Abstract
Background
Depression often manifests during adolescence when the development and networking of social and emotional brain areas is being influenced by hormones. The Wistar Kyoto (WKY) rat has been proposed as an animal model of adolescent depression with various face, construct, and predictive validities for clinical depression having been established.
Purpose
The influence of the estrous cycle on anxiety- and depression-like behaviors in female adolescents may be tested out further using this model.
Methods
Female adolescent WKY rats were tested for anxiety- and depression-like behaviors in the elevated plus maze and forced swim test (FST) during different phases of the estrous cycle with inbred, age-, and phase-matched Wistar rats as controls.
Results
Wistars in proestrus-estrus demonstrated reduced anxiety levels. WKY also demonstrated increased open arm time and entries and closed arm time, but less than Wistars, and as closed arm entries remained unaffected, it did not translate into a lowering of the anxiety levels. Risk taking and risk assessment behaviors were not affected by estrous phases in WKY, though exploratory behavior was reduced in proestrus-estrus. In Wistars, increased risk taking and decreased risk assessment behaviors were observed during proestrus-estrus. Increased immobility in the FST, indicative of learned helplessness was not influenced by phase in the WKY, which was at variance with Wistars that demonstrated phase-specific differences.
Conclusion
Results indicate a masking effect of indicative hormones in this putative model of adolescent depression, with implications for an unravelling of the steroid milieu in predisposed adolescent depression and for taking phase-specific time windows into account for therapeutic interventions.
Keywords: Females, Puberty, Elevated plus maze, Forced swim test
Introduction
The Wistar Kyoto (WKY) rat, which was inbred from the Wistar strain and generated for use as the normotensive control for the spontaneously hypertensive rat [1], has been suggested as an animal model of depression [2], as it demonstrates extreme stress sensitivity and manifests characteristic depression-like behaviors that are not stress-induced. WKYs, therefore, demonstrate hormonal, behavioral, and physiological measures similar to those found in symptom-presenting depressed patients [3,4,5], for instance, hyper-reactivity to stress and dysregulation of the hypothalamo-pituitary-adrenal (HPA) axis. When compared to the Wistar strain, adrenocorticotropic hormone and corticosterone levels are increased [6]. They also exhibit neurochemical abnormalities in several systems (dopaminergic and noradrenergic), with reduced levels of monoamines, as indicated in depression, as well as in peripheral hormones such as the thyroid stimulating hormone [7,8].
Thus, the WKY is said to be a good model for endogenous depressive behavior [8,9]. Their impaired adaptive capability makes them more susceptible to depressive behaviors. WKY rats show increased immobility in the forced swim test (FST), which is reflective of their helplessness, and are also prone to develop stress-induced anxiety-like characteristics [10]. This strain of rat was selected for our study as there are only few studies on the depressive aspects of this strain. More studies exist of it being used as a control for the spontaneously hypertensive rat. But they also harbor heterogeneity not found in other inbred strains, including greater behavioral and genetic variability [8].
Psychiatric disorders, such as depression manifestation during adolescence, have a genetic basis, though the wide-ranging changes occurring at the neuronal level, including remodelling of circuitry and influence of hormones, render the adolescent developing brain highly vulnerable to experiential inputs [11]. These changes that occur during puberty include increased levels of steroidal and other hormones, [12] increasing connectivity of social- and emotion-related areas, particularly the hippocampus, amygdala, prefrontal cortex, etc., that modulate behavior [13]. Female predisposition to depression arises from such organizational effects of sex hormones during brain development with enhanced depression-like behavior being shown in females [11].
As most animal studies on depression and anxiety use males [14], due to the variability of female behavioral and brain data induced by inherent hormonal fluxes [15], limited insights have been gained into stress-reactivity, depression, and anxiety profiles in females [16]. Furthermore, most female studies have used adults [3]. Very few studies use adolescent females [17]; most adolescent studies involving pups at 30–40 days of age (prepubertal) include both sexes [7,18] with no differences being shown between adolescents and adults [19]. However, sexually dimorphic gene expression differences exist prior to puberty with sex hormones acting as transcriptional regulators causing abundant gene expression differences between sexes [11].
Preclinical models are required for use in translational research, particularly in human affective disorders, such as anxiety and depression, where females are proven to be more vulnerable [11]. The effects of a dysfunctional HPA axis and precipitation by stressors are more profound in females [6]. A series of studies have demonstrated that fundamental differences exist in the behavioral and neural responses by males and females to environmental stressors [3,16]. Differences between males and females are more profound as a result of stress, with females demonstrating a greater corticosteroid response to acute stress than males, and increased susceptibility to learned helplessness following stress [1]. Such sex differences in factors that may trigger depression indicate that these should be considered systematically in developing an appropriate animal model.
In order to establish biologically based diagnostic criteria for anxiety and depression, animal models are indispensable as they provide a way to study functioning brain tissue. Although animal models of psychiatric disorders have limited ability to model human psychiatric symptoms, the potential to demonstrate the neural consequences of genetics, developmental, and potential environmental variables is great [20]. A common psychopathological pathway underlies the high comorbidity between depression and anxiety disorders [21], though expression of some neuropeptide biomarkers could enable a differentiation between anxiety and depression [22]. Moreover, in our earlier study, no correlations were obtained between anxiety- and depression-related behaviours in WKY [17], hinting at a probable separation of anxiety- and depression-related symptoms [23]. Core symptoms such as anhedonia and learned helplessness are not specific even in humans [24].
Though the classical clinical features of comorbid human depression and anxiety disorders are of the subjective order, a series of objective readouts (DSM-V) [23] have been developed in animal models of depression such as learned helplessness, FST, chronic restraint stress, anhedonia, etc. [16,24,25]. The FST is the primary behavioral paradigm that measures the helplessness in rodents and has been extensively used for the characterization of depression-like behavior in animal models as well as reversal of depression-like behavior on treatment with antidepressants [26], while the elevated plus maze (EPM) measures anxiety. The validity of using these behavioral paradigms to probe molecular changes associated with the development of depression (endogenous or environmentally induced) is substantiated by successful reduction of depression-like behaviors/anxiety levels by antidepressant treatments in both paradigms [27].
Sex differences in affective disorders and hormonal modulation of affective disorders provide an important window into the pathophysiology of anxiety and depression, the basis for the current study on estrous phase-specific changes in depression- and anxiety-like behaviors during adolescence. In the luteal phase of the estrous cycle, HPA responsiveness increases, and glucocorticoid feedback sensitivity and brain γ-aminobutyric acid content decreases indicating destabilization of homeostatic systems in predisposed individuals. Many studies have pointed to reduced vulnerability to the effects of stressors in females as compared to males. However, sex-related mood disorders, anxiety- and depression-like profiles predominate in females than in males [3]. Often gender-specific differences do not arise as estrus cycle is not taken into consideration, but it needs to be considered as a contributing factor in gender-specific differences in stress responses.
Estrous is a 4-day cycle which includes a diestrus 1 or metestrus phase during which mating occurs when the animal is said to be in heat or sexually active. Metestrus predominantly has activity of progesterone, while estrogen levels go down. Diestrus 2 proper is the corpus luteal phase. While metestrus lasts about 21 h, diestrus lasts for 57 h. During proestrus, under the influence of estrogen, ovarian follicles develop toward the end of proestrus. On the day of proestrus, estrogen peak is observed at around 11 a.m. Proestrus and estrus last 12 h each. A luteinizing hormone (LH) surge also occurs during estrus. By midnight, there is surge of progesterone, LH, and follicle stimulating hormone followed within 3–4 h by ovulation. In the absence of mating, the luteal phase progresses under the influence of progesterone [28,29,30]. Here, we screen for anxiety-like behaviors in the EPM and depression-like profile in the FST in 2 phases of estrus, viz., diestrus 1, 2, and proestrus-estrus in the WKY rat.
Methods
Breeding
Adult male and female Wistar Kyoto (WKY) and Wistar (WIS) rats were procured from the National Centre for Laboratory Animal Sciences, National Institute of Nutrition, Hyderabad, India. Wistars and WKYs were housed separately in groups on paddy husk bedding in polypropylene cages (43 × 27 × 15 cm) under standard laboratory conditions with food and water provided ad libitum. Six pairs of each strain (Wistar and WKY) were used for breeding. In order to synchronize the phases of the estrous cycle, females were housed adjacent to males for 2–3 days. Monogamous mating system was followed and after approximately 3 weeks, males were separated from the females. Within the next 3–4 days, litters ranging from 10 to 13 pups were delivered.
Sexing of Pups
On postnatal day 21, the pups were weaned from the dam and separated based on their sex. Sexing was carried out by manual measurement of the distance between anus and genital openings, with females being identified by the shorter distance. Each litter had an approximate male to female ratio of 6:4. One female pup from each litter was taken in order to avoid litter effects.
Experimental Subjects
Pups were maintained in groups of 6 per cage till they attained the age of postnatal day (P) 58 (∼P58 or P58 ± 1). The housing room was maintained on a 12-h light/dark cycle and ambient temperature of 25–27°C. The animals were handled and habituated to the recording laboratory environment consecutively for 3 days before the experiments began. All the experiments were conducted in the light cycle (8:00–13:00 h), and in accordance with the ethical guidelines for animal experimentation given by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India, and cleared by the Institutional Animal Ethics Committee.
Identification of Phases of Estrous Cycle
Vaginal lavage method was followed to determine the estrous phases in the rats from postnatal day 42 (P42) onward. Samples for the morphological studies were collected every day between 8.00 and 10.00 a.m. and 15 min before each behavioral recording (see below). Vaginal secretions were collected with micropipette containing 200 μL of 0.9% NaCl by inserting the tip into the vagina. One drop of vaginal fluid was placed onto a clean glass slide and smeared. Unstained smears were observed with a Leica DM2500 microscope and photomicrographs of the estrous cycle stages taken with a Leica Peltier cooled Digital Camera (DFC425). Alternatively, smears were also stained with hematoxylin-eosin to enable differentiation of the stages.
Behavioural Recordings and Analysis
The experiments were carried out during late adolescence (approximately postnatal day 60/∼P60) in both female Wistar and WKY rats. Animals were tested on selected behavioral paradigms for anxiety and depression, that is, EPM on P58 ± 1, habituated to the FST on P59 ± 1 and tested on P60 ± 1. Unconditioned/spontaneous behaviors in the EPM and FST were recorded using the Panasonic WV CP500 CCD video camera fed to a Piccolo frame grabber card and analyzed using Ethovision XT version 8.0® (Noldus, Netherlands). All recordings were done for 5 min under 7.8–8.0 L × light intensity.
Elevated Plus Maze
The EPM apparatus, made of acrylic (black) consisted of 2 open arms (50 × 10 cm) and 2 closed arms with no roof (50 × 10 × 40 cm) held at right angles to each other with an open square (10 × 10 cm) in the center. The maze was elevated 50 cm above the floor. The animals were placed in the center, facing the same open arm each time. The maze was cleaned thoroughly between each trial. EPM parameters, such as time and entries into the open arm and closed arm, distance travelled and latency, were automatically scored in real time by the system during data acquisition. Rearing and dipping frequencies were scored manually from the video recordings. Anxiety index, which was calculated as 1 − (open arm entry/total entry + open arm duration/total duration/2) [31], considers open arm entries and duration in relation to total entries and total duration. Non-classical, anxiety-related, or ethological parameters such as risk assessment was measured by head dips which were defined as an attempt to look “under” the maze with the head pointing toward the floor from the open arm [32], or stretch-attend postures (SAPs) defined as stretching of the body to look into the open arms of the apparatus with only head and forelimbs forward before entering (or not) from the secured area, such as closed arm [32]. Rearing frequency, which is a measure for exploratory behavior, was defined as the animal rising on the hind limbs, both touching and not touching the wall, as protected and unprotected [33].
Forced Swim Test
The apparatus was made of a round bucket with a diameter of 40 cm and height of 44 cm. Water was filled up to 32 cm from the base with water temperature maintained between 25 and 28°C. FST was carried out as per the procedure previously described [34,35] with minor modifications [14,17]. The procedure consisted of 2 days of testing. On the first day, the animal was habituated in the FST apparatus for 10 min and the testing was carried out for 5 min 24 h after the habituation exposure. After each trial, the animal was wiped with a towel and the body dried under the lamp to prevent hypothermia. In the FST, the time spent by the animals in passive behavior (floating) called immobility, which was defined as the lack of motion of the whole body, and active behaviors (swimming and climbing) along with the latency to the first immobility were scored automatically in real time by the system during data acquisition. Ethovision settings that differentiated between immobility, swimming, and climbing behaviors have been validated manually [14,17,36].
Statistical Analysis
Data are expressed as mean ± SEM. Two-way analysis of variance (ANOVA) was carried out to determine the main effects of strain and phase as well as strain × phase interactions. Post tests were used to determine significant differences between individual means, either of strain or of estrous cycle phases. The level of significance was defined as p ≤ 0.05.
Results
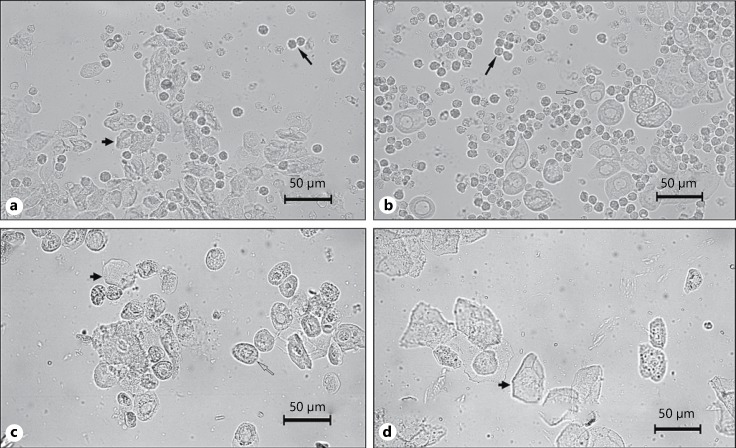
The characterization of each phase in the estrous cycle was made based on the proportion of 3 different cell types observed in the vaginal smear, namely epithelial cells (round and nucleated cells), cornified cells (anucleated irregular cells), and leucocytes (small round cells). Metestrus (D1) smear consisted of same proportion of epithelial cells, cornified cells and leucocytes (Fig. 1a), diestrus (D2) smear majorly consisted of leucocytes (Fig. 1b), proestrus (P) smear consisted of a predominance of nucleated epithelial cells (Fig. 1c), and estrus (E) smear primarily consisted of cornified cells (Fig. 1d). Only the females which were cycling normally with a mean duration of 4–5 days were used for the behavioral studies. Rats in metestrus and diestrus (D1 + D2) were considered as 1 group, while rats in proestrus and estrus (P + E) were considered as the other group.
Fig. 1.
Photomicrographs of stages of the estrous cycle: a metestrus or diestrus 1; b diestrus 2; c proestrus; d estrus. Long arrows point to leucocytes; short arrows point to cornified cells; open long arrows point to nucleated epithelial cells.
Elevated Plus Maze
During late adolescence (P58 ± 1), all the behavioral variables assessed in the EPM demonstrated significant strain-specific and/or estrous phase-specific differences. Overall, rats did not demonstrate any significant differences in locomotion between strains or phases, though generally they showed increased locomotion during the proestrus-estrus (P + E) than in the diestrus (D1 + D2) phase as demonstrated by a trend toward significance in the main effect of phase (2-way ANOVA: phase: F(1,18) = 3.48; p = 0.0785; strain: F(1,18) = 0.02; p = 0.8941; strain × phase interaction: F(1,18) = 0.00; p = 0.9638).
The P + E phase induced an overall decrease in anxiety levels by increasing open arm time and open arm entries with a corresponding decrease in closed arm time and closed arm entries in both strains. Ethological parameters such as head dips and SAPs were also increased in P + E phase, while rearing frequency, which is indicative of exploratory behavior, was variably affected. For strain-specific and phase-specific differences in open and closed arm entries and duration, locomotion, anxiety index, rearing frequencies, head dips, and SAPs (Table 1).
Table 1.
Anxiety-related profiles of late adolescent Wistar and WKY rats in elevated plus maze during different phases of the estrous cycle
| Variables | D1 + D2 |
P + E |
||
|---|---|---|---|---|
| WIS | WKY | WIS | WKY | |
| Time, % | ||||
| Open arm | 14.16±1.08 | 15.28±4.91 | 45.18±4.10$$$ | 25.28±2.37** |
| Closed arm | 69.26±5.04 | 83.83±4.66 | 32.92±2.48$$$ | 51.99±4.83*, $$$ |
| Entries, % | ||||
| Open arm | 41.87±3.67 | 28.88±2.11 | 60.10±2.77$$ | 42.55±6.64* |
| Closed arm | 55.76±3.43 | 71.12±2.11** | 42.28±1.69$ | 66.30±5.63*** |
| Anxiety index | 0.71±0.02 | 0.79±0.03 | 0.49±0.03$$$ | 0.67±0.06** |
| Locomotion, m | 16.33±1.68 | 16.15±1.63 | 20.24±2.02 | 19.87±2.74 |
| Ethological parameters, n | ||||
| Nose dips | 7.17±0.75 | 6.80±0.86 | 11.43±1.29$ | 7.17±0.87* |
| Rearings | 10.00±1.08 | 24.40±3.12*** | 14.20±1.07 | 14.50±1.44$$ |
| Stretch-attend postures | 1.80±0.20 | 2.75±0.25 | 0.33±0.21$$ | 3.25±0.48** |
Data are expressed as mean ± SEM. n = 6 in each group. One-way ANOVA.
Indicates significant differences between the strains – Wistar (WIS) and Wistar Kyoto (WKY) during diestrus (D1 + D2) and proestrusestrus (P + E) phases of estrous cycle.
Indicates significant differences between the strains – Wistar (WIS) and Wistar Kyoto (WKY) during diestrus (D1 + D2) and proestrusestrus (P + E) phases of estrous cycle.
Indicates significant differences between the strains – Wistar (WIS) and Wistar Kyoto (WKY) during diestrus (D1 + D2) and proestrusestrus (P + E) phases of estrous cycle.
Indicates significant differences in the same strain during diestrus (D1 + D2) and proestrus-estrus (P + E) phases of estrous cycle. p < 0.05, p < 0.01, p < 0.001.
Indicates significant differences in the same strain during diestrus (D1 + D2) and proestrus-estrus (P + E) phases of estrous cycle. p < 0.05, p < 0.01, p < 0.001.
Indicates significant differences in the same strain during diestrus (D1 + D2) and proestrus-estrus (P + E) phases of estrous cycle. p < 0.05, p < 0.01, p < 0.001.
Open/Closed Arm Time
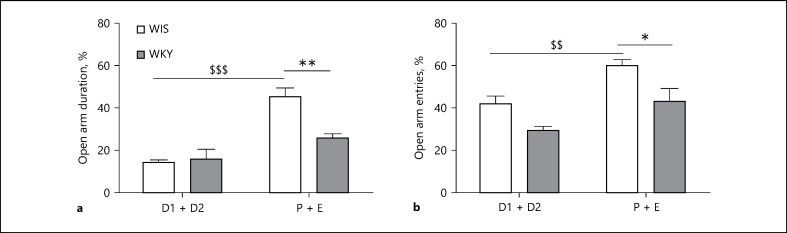
A 2-way ANOVA returned significant main effects of strain for open arm time (F(1,15) = 5.63; p = 0.0315) indicating that WKYs in P + E phase exhibited a significant (p < 0.01) decrease in open arm duration when compared to phase-matched Wistars (Fig. 2a). A significant main effect of phase (F(1,15) = 26.88; p = 0.0001) was also observed with Wistars exhibiting a significant (p < 0.001) increase in open arm duration during the P + E phase when compared to the D1 + D2 phase (2-way ANOVA: strain × phase interaction: F(1,15) = 7.06; p = 0.0179).
Fig. 2.
Anxiety-related behaviors in EPM of Wistar (WIS) and Wistar Kyoto (WKY) female rats at P 60 during diestrus (D1 + D2) and proestrus-estrus phases (P + E) of estrous cycle. a Open arm duration. ** Significant differences between WIS and WKY during proestrus-estrus phases (P + E) of estrous cycle, p < 0.01, t = 3.374. $$$ Significant difference in Wistars during diestrus (D1 + D2) and proestrus-estrus phase (P + E) − p < 0.001, t = 6.142. b Open arm entries. * Significant differences between WIS and WKY during proestrus-estrus phases (P + E) of estrous cycle, p < 0.05, t = 3.093. $$ Significant difference in Wistars during diestrus (D1 + D2) and proestrus-estrus phase (P + E) − p < 0.01, t = 3.214.
Closed arm time (%) results showed a significant main effect for strain (F(1,14) = 15.03; p = 0.0017) with WKYs demonstrating significant increased (p < 0.05) closed arm time in the P + E phase when compared to Wistars. A significant main effect for phase (F(1,14) = 61.74; p < 0.0001) was also observed with WKYs demonstrating a significant (p < 0.001) increase in closed arm time during the P + E phase, while Wistars demonstrated a significant (p < 0.001) decrease on testing in the same phase (2-way ANOVA: strain × phase interaction: F(1,14) = 0.27; p = 0.6129).
Open/Closed Arm Entries
A significant main effect of strain (F(1,21) = 13.95; p = 0.0012) in open arm entries was observed with WKYs exhibiting a significant (p < 0.05) decrease in open arm entries during P + E phase when compared to phase-matched Wistars (Fig. 2b). A significant main effect of phase (F(1,21) = 15.23; p = 0.0008) in open arm entries was also observed with Wistars demonstrating a significant (p < 0.01) increase in open arm entries in P + E phase when compared to D1 + D2 phase (1-way ANOVA: strain × phase interaction: F(1,21) = 0.31; p = 0.58).
Significant main effects of strain (F(1,17) = 40.31; p < 0.0001) and phase (F(1,17) = 8.70; p = 0.0090) were obtained in closed arm entries with WKY rats demonstrating significantly increased closed arm entries during both D1 + D2 (p < 0.01) and P + E (p < 0.001) phases when compared to age- and phase-matched Wistars (one-way ANOVA: strain × phase interaction: F(1,17) = 1.95; p = 0.1807).
A significant main effect of strain (F(1,20) = 12.22; p = 0.0023) was demonstrated in anxiety levels with increased (p < 0.01) anxiety levels in WKY during the P + E phase when compared to age- and phase-matched Wistars. A significant main effect of phase (F(1,20) = 21.29; p = 0.0002) was also demonstrated with Wistars showing a decrease (p < 0.001) in anxiety levels during P + E phase as compared to D1 + D2 phase (2-way ANOVA: strain × phase interaction: F(1,20) = 1.83; p = 0.1910).
Ethological Parameters
A significant main effect of strain was observed in head dips (F(1,20) = 5.10; p = 0.0352) with head dipping behavior being significantly decreased (p < 0.05) in WKY during the P + E phase when compared to age- and phase-matched Wistars. A significant main effect of phase (F(1,20) = 5.10; p = 0.0352) was also observed in Wistars with more head dips during P + E phase (p < 0.01) when compared to D1 + D2 phase (2-way ANOVA: strain × phase interaction: F(1,20) = 3.61; p = 0.0718).
A significant main effect of strain (F(1,14) = 13.08; p = 0.0028) was observed for rearing behavior, with WKYs demonstrating significantly (p < 0.001) increased rearing frequency during D1 + D2 phase when compared to age- and phase-matched Wistars. WKY rats demonstrated a significantly lower (p < 0.01) rearing frequency during P + E phase than during D1 + D2 phase (2-way ANOVA: phase: F(1,14) = 1.97; p = 0.1826; strain x phase interaction: F(1,14) = 4.52; p = 0.05).
A significant main effect of strain (F(1,15) = 45.94; p < 0.0001) in SAPs was observed with significantly increased SAPs in WKYs during P + E phase (p < 0.01) when compared to age- and phase-matched Wistars. A significant main effect of phase (F(1,15) = 2.87; p = 0.1108) was also demonstrated with a significant decrease (p < 0.01) in Wistars during P + E phase when compared to the diestrus phase (2-way ANOVA: strain × phase interaction: F(1,15) = 11.88; p = 0.0036).
Forced Swim Test
More than half of the test period was spent in climbing, while the remaining constituted time spent in swimming and being immobile. No differences in swimming behavior between strains and between phases of the estrous cycle were observed (Table 2). Climbing behavior increased significantly (p < 0.01) in Wistars during P + E phase when compared to diestrus phase (2-way ANOVA: main effect of phase: F(1,20) = 8.64; p = 0.0081; main effect of strain: F(1,20) = 0.31; p = 0.5866, strain × phase interaction: F(1,20) = 3.24; p = 0.0868).
Table 2.
Behaviors of late adolescent Wistar and WKY rats in the forced swim test during different phases of the Estrous cycle
| Variables | D1 + D2 |
P + E |
||
|---|---|---|---|---|
| WIS | WKY | WIS | WKY | |
| Time, % | ||||
| Immobility | 16.80±2.09 | 22.16±3.52 | 7.880±0.97$ | 16.39±2.07* |
| Swimming | 20.49±1.89 | 20.52±2.08 | 21.27±0.41 | 22.20±2.84 |
| Climbing | 56.38±3.48 | 62.32±3.80 | 78.96±2.61$$ | 67.75±8.30 |
| Latency, s | 12.48±1.10 | 11.13±2.84 | 59.35±8.86$$$ | 34.70±4.69**, $$ |
Data are expressed as mean ± SEM. n = 6 in each group. Data are expressed as mean ± SEM. One-way ANOVA statistics are shown.
Indicates significant differences between the strains – Wistar (WIS) and Wistar Kyoto (WKY) rats during diestrus (D1 + D2) and proestrus-estrus phases (P + E) of estrous cycle.
Indicates significant differences between the strains – Wistar (WIS) and Wistar Kyoto (WKY) rats during diestrus (D1 + D2) and proestrus-estrus phases (P + E) of estrous cycle.
Indicates significant differences in the same strain during diestrus (D1 + D2) and proestrus-estrus phases (P + E) of estrous cycle. p < 0.05, p < 0.01, p < 0.001.
Indicates significant differences in the same strain during diestrus (D1 + D2) and proestrus-estrus phases (P + E) of estrous cycle. p < 0.05, p < 0.01, p < 0.001.
Indicates significant differences in the same strain during diestrus (D1 + D2) and proestrus-estrus phases (P + E) of estrous cycle. p < 0.05, p < 0.01, p < 0.001.
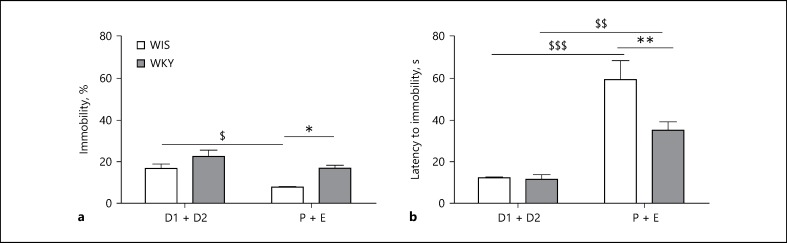
A significant main effect of phase (F(1,15) = 11.58; p = 0.0039) for time spent immobile was demonstrated with a significantly reduced immobility in Wistars during the P + E phase in relation to the D1 + D2 phase (Fig. 3a). A significant main effect of strain (F(1,15) = 10.31; p = 0.0058) was also observed with WKYs exhibiting significantly increased (p < 0.05) immobility in the P + E phase when compared to age- and phase-matched Wistars (one-way ANOVA: strain × phase interaction: F(1,15) = 0.53; p = 0.4767).
Fig. 3.
Depression-like behaviors of Wistar (WIS) and Wistar Kyoto (WKY) female rats at P60 during diestrus (D1 + D2) and proestrus-estrus phases (P + E) of estrous cycle in FST. a Immobility duration. * Significant difference between WIS and WKY during proestrus-estrus phases (P + E) of estrous cycle, t = 2.956; p < 0.05. $ Significant difference in Wistar during diestrus (D1 + D2) and proestrus-estrus phase (P + E), t = 3.237; p < 0.05. b Latency to immobility. ** Significant difference between WIS and WKY during proestrus-estrus phases (P + E) of estrous cycle, p < 0.01, t = 3.799. $$$ Significant difference in Wistar during diestrus (D1 + D2) and proestrus-estrus phase (P + E), t = 8.342; p < 0.001. $$ Significant difference in WKYs during diestrus (D1 + D2) and proestrus-estrus phase (P + E), t = 3.716; p < 0.01.
Latency to immobility, which is the time taken by the animal in seconds to exhibit first immobility was very significantly increased when tested in the P + E phase in both strains. Significant main effects of strain (F(1,18) = 10.13; p = 0.0051) and phase (F(1,18) = 74.44; p < 0.0001) were exhibited with strain x phase interaction (F(1,18) = 8.15; p = 0.0105). Both WKY and Wistars demonstrated phase-specific differences with significant increase (p < 0.01) in WKY and in Wistars (p < 0.001) in the P + E phase. However, the latency of WKYs was significantly lower (p < 0.01) than that of Wistars (Fig. 3b) for strain-specific and phase-specific differences in immobility, swimming, and climbing behaviors and latency to immobility (Table 2).
Discussion
Puberty, which is marked by the onset of the estrous cycle begins around P42 in WKY, so P58–60 ± 1, during which these tests were conducted would correspond to late adolescence, as some [15] consider with adolescence extending upto P60, though others [37] already consider P60 as young adult. The WKY rats demonstrated no phase-specific differences in open arm duration or entries with no corresponding change in anxiety levels, indicating that hormonal modulation, particularly of estrogen, may be masking the anxiety profiles normally demonstrated in the WKY. This is significant, as the Wistar readouts suggest that behavioral indices of anxiety do vary across the various phases of the estrous cycle, with the proestrus-estrus phase demonstrating significantly increased open arm duration and entries and therefore reduced anxiety levels. During diestrus too, WKYs demonstrate reduced open arm entries when compared to age- and phase-matched Wistars. The modulatory effects that ovarian hormones have on behavioral indices of anxiety have been studied, but not in a depression model. Estradiol (E2) is high in proestrus, while no significant difference exists in progesterone levels. E2 was found to significantly affect open arm time [38] though no other effects have been demonstrated. Our results demonstrate additional phase-specific differences in anxiety-related measures as well as ethological measures in Wistars and we have extended the same to a putative depression model, the WKY.
Irrespective of the phase they were tested in, the WKY demonstrated increased closed arm entries. Closed arm time was reduced during proestrus-estrus, with a corresponding decrease in rearing frequency. During diestrus, on the contrary, WKY demonstrated more rearing than Wistars, which is again a consequence of increased closed arm time. Among the non-classical ethological measures, testing during proestrus-estrus phase induced a significant increase in head dips (increased risk-taking behavior) in Wistars, while they were significantly reduced in WKY. SAPs, where the animal's body is still in the “safe” zone (here closed arm) and therefore indicative of risk assessment were higher in both phases in the WKY indicating higher levels of behavioral inhibition, which also manifests itself by a reserved response or general inactivity in the face of novelty [39]. This low behavioral activity was accompanied by reduced emission of ultrasonic vocalizations, which are important social communication tools for rodents and have been correlated to lower basal levels of serotonin when compared to other strains [39].
E2 administered during diestrus had the same effect on open arm time as when tested during proestrus, leading to lowered anxiety levels in proestrus than diestrus indicating that E2 modulates this response [38]. That open arm exploration of the plus maze varied according to the stages of the estrous cycle and has also been shown by others [40], where light intensity modulations were used. A high light intensity induced a small increase in open arm exploration in metestrus, while low light intensities with anxiolytic-like effects increased open arm exploration in proestrus and estrus. Sensitivity to this effect may have been higher during proestrus and estrus proving that it may prove detrimental to drug studies if phases of the estrous cycle are ignored in the analysis. Progesterone was found to increase open arm exploration under high light conditions. Assessing reproductive status or stages in rats is important, as they exhibit a clear and well-defined estrous cycle with reduced disruptions even with the routine stress in the animal facility [34,41].
In the FST, the WKY demonstrated increased immobility, irrespective of the phase tested in, exhibiting learned helplessness in a stress-inducing context, an effect which was more prominent in proestrus-estrus phase, despite increased levels of ovarian hormones, indicating again a masking effect of E2, since the Wistars demonstrated a decrease in immobility. The WKY, therefore, has been suggested as a model to define an organism's response to stress and that the greater vulnerability of the proestrus-estrus WKY rat indicates that the steroid hormone milieu may be responsible for behavioral differences in addition to the ones programmed in early development [3]. However, swimming behavior was not affected by phase and was comparable in both strains. Climbing was significantly increased in proestrus-estrus in Wistars, while in WKY again no change was observed. Latency to immobility, which was increased in both strains in proestrus-estrus could have been modulated by ovarian hormones. The strain differences observed could be due to the WKY being more inherently susceptible to stress as shown in ulcers induced by restraint stress [3] indicating that strain-specific differences in stress reactivity do exist.
The behavioral response in FST is compared with despair or helplessness in depressed humans and though it has low face validity, it has demonstrated high predictive validity for antidepressant screening [42]. Other studies, however, suggest that antidepressants either had no effect on stress-induced increase in anxiety behaviors [20] or the antidepressant response in WKY was inconclusive [27]. Phase-specific FST behaviors could operationally define behavioral depression as adult WKY females were found to be emotionally reactive, and based on the phase tested in, demonstrated variable stress responses and behavioral depression [3]. Proestrus-estrus females were found to be less active in the FST, demonstrating an increased depression-like profile indicating that established pathways are modulated by hormones [3]. The results of increased learned helplessness or behavioral despair observed here in late adolescent WKYs was not evident in early and mid-adolescent WKY females when they were compared to the progenitor Wistar strain [17]. Whether the ovarian hormonal milieu enhances the observed stress response or as discussed earlier is masked in WKY remains to be established. However, the results do indicate that the WKY do constitute models of adolescent depression with key criteria from our own [43] and other studies [2,7] validating this. Moreover, when exposed to FST after acute stress, females demonstrate a greater corticosteroid response than males indicating increased susceptibility to learned helplessness.
Evidence supporting face validity would be provided by increased anxiety index, increased learned helplessness, and increased anhedonia. Two of these have been demonstrated here for adolescent WKY females. Other studies have returned hypoactivity in open field [44] and increased locomotor activity under methylphenidate administration which increases immediate early gene NGFI-B mRNA acting via dopamine receptor subtype 2 (D2) in accumbens and striatum [45]. During adolescence, there is an increased expression of glucocorticoids, and estrogen has been shown to suppress neuronal overproduction in female prefrontal cortex, so that synaptic pruning during adolescence may unmask underlying predispositions [46].
From the results of this study, it emerges that the female adolescent WKY rat is stress-sensitive, exhibits anxiety-related and depression-like behaviors indicative of endogenous hormonal abnormalities and therefore can be used as an animal model of depression. In particular, hormonal-based differences have been dissected out here in the estrous cycle, underlying the fact that animal models are indispensable for such as venture, especially when there is a need to understand underlying neurobiological phenomena and the search for new treatment approaches.
Authorship Contributions
D.D. and M.S. conceptualized the work and wrote the manuscript; D.D. did the experiments, analysis, and statistics.
Disclosure Statement
The authors declare compliance to ICMJE recommendations and declare no conflict of interest.
Source of Funding
University Grants Commission, Govt. of India (F. No. 41/545/2012 (SR).
Acknowledgments
Grant support from DST (CSI/05/2009) and DBT (BT/PR4676/MED/30/735/2012), Govt. of India toward equipment is acknowledged.
References
- 1.Sterley T, Howells FM, Russell VA. Effects of early life trauma are dependent on genetic predisposition: a rat study. Behav Brain Funct. 2011;7:11. doi: 10.1186/1744-9081-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braw Y, Malkesman O, Dagan M, et al. Anxiety-like behaviors in pre-pubertal rats of the flinders sensitive line (FSL) and Wistar-Kyoto (WKY) animal models of depression. Behav Brain Res. 2006;167:261–269. doi: 10.1016/j.bbr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Pare WP, Redei E. Sex differences and stress response of WKY Rats. Physiol Behav. 1993;54:1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- 4.van den Bergh FS, Bloemarts E, Chan JS, et al. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Rauhut AS, Zentner IJ, Mardekian SK, et al. Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine. Physiol Behav. 2008;93:177–188. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Solberg LC, Olson SL, Turek FW, et al. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R786–R794. doi: 10.1152/ajpregu.2001.281.3.R786. [DOI] [PubMed] [Google Scholar]
- 7.Braw Y, Malkesman O, Merenlender A, et al. Withdrawal emotional-regulation in infant rats from genetic animal models of depression. Behav Brain Res. 2008;193:94–100. doi: 10.1016/j.bbr.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8:925–932. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- 9.O'Neil MF, Moore NA. Animal models of depression: are there any? Hum Psychopharmacol. 2003;18:239–254. doi: 10.1002/hup.496. [DOI] [PubMed] [Google Scholar]
- 10.McAuley JD, Stewart AL, Webber ES, et al. Wistar-Kyoto rats as an animal model of anxiety vulnerability: support for a hypervigilance hypothesis. Behav Brain Res. 2009;204:162–168. doi: 10.1016/j.bbr.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordero MI, Ansermet F, Sandi C. Long-term programming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology. 2013;38:2758–2769. doi: 10.1016/j.psyneuen.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Pignatelli D, Xiao F, Gouveia AM, et al. Adrenarche in the rat. J Endocrinol. 2006;191:301–308. doi: 10.1677/joe.1.06972. [DOI] [PubMed] [Google Scholar]
- 13.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Shetty RA, Sadananda M. Immediate and delayed anxiety- and depression-like profiles in the adolescent Wistar-Kyoto rat model of endogenous depression following postweaning social isolation. Behav Brain Res. 2017;320:323–332. doi: 10.1016/j.bbr.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 16.Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50:534–538. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza D, Sadananda M. Anxiety- and depressive-like profiles during early- and mid-adolescence in the female Wistar Kyoto rat. Int J Dev Neurosci. 2017;56:18–26. doi: 10.1016/j.ijdevneu.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Malkesman O, Braw Y, Maayan R, et al. Two different putative genetic animal models of childhood depression. Biol Psychiatry. 2006;59:17–23. doi: 10.1016/j.biopsych.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Mehta NS, Wang L, Redei EE. Sex differences in depressive, anxious behaviors and hippocampal transcript levels in a genetic rat model. Genes Brain Behav. 2013;12:695–704. doi: 10.1111/gbb.12063. [DOI] [PubMed] [Google Scholar]
- 20.Deussing JM. Animal models of depression. Drug Discov Today Dis Model. 2006;3:375–383. [Google Scholar]
- 21.Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychol Bull. 2014;140:816–845. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann ID, Wegener G, Homberg JR, et al. Animal models of depression and anxiety: what do they tell us about human condition? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1357–1375. doi: 10.1016/j.pnpbp.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 5. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 24.Bessa JM, Mesquita AR, Oliveira M, et al. A trans-dimensional approach to the behavioral aspects of depression. Front Behav Neurosci. 2009;3:1. doi: 10.3389/neuro.08.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czéh B, Fuchs E, Wiborg O, et al. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol. 2013;4:161. doi: 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armario A, Nadal R. Individual differences and the characterization of animal models of psychopathology: a strong challenge and a good opportunity. Front Pharmacol. 2013;4:137. doi: 10.3389/fphar.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whishaw IQ, Kolb B. A Handbook with Tests. Oxford: Oxford University Press; 2005. The Behavior of the Laboratory Rat. [Google Scholar]
- 29.Paccola CC, Resende CG, Stumpp T, et al. The rat estrous cycle revisited: a quantitative and qualitative analysis. Anim Reprod. 2013;10:677–683. [Google Scholar]
- 30.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 31.Cohen H, Matar MA, Buskila D, et al. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Melo TG, Izídio GS, Ferreira LS, et al. Antidepressants differentially modify the extinction of an aversive memory task in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:33–40. doi: 10.1016/j.pnpbp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Estanislau C, Morato S. Behavior ontogeny in the elevated plus-maze: prenatal stress effects. Int J Dev Neurosci. 2006;24:255–262. doi: 10.1016/j.ijdevneu.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 35.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 36.Noldus Information Technology . Evaluation of Porsolt Swim Test Activity: Using the Mobility Parameter of Ethovision. Netherlands: Noldus; 2004. [Google Scholar]
- 37.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 38.Marcondes FK, Miguel KJ, Melo LL, et al. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 39.Howells FM, Bindewald L, Russell VA. Cross-fostering does not alter the neurochemistry or behavior of spontaneously hypertensive rats. Behav Brain Funct. 2009;5:24. doi: 10.1186/1744-9081-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mora S, Dussaubat N, Díaz-velíz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 41.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kokras N, Antoniou K, Mikail HG, et al. Forced swim test: what about females? Neuropharmacology. 2015;99:408–421. doi: 10.1016/j.neuropharm.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 43.D'Souza D, Sadananda M. Anxiety-related behaviours and neurochemical profiles in adolescent female Wistar Kyoto Rats. International Symposium on Neurosciences and XXXI Annual Conference of Indian Academy of Neurosciences. 2013:79. [Google Scholar]
- 44.Pare WP, Kluczynski J. Differences in the stress response of Wistar-Kyoto (WKY) rats from different vendors. Physiol Behav. 1997;62:643–648. doi: 10.1016/s0031-9384(97)00191-1. [DOI] [PubMed] [Google Scholar]
- 45.Drolet G, Proulx K, Pearson D, et al. Comparisons of behavioral and neurochemical characteristics between WKY, WKHA, and Wistar rat strains. Neuropsychopharmacology. 2002;27:400–409. doi: 10.1016/S0893-133X(02)00303-2. [DOI] [PubMed] [Google Scholar]
- 46.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]