes varied.14, 15, 16, 17, 18 In these trials, the association between muscle mass and CRF was not studied. Current data on the association between muscle mass and CRF are scarce and conflicting.19, 20
In this study, we aim to accurately define to what extent muscle mass is related to CRF in patients with advanced cancer. We performed a cross‐sectional study of the association between muscle mass and CRF in patients with advanced cancer. A direct relation between muscle mass and CRF would further support the hypothesis that improving muscle mass is an important target for reducing CRF.
Methods
A cross‐sectional study was performed in patients with advanced cancer scheduled for treatment with chemotherapy between June 2011 and July 2014. This study was embedded in a large observational study in patients with advanced cancer (Netherlands trial register NTR3094), which resulted in two publications.21, 22 Patients with colorectal, breast, prostate, or lung cancer, normal blood counts, and no chemistry abnormalities who were scheduled to start a new line of chemotherapy were included in this trial. Exclusion criteria were peripheral edema or ascites, chemotherapy in the previous month, pregnancy, and insufficient comprehension of the Dutch language to complete questionnaires. After providing informed consent, patient characteristics and data on comorbidity and prior treatments were collected from the patients' medical files. All data were anonymized and stored in the web‐based database system Open Clinica (version 3.3). This observational study was approved by the ethics committee of the VU University medical center.
Assessments
Fatigue was assessed with the Functional Assessment of Chronic Illness Therapy (FACIT)‐fatigue.23 The FACIT‐fatigue is a validated questionnaire focusing on physical fatigue and the functional consequences of fatigue. It has an excellent internal consistency (Cronbach's alpha 0.93–0.95) and good test–retest reliability (r 0.72–0.90).23, 24, 25 The 13 items are scored on a 4‐point Likert scale, resulting in a total score between 0 and 52 points, with lower scores indicating higher levels of CRF. A score <34 on the FACIT‐fatigue was previously defined as a cut‐off for fatigue.26, 27 This cut‐off is concordant with the ICD‐10 criteria for CRF, which state that significant fatigue, diminished energy, and an increased need to rest (disproportionate to any recent change in activity level) should be present during at least two consecutive weeks in the past month, or that one or two of these symptoms are present combined with five or more out of 10 other symptoms that directly influence daily activities.27
Skeletal muscle area (cm2) was measured with SliceOmatic Software V 5.0 (Tomovision, Magog, Canada) using routine computed tomography (CT) scans conducted for diagnostic purposes before start of chemotherapy treatment. The third lumbar vertebra (L3) was used as a standard landmark.28, 29 The first image extending from L3 to the iliac crest was chosen to measure total muscle cross‐sectional area. The L3 region contains psoas, para‐spinal muscles (erector spinae, quadratus lumborum), and the abdominal wall muscles (transversus abdominus, external and internal obliques, rectus abdominus). These muscles were identified based on their anatomic features by a trained researcher. The structures of those specific muscles were quantified based on pre‐established thresholds of Hounsfield Units (HU) (−29 to +150) of skeletal muscle tissue.30 Cross‐sectional areas (cm2) of the sum of all these muscles were computed by summing tissue pixels and multiplying by the pixel surface area. In patients with lung cancer, no routine CT scan of the abdomen including L3 is being made. Therefore, there were no CT images available on level L3 for the lung cancer patients; T4 was used as an alternative for the assessment of the skeletal muscle area. This region contains pectoral muscles, external intercostal, serratus anterior, teres major, subscapularis, infraspinatus, rhomboid major, erector spinae, and trapezius muscles. Measurements at the T4 level are currently being validated; therefore, these results were analysed separately and should be interpreted with caution.
Skeletal muscle index (SMI) was calculated as the ratio of skeletal muscle area (cm2)/height (m)2. Body height was measured using a stadiometer; the patient was standing barefoot, and height was determined to the nearest cm. Low SMI on level L3 was defined as <41cm2/m2 for women, <43 cm2/m2 for men with a body mass index (BMI) <25 kg/m2, and <53 cm2/m2 for men with BMI ≥25 kg/m2. 31 For level T4 no reference values are available.
Statistical analysis
Descriptive results are presented by their mean and standard deviation, or median and interquartile range (IQR) whenever appropriate. Univariate and multiple linear regression were used study the association between CRF and SMI, adjusted for age; gender; tumour type; chemotherapy treatment line (first vs. second and higher); Charlson Comorbidity Index; and chemotherapy, hormone therapy, targeted therapy, or high dose corticosteroid treatment (equivalent to prednisone >10 mg/day over 3 weeks) in the past 6 months. These factors were chosen as they may influence CRF and/or SMI.32, 33 To study whether the association between SMI and fatigue was modified by age, gender, and hormone therapy, we added the interaction terms of SMI with these variables into the regression equation. All statistical tests were two sided, and P < 0.05 was considered statistically significant. Analyses were performed with IBM SPSS version 22.0.0.0 (IBM, Armonk, NY, USA).
Results
In total, 302 patients were included, of whom 233 patients had both completed the FACIT‐fatigue questionnaire and had a CT scan that met the required conditions for assessment of the skeletal muscle area (Figure 1). The L3 group (n = 151) comprised patients with colorectal, prostate, and breast cancer. The T4 group (n = 82) comprised patients with lung cancer. Patients were included with a CT scan within 90 days of their assessment; however, approximately 80% of the patients had a CT scan made within the last 30 days as was considered appropriate for their cancer treatment by their oncologist. The median number of days between the CT scan and FACIT‐fatigue scoring was 15 days (IQR 7–28). Patient characteristics are shown in Table 1.
Figure 1.
Study flow chart.
Table 1.
Patient characteristics
|
All patients n = 233 n (%) |
L3 group n = 151 n (%) |
T4 group n = 82 n (%) |
||
|---|---|---|---|---|
| Age | Mean (SD) | 63.6 (9.9) | 64.5 (9.8) | 61.9 (9.9) |
| Gender |
Male Female |
129 (55) 104 (45) |
89 (59) 62 (41) |
40 (49) 42 (51) |
| Cancer type |
Lung Colorectal Prostate Breast |
82 (35) 75 (32) 44 (19) 32 (14) |
— 75 (50) 44 (29) 32 (21) |
82 (100) — — — |
| Brain metastasis | 18 (8) | 3 (2) | 15 (18) | |
| Chemotherapy in past 6 months | 35 (15) | 26 (17) | 9 (11) | |
| Radiotherapy in past 6 months | 9 (4) | 3 (2) | 6 (7) | |
| Hormone therapy in past 6 months | 31 (13) | 31 (21) | 0 (0) | |
| Targeted therapy in past 6 months | 18 (8) | 14 (9) | 4 (5) | |
| Corticosteroid use in past 6 months1 | 24 (10) | 16 (11) | 8 (10) | |
| ECOG performance status |
0 1 2 Unknown |
57 (25) 94 (40) 17 (7) 65 (28) |
46 (31) 83 (55) 14 (9) 8 (5) |
11 (13) 11 (13) 3 (4) 57 (70) |
| Charlson Comorbidity Index2 |
0 1–2 3–4 ≥5 |
165 (71) 62 (27) 4 (2) 2 (1) |
112 (74) 35 (23) 3 (2) 1 (1) |
53 (65) 27 (33) 1 (1) 1 (1) |
| Chemotherapy line |
First Second Third >Third |
183 (79) 32 (14) 10 (4) 8 (3) |
114 (76) 24 (16) 7 (5) 6 (4) |
69 (84) 8 (10) 3 (4) 2 (2) |
| C‐reactive proteine (mg/L) |
Median (IQR) |
12 (4–33) | 9 (4–28) | 13 (4–35) |
| Albumin (g/L) | Mean (SD) | 35 (4) | 36 (4) | 35 (5) |
| Haemoglobin (mmol/L) | Mean (SD) | 8.0 (1.0) | 7.9 (1.0) | 8.2 (1.0) |
| Creatinin (μmol/L) |
Median (IQR) |
68 (57–83) | 68 (58–83) | 70 (59–84) |
| BMI (kg/m2) | Mean (SD) | 25.6 (4.0) | 26.2 (3.8) | 24.6 (4.1) |
| BMI males (kg/m2) | Mean (SD) | 26.0 (3.7) | 26.6 (3.4) | 24.7 (4.1) |
| BMI females (kg/m2) | Mean (SD) | 25.1 (4.3) | 25.6 (4.3) | 24.4 (4.2) |
Characteristics of the patients included in the study. BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range (25th and 75th percentile); SD, standard deviation of the mean.
1. >10 mg prednisolon/day for at least 3 weeks.
2. Charlson Comorbidity Index, not adjusted for age, primary cancer diagnosis was not counted as comorbidity.
The median score on the FACIT‐fatigue questionnaire was 36 (IQR 26–44); 96 patients (41%) were diagnosed with CRF (FACIT‐fatigue score <34, Table 2). The median SMI was 43 cm2/m2 in the L3 group and 59 cm2/m2 in the T4 group (Table 2). In the L3 group, 58% of the men and 61% of the women had a low SMI.
Table 2.
SMI and FACIT‐fatigue scores
| Skeletal muscle area (cm2) median (IQR) | Skeletal muscle index (cm2/m2) median (IQR) | FACIT‐fatigue score median (IQR) | FACIT‐fatigue score < 34 n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study sample | T4 | L3 | T4 | L3 | Overall | T4 | L3 | Overall | T4 | L3 |
| Total group | 177 (149–210) | 133 (109–153) | 59 (53–69) | 43 (38–50) | 36 (26–44) | 35 (23–43) | 38 (29–45) | 96 (41) | 37 (45) | 59 (39) |
| T4 n = 82 | ||||||||||
| L3 n = 151 | ||||||||||
| Men | 208 (189–236) | 148 (133–167) | 67 (59–73) | 46 (42–52) | 38 (26–45) | 33 (21–41) | 40 (31–47) | 49 (38) | 20 (50) | 29 (33) |
| T4 n = 40 | ||||||||||
| L3 n = 89 | ||||||||||
| Women | 153 (132–165) | 108 (101–124) | 55 (51–62) | 39 (36–45) | 36 (25–43) | 37 (25–44) | 34 (27–43) | 47 (45) | 17 (41) | 30 (48) |
| T4 n = 42 | ||||||||||
| L3 n = 62 | ||||||||||
FACIT, Functional Assessment of Chronic Illness Therapy; IQR, interquartile range; SMI, skeletal muscle index.
Skeletal muscle data and FACIT‐fatigue scores of the patients included in the study. Data are presented for the entire group and separately for patients with a CT scan on the level of the third lumbar or fourth thoracic vertebrae, and for men and women separately. For the FACIT‐fatigue questionnaire, a score below 34 points correlates with clinically significant fatigue.
The univariate association between SMI and CRF in the L3 group had a B of 0.230 (95% CI −0.004–0.464, P 0.054) (Figure 2 and Table 3). In the multivariate analysis, this association was modified by gender (B interaction 0.716, 95% CI 0.172–1.261, P 0.010). Age and hormone therapy did not modify the association between SMI and CRF. Subsequent stratified analysis showed a statistically significant positive association between SMI and CRF in men (B 0.345, 95% CI 0.017–0.672, P 0.039) in the L3 group. In women (n = 62), higher SMI seemed to be associated with more CRF, but this was not statistically significant (B −0.401, 95% CI −0.867–0.065, P 0.090) (Table 3). No significant association between SMI and CRF was found in the T4 group (B 0.082, 95% CI −0.167–0.330, P 0.515). The association between SMI and CRF in the T4 group was modified by age (B interaction 0.024, 95% CI 0.005–0.043, P 0.014). Skeletal muscle index and CRF were not associated in any of the stratified analyses [several subgroups were defined: split at median age (61.9), and three groups age < 50, 50–70, and >70].
Figure 2.
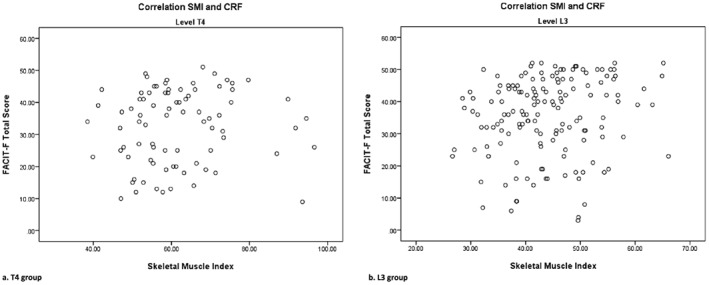
Correlation of skeletal muscle index (SMI) and Functional Assessment of Chronic Illness Therapy (FACIT)‐fatigue.
Table 3.
Univariate and multivariate linear analysis
| Study sample | B | 95% CI lower bound | 95% CI upper bound | Sign. |
|---|---|---|---|---|
| L3—Total group | ||||
| Univariate | 0.230 | −0.004 | 0.464 | 0.054 |
| Multivariate | 0.142 | −0.118 | 0.403 | 0.282 |
| L3—Men | ||||
| Univariate | 0.447 | 0.143 | 0.751 | 0.004 |
| Multivariate | 0.345 | 0.017 | 0.672 | 0.039 |
| L3—Women | ||||
| Univariate | −0.422 | −0.856 | 0.012 | 0.056 |
| Multivariate | −0.401 | −0.867 | 0.065 | 0.090 |
| T4—Total group | ||||
| Univariate | 0.065 | −0.139 | 0.269 | 0.530 |
| Multivariate | 0.082 | −0.167 | 0.330 | 0.515 |
Univariate and multivariate analysis studying the association between skeletal muscle index (SMI) and Functional Assessment of Chronic Illness Therapy (FACIT)‐fatigue scores. Multivariate analysis was performed with the following covariates: sex (L3‐total group and T4 group); age; tumour type (L3 group); chemotherapy*; hormone therapy*; targeted therapy*; corticosteroid treatment (>10 mg prednisolon/day for at least 3 weeks); first vs. ≥second line of chemotherapy; and Charlson Comorbidity Index (not age adjusted).
As prior treatment in the past six months.
Discussion
Cancer‐related fatigue is a highly prevalent symptom that deteriorates quality of life. Both the current concepts of the multifactorial pathophysiology of CRF and the efficacy of training interventions support the hypothesis that muscle wasting is associated with CRF. In the current study, 41% of the patients were diagnosed with CRF, and more than half of the patients in the L3 group had a low SMI.31 We found a significant association between higher muscle mass assessed at level L3 and lower fatigue levels in men, but not in women.
The significant association between muscle mass at level L3 and CRF in men is in line with a previous study by Kilgour et al. 20 who performed a cross‐sectional study among 84 patients with inoperable (stage III–IV) gastro‐intestinal or non‐small cell lung cancer. Kilgour et al. have found a significant univariate negative correlation (B − 4.8, P < 0.01) between fatigue measured with the Brief Fatigue Inventory and muscle mass measured with dual‐energy X‐ray absorptiometry (skeletal muscle mass index). In the multivariate analysis, the association between muscle mass and fatigue was modified by gender. Subgroup analysis showed that fatigue scores had a strong negative correlation with muscle mass in men (r 0.60, P < 0.001) but not in women. In the current study, we were able to confirm these results in a larger group of patients with more diverse tumour types, while using other, also well‐validated instruments to measure CRF and muscle mass. Kilgour et al. suggested that the different results for men and women were possibly related to absolute differences in muscle mass between men and women, or differences in BMI or circulating sex hormones. In the current study, the median muscle mass index for men in the L3 group is on the lower end of the skeletal muscle mass index range in the study by Kilgour et al. when using the equation provided by Mourtzakis et al.,29 but still a relation with fatigue is found in these men. The lower SMI of women is therefore possibly not the only explanation for this result. Body mass index did also not differ between the men and women in the L3 group in the current study. No measurements of sex hormones were performed in the current study. Approximately half of the males in the L3 group were diagnosed with prostate cancer and were pretreated with androgen deprivation therapy, but the diagnosis of prostate cancer was not a predictive factor for fatigue.
Muscle mass and fatigue for women in the L3 group were not only unrelated, but we even found a trend towards higher fatigue levels in women with higher muscle mass. Almost half of these women were diagnosed with breast cancer and frequently pretreated with hormone or chemotherapy, which may have resulted in a selection bias for women with less fatigue, or who have already participated in exercise programs during/after previous treatment lines.
An important strength of this study is the use of reliable, validated instruments to assess CRF23, 24 as well as the objective assessment of muscle mass34 in a relatively large group of patients with advanced cancer. Although the assessment of muscle mass at L3 level is well validated,35 muscle mass assessment at T4 level needs validation. No correlation between muscle mass and fatigue was present in the T4 group. Although this result might be influenced by a limited sample size in the gender specific subgroups or differences in tumour types, it could also be possible that the measurement of muscle mass at level T4 is less reliable.
A possible limitation from our study is the fact that most likely other factors influence fatigue in patients with advanced cancer. In the current study, the effect of pain and distress, which can also influence fatigue scores,1 was not evaluated. Furthermore, central aspects of CRF, such as the effect of serotonin and cortisol levels on the functioning of the suprachiasmatic nucleus, may play a major role in its severity, independent from their effect on muscle mass.4 The positive effect of endurance exercise on CRF may be related to these central effects.6 The study of Lovgren et al. in lung cancer patients also suggests that women experience more problems with emotional functioning than men, which may also result in higher levels of fatigue.36
Second, we may have included a relatively fit group of patients with a good performance status and low comorbidity. Therefore, patients with an extremely low SMI or high fatigue levels may have been excluded on forehand because of a poor performance status. This bias should be taken into account when generalizing these results to other patient groups. From the current data, it could not be extrapolated whether this may have also been a factor contributing to the absence of a correlation between SMI and CRF in women.
Third, because this is a cross‐sectional study, causality cannot be assumed. Future studies need to confirm that an increase in muscle mass actually leads to a reduced fatigue level in men with advanced cancer. This may be of major clinical importance as fatigue is one of the most common symptoms in patient with advanced cancer, for whom quality of life is generally (one of) the most important factor(s) in treatment decisions. From our perspective, patients with progressively advanced cancer do often have benefit from effective palliative chemotherapy in the sense of improving their condition by reducing their tumour burden. Combining cancer treatment with exercise and/or dietary interventions that increase muscle mass may help to further improve treatment tolerance and results.
Conclusions
We found a significant association between muscle mass and CRF in men with advanced cancer, but not in women. These findings may lead to the suggestion that exercising may potentially be beneficial to reduce or prevent fatigue in men by increasing muscle mass. The absence of a relation between SMI and CRF in women and the limited strength of the association in men highlight that CRF is a multifactorial problem. To further reduce CRF in both men and women with cancer, multifactorial assessments need to be performed in order to develop effective treatment strategies.
Conflict of interest
All authors declare that they have no conflict of interest.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.37
Neefjes, E. C. W. , van den Hurk, R. M. , Blauwhoff‐Buskermolen, S. , van der Vorst, M. J. D. L. , Becker‐Commissaris, A. , de van der Schueren, M. A. E. , Buffart, L. M. , and Verheul, H. M. W. (2017) Muscle mass as a target to reduce fatigue in patients with advanced cancer. Journal of Cachexia, Sarcopenia and Muscle, 8: 623–629. doi: 10.1002/jcsm.12199.
References
- 1. Berger A. Cancer‐related fatigue NCCN guidelines. 2015. 22‐1‐2015.
- 2. Hofman M, Ryan JL, Figueroa‐Moseley CD, Jean‐Pierre P, Morrow GR. Cancer‐related fatigue: the scale of the problem. Oncologist 2007;12:4–10. [DOI] [PubMed] [Google Scholar]
- 3. Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer 2002;38:27–43. [DOI] [PubMed] [Google Scholar]
- 4. Neefjes EC, van der Vorst MJ, Blauwhoff‐Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer‐related fatigue. Oncologist 2013;18:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bower JE. Cancer‐related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer‐related fatigue. Oncologist 2007;12:22–34. [DOI] [PubMed] [Google Scholar]
- 7. David MP, Walsh D. Mechanisms of fatigue. J Support Oncol 2010;8:164–174. [PubMed] [Google Scholar]
- 8. Shafqat A, Einhorn LH, Hanna N, Sledge GW, Hanna A, Juliar BE, et al. Screening studies for fatigue and laboratory correlates in cancer patients undergoing treatment. Ann Oncol 2005;16:1545–1550. [DOI] [PubMed] [Google Scholar]
- 9. al‐Majid S, Do MC. Cancer‐induced fatigue and skeletal muscle wasting: the role of exercise. Biol Res Nurs 2001;2:186–197. [DOI] [PubMed] [Google Scholar]
- 10. Cantarero‐Villanueva I, Fernandez‐Lao C, Cuesta‐Vargas AI, Del Moral‐Avila R, Fernandez‐de‐Las‐Penas C, Arroyo‐Morales M. The effectiveness of a deep water aquatic exercise program in cancer‐related fatigue in breast cancer survivors: a randomized controlled trial. Arch Phys Med Rehabil 2013;94:221–230. [DOI] [PubMed] [Google Scholar]
- 11. Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta‐analysis. Med Sci Sports Exerc 2013;45:2080–2090. [DOI] [PubMed] [Google Scholar]
- 12. Kampshoff CS, Chinapaw MJ, Brug J, Twisk JW, Schep G, Nijziel MR, et al. Randomized controlled trial of the effects of high intensity and low‐to‐moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Med 2015;13:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 2015;33:1918–1927. [DOI] [PubMed] [Google Scholar]
- 14. Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CR, Morrow GR. A 4‐week home‐based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol 2009;7:158–167. [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen PA, Dechet CB, Porucznik CA, LaStayo PC. Comparing eccentric resistance exercise in prostate cancer survivors on and off hormone therapy: a pilot study. PMR 2009;1:1019–1024. [DOI] [PubMed] [Google Scholar]
- 16. Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010;28:340–347. [DOI] [PubMed] [Google Scholar]
- 17. Hanson ED, Sheaff AK, Sood S, Ma L, Francis JD, Goldberg AP, et al. Strength training induces muscle hypertrophy and functional gains in black prostate cancer patients despite androgen deprivation therapy. J Gerontol A Biol Sci Med Sci 2013;68:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis 2013;16:328–335. [DOI] [PubMed] [Google Scholar]
- 19. Jones LW, Friedman AH, West MJ, Mabe SK, Fraser J, Kraus WE, et al. Quantitative assessment of cardiorespiratory fitness, skeletal muscle function, and body composition in adults with primary malignant glioma. Cancer 2010;116:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilgour RD, Vigano A, Trutschnigg B, Hornby L, Lucar E, Bacon SL, et al. Cancer‐related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle 2010;1:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blauwhoff‐Buskermolen S, de van der Schueren MA, Verheul HM, Langius JA. ‘Pre‐cachexia’: a non‐existing phenomenon in cancer? Ann Oncol 2014;25:1668–1669. [DOI] [PubMed] [Google Scholar]
- 22. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 23. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997;13:63–74. [DOI] [PubMed] [Google Scholar]
- 24. Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res 2004;56:157–170. [DOI] [PubMed] [Google Scholar]
- 25. Barsevick AM, Cleeland CS, Manning DC, O'Mara AM, Reeve BB, Scott JA, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage 2010;39:1086–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manir KS, Bhadra K, Kumar G, Manna A, Patra NB, Sarkar SK. Fatigue in breast cancer patients on adjuvant treatment: course and prevalence. Indian J Palliat Care 2012;18:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Belle S, Paridaens R, Evers G, Kerger J, Bron D, Foubert J, et al. Comparison of proposed diagnostic criteria with FACT‐F and VAS for cancer‐related fatigue: proposal for use as a screening tool. Support Care Cancer 2005;13:246–254. [DOI] [PubMed] [Google Scholar]
- 28. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol(1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 29. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 30. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 31. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McGargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 32. Jones LW, Friedman AH, West MJ, Mabe SK, Fraser J, Kraus WE, et al. Quantitative assessment of cardiorespiratory fitness, skeletal muscle function, and body composition in adults with primary malignant glioma. Cancer 2010;116:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 34. Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 2009;3:269–275. [DOI] [PubMed] [Google Scholar]
- 35. MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross‐sectional body composition analysis. Curr Opin Support Palliat Care 2011;5:342–349. [DOI] [PubMed] [Google Scholar]
- 36. Lövgren M, Tishelman C, Sprangers M, Koyi H, Hamberg K. Symptoms and problems with functioning among women and men with inoperable lung cancer—a longitudinal study. Lung Cancer 2008;60:113–124. [DOI] [PubMed] [Google Scholar]
- 37. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


