Abstract
Background
The aim of this study is to compare the perioperative and oncologic outcomes of open and laparoscopic approaches for concomitant resection of synchronous colorectal cancer and liver metastases.
Methods
Between 2006 and 2015, all patients undergoing combined resection of primary colorectal cancer and liver metastases were included in the study (n=43). Laparoscopic and open groups were compared regarding clinical, perioperative and oncologic outcomes.
Results
There were 29 patients in the open group and 14 patients in the laparoscopic group. The groups were similar regarding demographics, comorbidities, histopathological characteristics of the primary tumor and liver metastases. Postoperative complication rate (44.8% vs. 7.1%, P=0.016) was higher, and hospital stay (10 vs. 6.4 days, P=0.001) longer in the open compared to the laparoscopic group. Overall survival (OS) was comparable between the groups (P=0.10); whereas, disease-free survival (DFS) was longer in laparoscopic group (P=0.02).
Conclusions
According to the results, in patients, whose primary colorectal cancer and metastatic liver disease was amenable to a minimally invasive resection, a concomitant laparoscopic approach resulted in less morbidity without compromising oncologic outcomes. This suggests that a laparoscopic approach may be considered in appropriate patients by surgeons with experience in both advanced laparoscopic liver and colorectal techniques.
Keywords: Synchronous liver metastases, concomitant resection, laparoscopic
Introduction
Ten to twenty-five percent of colorectal cancer patients present with liver metastases (1-3). Surgical resection provides the patient with the best chance of cure. Nevertheless, in patients presenting with resectable disease in a synchronous fashion, there is debate regarding the timing of both procedures. While a staged colorectal and hepatic resection has been preferred in general to reduce the risk of postoperative morbidity and mortality (4-6), several recent studies have reported that simultaneous resection of colorectal primary and liver metastases is safe and feasible (1-3,7).
Laparoscopic resection of primary colorectal cancer has become established. Minimally invasive techniques have also been developed for liver resection over the last decade (8-10). However, data regarding outcomes on laparoscopic simultaneous colorectal cancer resection with liver metastases are limited. In the present study, we aimed to investigate the perioperative and oncological outcomes of laparoscopic resection of primary colorectal tumor and liver metastases, with a comparison to the open approach.
Methods
Patients and methods
This was a retrospective analysis of prospectively collected data. Between February 2006 and October 2015, 43 patients who underwent concomitant resection of the primary colorectal cancer and metastatic liver tumor at the Departments of Colorectal and General Surgery, Cleveland Clinic, were identified through an IRB-approved institutional database. The approach was open in 29 and laparoscopic in 14 patients. The choice of a laparoscopic versus open approach was dependent on surgeon preference and extent of the primary and liver tumors. These two groups were compared in terms of clinical, perioperative and oncologic outcomes. Patient demographics, primary and metastatic tumor characteristics (number, size, location), intraoperative data (type of colectomy and hepatectomy, operative time, blood loss, transfusions), and postoperative hospital course (30-day morbidity, mortality, and length of hospital stay) were analyzed. Both disease-free and overall-survival were calculated.
All patients underwent CT scans of the chest, abdomen and pelvis preoperatively for staging. Pelvic MRI was also performed in those with rectal cancer.
Our techniques for resecting the primary colorectal cancer and liver metastases have been reported extensively elsewhere (8,11). Intraoperative ultrasonography of the liver was performed in each case. Minor liver resection was defined as a resection of one or two segments, whereas resection of three or more segments was classified as a major hepatectomy.
Patients were followed with blood chemistry, serum Carcino embryonic antigen (CEA), and CT scans of the abdomen-pelvis quarterly for the first 2 years and then biannually. Chest CT was obtained at least once a year.
Statistical analysis
Categorical variables were reported as frequency (%) and quantitative variables were reported as mean ± standard error mean. Categorical variables were compared using chi-square test or Fisher’s exact test, while quantitative and ordinal variables were compared using the t-test/Wilcoxon rank sum test. Overall and disease-free survival (DFS) rates were calculated with the Kaplan-Meier method. A P<0.05 was considered statistically significant.
Results
Table 1 shows the clinical characteristics of the patients in each group. The groups were similar in regards to age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score, preoperative CEA, cardiopulmonary comorbidities, primary tumor size and location, size and number of liver tumors, and distribution of metastases throughout the liver (unilobar or bilobar). There was no difference between the groups regarding the receipt of neoadjuvant chemotherapy directed to the liver (P=0.16). The types of colorectal procedures were similar between the two groups (P=0.81) (Table 2). In the same fashion, the type of liver resections performed (hemihepatectomy, bi-segmentectomy and wedge/segmentectomy) was similar between the groups (P=0.83). There were no conversions to open in the laparoscopic group. In the open group, a Pringle maneuver was used in 12 patients (41.4%) versus none of the patients in the laparoscopic group (P=0.004).
Table 1. Demographic and clinical characteristics of patients in each group (continuous data are given as mean ± standard error of the mean).
| Parameter | Open (n=29) | Laparoscopic (n=14) | P-value |
|---|---|---|---|
| Age¥ (years) | 57.7±2.5 | 56.3±3.3 | 0.44 |
| Gender (F/M) | 13/16 | 8/6 | 0.45 |
| BMI¥ (kg/m2) | 27.5±1.2 | 25.1±0.8 | 0.44 |
| ASA score (I/II/III/IV) | 0/6/21/2 | 0/0/12/2 | 0.15 |
| Preop CEA (mcg/L) | 14.3±4.9 | 36.9±30.4 | 0.28 |
| Cardiopulmonary comorbidity | 16 (55.2) | 10 (71.4) | 0.31 |
| Colon | 14 (48.3) | 6 (42.9) | 0.74 |
| Rectum | 15 (51.7) | 8 (57.1) | |
| Primary tumor size¥ (cm) | 3.7±0.5 | 3.7±0.7 | 0.93 |
| Liver tumor size (cm) | 2.7±0.2 | 2.4±0.7 | 0.06 |
| Number of liver tumors¥ | 2.1±0.2 | 1.6±0.3 | 0.25 |
| Unilobar metastases | 19 (65.5) | 12 (85.7) | 0.28 |
| Bilobar metastases | 10 (34.5) | 2 (14.3) |
Values are expressed as absolute numbers (percentages) unless indicated otherwise. ¥, Standard deviation; BMI, body mass index; ASA, American society of anesthesiologists score; CEA, carcino embryonic antigen.
Table 2. Operative and perioperative details of the study patients (continuous data are given as mean ± standard error of the mean).
| Parameter | Open (N=29) | Laparoscopic (N=14) | P-value |
|---|---|---|---|
| Colorectal resection | 0.81 | ||
| LAR | 17 (58.6) | 8 (57.1) | |
| HC | 12 (41.4) | 6 (42.9) | |
| Liver resections | 0.83 | ||
| Minor/major | 25/4 | 12/2 | |
| Hemi hepatectomy (R/L) | 4 | 2 | |
| Bi-segmentectomy | 5 | 1 | |
| Wedge/segmentectomy | 20 | 11 | |
| Operative time (minutes) | 341±27 | 321±35 | 0.85 |
| EBL (mL) | 578±116 | 347±37 | 0.70 |
| PRBC transfusion | 10 (34.5) | 1 (7.1) | 0.07 |
| Postoperative complication | 13 (44.8) | 1 (7.1) | 0.016 |
| Pathological examination | |||
| T (1/2/3/4) | 4/3/19/3 | 2/0/11/1 | 0.85 |
| N (0/1/2) | 12/13/4 | 4/7/3 | 0.69 |
| Liver resection margin | |||
| R0 | 27(93.1) | 12 (85.7) | 0.59 |
| R1 | 2 (6.9) | 2 (14.3) | |
| Hospital stay¥ (days) | 10±0.9 | 6.4±0.8 | 0.001 |
| Neoadjuvant chemotherapy | 19 (65.5) | 6 (42.9) | 0.16 |
| Adjuvant chemotherapy | 20 (80.0) | 11 (84.6) | 0.99 |
Values are expressed as absolute numbers (percentages) unless indicated otherwise. ¥, Standard Deviation; LAR, low anterior resection; HC, hemicolectomy; EBL, estimated blood loss; PRBC, packed red blood cells.
There were no differences between the two groups regarding operative time, estimated blood loss, blood transfusion, pathologic characteristics of primary tumor, and surgical margin status for either the primary or metastatic liver tumor resection. Postoperative complication rate (44.8% vs. 7.1%, P=0.016) was higher, and hospital stay (10 vs. 6.4 days, P=0.001) longer in the open compared to the laparoscopic group. The complications in the open group were ileus (n=5), pulmonary (n=3), organ space surgical site infection (SSI) (n=3), cardiac (n=2), fascial dehiscence (n=2), superficial SSI (n=1), liver abscess (n=1), rectovaginal fistula (n=1), in contrast to an anastomotic leak in one patient in the laparoscopic group. One patient in the open group died on postoperative day 7 due from pulmonary emboli. There was no mortality in the laparoscopic group. Time to regular diet (4.0±0.5 vs. 6.4±0.5 days) and flatus (2.9±0.3 vs. 4.5±0.3 days) was shorter in the laparoscopic compared to the open group (P=0.001 and P=0.003, respectively) (Figures 1,2).
Figure 1.

Time to regular diet in the study groups (P=0.001).
Figure 2.
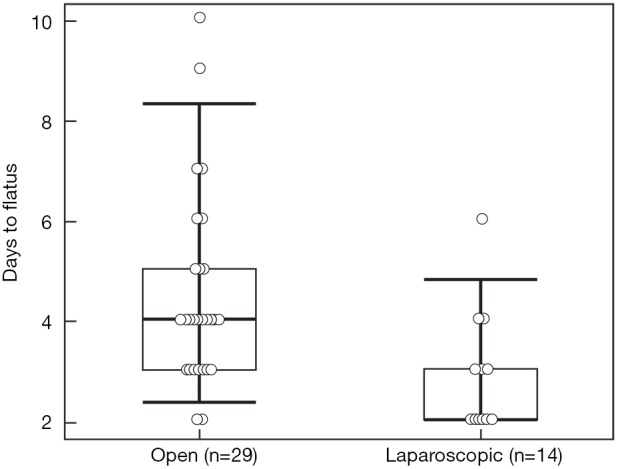
Time to flatus in the open and laparoscopic groups (P=0.003).
The median follow up in the open group was 24.2±3.7 and in the laparoscopic group 20.3±6.6 months (P=0.34). Overall survival (OS) was comparable (P=0.10), but DFS longer in the laparoscopic group (P=0.028) (Figures 3,4). Two groups were comparable in terms of recurrence rates [41.3% (n=12) vs. 14.2% (n=2), P=0.08]. Out of 12 recurrences in the open group, 11 of them were new liver disease and one patient had local liver recurrence. Seven patients had extra hepatic recurrence. Both of the two recurrences in the laparoscopic group were new liver disease.
Figure 3.
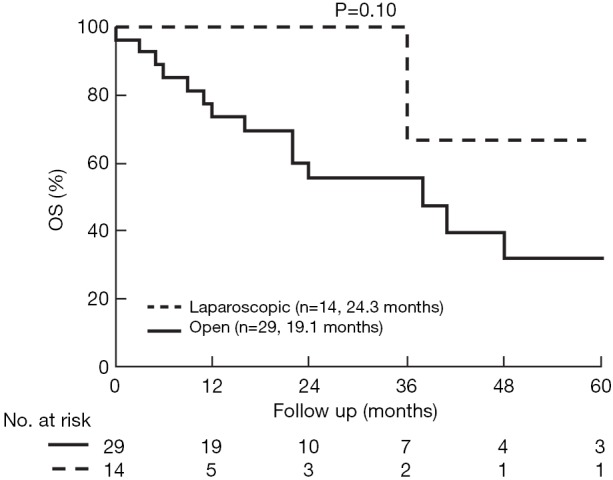
Kaplan-Meier OS in the study groups. Median OS was 24.3 and 19.1 months, respectively (P=0.10). OS, overall survival.
Figure 4.
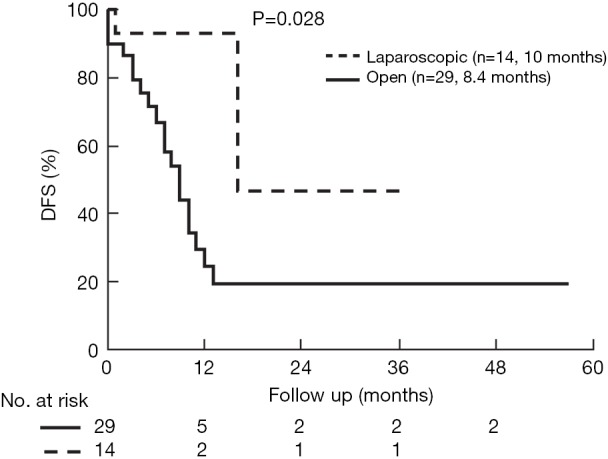
Kaplan Meier DFS in the open and laparoscopic groups. Median DFS was 10 and 8.4 months, respectively (P=0.028). DFS, disease free survival.
Discussion
This study focused on a comparison of open versus laparoscopic one-stage management of synchronous liver metastasis and primary colorectal cancer. Our results showed that in patients amenable to a laparoscopic resection of both sites, the perioperative outcomes were more favorable when compared to a series of patients who underwent similar types of primary and liver resections. This report is another contribution to the small volume of existing data on laparoscopic 1-stage management of synchronous liver metastases from colorectal cancer.
The management of patients with synchronous colorectal primary and metastatic liver tumors is challenging. The optimal timing and surgical approach remains controversial (2-6,8,12,13). It is unknown if staged resection is more advantageous to a 1-stage operation in regards to morbidity and mortality. With the advances in patient care, a 1-stage approach seems attractive to reduce costs and the need for two separate admissions. The performance of the 1-stage resection laparoscopically might offer significant advantages to the patient regarding postoperative recovery (14-17). However, there are little data on the feasibility, safety, and oncologic equivalency of this approach.
Our review of the literature identified few studies that have specifically compared laparoscopic and open approach for synchronous resection of primary and liver metastatic colorectal cancer. Iwashashi et al. studied 21 patients who underwent laparoscopic resection and compared to 21 matched patients who underwent open resection. With similar demographics, histopathological and procedural characteristics, blood loss was less in the laparoscopic group. The complications in the laparoscopic group also tended to be less compared to the open group. With a shorter hospital stay in the laparoscopic group, 5-year overall and DFS was similar to the open group (17). Our results are similar to this study, with a lower rate of complications and shorter hospital stay in the laparoscopic group. In the second publication from China (14), 13 patients who underwent 1-stage laparoscopic colorectal and hepatic resection were compared to 13 patients who had open resections. In this report, the operative time and hospital stay were less in the laparoscopic group. Patients undergoing laparoscopic procedures resumed off-bed activities, bowel movement, and oral intake earlier than those undergoing open procedures. The 1-, 3- and 5-year survival rates were comparable between the groups. In our study, the resumption of regular diet and return of bowel function were faster in the laparoscopic group.
The use of neoadjuvant chemotherapy before combined liver and colorectal surgery is evolving. It has been shown that patients with colorectal cancer metastases who undergo perioperative chemotherapy have an improved progression-free survival compared to surgery alone (18), albeit with increased morbidity related to liver toxicity. In our study, the rate of neoadjuvant chemotherapy was similar between two groups. We prefer upfront surgical approach to the primary tumor without neoadjuvant chemotherapy in patients with symptomatic or nearly obstructing primary colorectal cancer. In these patients, the resection of liver metastases concomitantly is controversial. Our approach in these patients involves performing minor liver resections concomitantly, but leaving major hepatic resections to a second stage after chemotherapy. We have established a multidisciplinary tumor board in the recent years with an aim to standardize the management of these patients. Nevertheless, with similar primary and metastatic liver tumor characteristics, there was better DFS in laparoscopic group compared to open group.
There were differences in the surgical technique between open and laparoscopic liver resections in the current study, with the main advantage of the latter approach being the avoidance of inflow occlusion (Pringle maneuver). Pringle maneuver is proposed to cause transient portal hypertension and impair anastomotic healing related to an onset of intestinal edema (19,20). Many laparoscopic hepatectomy series have reported a low utilization of inflow occlusion owing to decrease in liver flow related pneumoperitoneum and the use of advanced energy devices (15). In the current study, Pringle maneuver was performed in 41.4% of patients in the open group with none in the laparoscopic group.
Another point of discussion is the issue of which organ system should be approached first during concomitant resections. At our institution, we prefer to approach the organ system whose resection would require a more extensive procedure first. Additionally, if the primary colorectal tumor is symptomatic or the possibility of a near-future bowel obstruction is anticipated, colorectal tumor is resected first. However if the liver lesions require a major liver resection and the primary site is small (i.e., a non-obstructing right colon tumor), we will perform hepatectomy first. There is a scarce amount of data on this topic in the literature, however a few authors have suggested liver resection first to avoid the detrimental effects of prolonged portal vein occlusion on the colonic anastomosis (21).
In our study overall morbidity was found to be significantly lower in the laparoscopic patients. Our results are consistent with the literature and this is an essential benefit of the minimally invasive approach.
The limitations of our study were the retrospective nature, small sample size and short mid-term follow up. Our results are encouraging and hence we recommend a concomitant laparoscopic approach in appropriate patients with tumors amenable to minimally invasive surgery by surgeons with experience in both procedures. We hope that this study can encourage larger prospective studies to be performed.
Acknowledgements
None.
Ethical Statement: The study was approved by institutional/regional/national ethics committee/ethics board of Cleveland Clinic (No. 14-1493).
Footnotes
Conflicts of Interest: Dr. Eren Berber is a consultant to Ethicon and Aesculap Inc. His consultation activities involve product development for both open and laparoscopic surgical procedures. The other authors have no conflicts of interest to declare.
References
- 1.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 2007;14:3481-91. 10.1245/s10434-007-9522-5 [DOI] [PubMed] [Google Scholar]
- 2.Martin RC, 2nd, Augenstein V, Reuter NP, et al. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg 2009;208:842-50;discussion 850-2. 10.1016/j.jamcollsurg.2009.01.031 [DOI] [PubMed] [Google Scholar]
- 3.Lyass S, Zamir G, Matot I, et al. Combined colon and hepatic resection for synchronous colorectal liver metastases. J Surg Oncol 2001;78:17-21. 10.1002/jso.1117 [DOI] [PubMed] [Google Scholar]
- 4.Thelen A, Jonas S, Benckert C, et al. Simultaneous versus staged liver resection of synchronous liver metastases from colorectal cancer. Int J Colorectal Dis 2007;22:1269-76. 10.1007/s00384-007-0286-y [DOI] [PubMed] [Google Scholar]
- 5.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg 2000;231:743-51. 10.1097/00000658-200005000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura F, Miyazaki M, Suwa T, et al. Reduced hepatic acute-phase response after simultaneous resection for gastrointestinal cancer with synchronous liver metastases. Br J Surg 1996;83:1002-6. 10.1002/bjs.1800830738 [DOI] [PubMed] [Google Scholar]
- 7.Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 2003;197:233-41; discussion 241-2. 10.1016/S1072-7515(03)00390-9 [DOI] [PubMed] [Google Scholar]
- 8.Capussotti L, Ferrero A, Viganò L, et al. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol 2007;14:195-201. 10.1245/s10434-006-9055-3 [DOI] [PubMed] [Google Scholar]
- 9.Abu Hilal M, Underwood T, Zuccaro M, et al. Short- and medium-term results of totally laparoscopic resection for colorectal liver metastases. Br J Surg 2010;97:927-33. 10.1002/bjs.7034 [DOI] [PubMed] [Google Scholar]
- 10.Farges O, Jagot P, Kirstetter P, et al. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg 2002;9:242-8. 10.1007/s005340200026 [DOI] [PubMed] [Google Scholar]
- 11.Ratti F, Catena M, Di Palo S, et al. Laparoscopic Approach for Primary Colorectal Cancer Improves Outcome of Patients Undergoing Combined Open Hepatic Resection for Liver Metastases. World J Surg 2015;39:2573-82. 10.1007/s00268-015-3127-0 [DOI] [PubMed] [Google Scholar]
- 12.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-62. [DOI] [PubMed] [Google Scholar]
- 13.Weber JC, Bachellier P, Oussoultzoglou E, et al. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br J Surg 2003;90:956-62. 10.1002/bjs.4132 [DOI] [PubMed] [Google Scholar]
- 14.Hu MG, Ou-yang CG, Zhao GD, et al. Outcomes of open versus laparoscopic procedure for synchronous radical resection of liver metastatic colorectal cancer: a comparative study. Surg Laparosc Endosc Percutan Tech 2012;22:364-9. 10.1097/SLE.0b013e31825af6b2 [DOI] [PubMed] [Google Scholar]
- 15.Lupinacci RM, Andraus W, De Paiva Haddad LB, et al. Simultaneous laparoscopic resection of primary colorectal cancer and associated liver metastases: a systematic review. Tech Coloproctol 2014;18:129-35. 10.1007/s10151-013-1072-1 [DOI] [PubMed] [Google Scholar]
- 16.Hatwell C, Bretagnol F, Farges O, et al. Laparoscopic resection of colorectal cancer facilitates simultaneous surgery of synchronous liver metastases. Colorectal Dis 2013;15:e21-8. 10.1111/codi.12068 [DOI] [PubMed] [Google Scholar]
- 17.Iwahashi S, Shimada M, Utsunomiya T, et al. Laparoscopic hepatic resection for metastatic liver tumor of colorectal cancer: comparative analysis of short- and long-term results. Surg Endosc 2014;28:80-4. 10.1007/s00464-013-3165-3 [DOI] [PubMed] [Google Scholar]
- 18.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208-15. 10.1016/S1470-2045(13)70447-9 [DOI] [PubMed] [Google Scholar]
- 19.Gonce ME, Brackett DJ, Squires RA, et al. Development of circulatory and metabolic shock following transient portal triad occlusion. J Surg Res 1995;59:534-43. 10.1006/jsre.1995.1203 [DOI] [PubMed] [Google Scholar]
- 20.Figueras J, Llado L, Ruiz D, et al. Complete versus selective portal triad clamping for minor liver resections: a prospective randomized trial. Ann Surg 2005;241:582-90. 10.1097/01.sla.0000157168.26021.b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patriti A, Ceccarelli G, Bartoli A, et al. Laparoscopic and robot-assisted one-stage resection of colorectal cancer with synchronous liver metastases: a pilot study. J Hepatobiliary Pancreat Surg 2009;16:450-7. 10.1007/s00534-009-0073-y [DOI] [PubMed] [Google Scholar]


