Abstract
Background
Neonatal bacterial infections have been reported to cause white matter loss, although studies concerning the influence of infection on the expression of myelin and aging are still in their emerging state.
Purpose
The present study aimed to investigate the effects of perinatal lipopolysaccharide (LPS) exposure on the myelination at different age points using histochemical and immunocytochemical techniques and the relative motor coordination.
Methods
A rat bacterial infection model was established by exposing the neonatal rats with LPS (0.3 mg/kg body weight, i.p., on postnatal day (PND) 3 followed by a booster at PND 5) and its impact was studied on the myelination and motor coordination in young, adult, and senile rats.
Results
The results obtained suggest that the administration of LPS induces demyelination, predominantly in cortex and corpus callosum. Low expression level of myelin oligodendrocyte glycoprotein (MOG) was observed at all time points, with prominence at 12, 18, and 24 months of age. In addition, reduced staining with luxol fast blue stain was also recorded in the experimentals. With the increasing demyelination and declining motor ability, LPS exposure also seemed to accelerate normal aging symptoms.
Conclusion
There is a direct correlation of myelin damage and poor motor coordination with age. This would provide a better incite to understand inflammation-associated demyelinating changes in age-associated neurodegenerative disorders. Since, no long-term studies on behavioral impairments caused by LPS-induced demyelination in the central nervous system has been reported so far, this work would help in the better understanding of the long-term pathological effects of bacterial-induced demyelination.
Keywords: Lipopolysacchride, Myelin oligodendrocyte glycoprotein, Demyelination, Motor behavior
Introduction
Demyelinating diseases like multiple sclerosis (MS) and cerebral palsy are major concerns for neurological research as these diseases are the main threat for neurological mortality. Previously, white matter damage was reported to be associated with MS only [1], but now it is found that it also increases the risk of acquiring psychiatric and neurobehavioral disorders as in schizophrenic [2] and Alzheimer's patients [3]. Hence, demyelination and white matter degeneration is getting increasing importance in scientific research [4].
The transmission of signals in the nervous system is largely by way of long nerve cell processes, which are insulated with myelin sheath synthesized by myelinating cells. These specialized myelin-forming cells can be the target of specific diseases, leading to loss of function of effective signalling between nerve cells [5]. Demyelination has been reported in a multitude of conditions, viz., malnutrition, exposure to toxicants, viral and bacterial infections, trauma, genetic deficits, etc. Inflammation is increasingly being linked to several brain disorders, but how neuroinflammation may influence myelin damage remains to be established [6,7,8]. Bacterial Infections are the most common cause of the human infectious diseases. Many bacteria are pathogenic to humans and carry virulent genes or RNA or cell wall protein that causes pathogenic inflammatory responses in the host leading to death following severe sepsis [9]. Few important demyelinating diseases caused by bacterial infection are MS by Chlamydia pneumonia and gut bacteria, acute disseminated encephalomyelitis by Mycoplasma pneumonia and Campylobacter jejuni, acute hemorrhagic leucoencephalitis by M. Pneumonia, progressive multifocal leukoencephalopathy by M. Pneumonia, and rheumatoid arthritis by Escherichia coli [7,10]. Lipopolysaccharide (LPS), an endotoxin of gram-negative bacteria has the potential to activate innate immune response in a host by binding to the toll-like receptor-4 [11]. It activates the glial cells, that is, astrocytes and microglia and upregulates the release of a cascade of pro-inflammatory cytokines and interleukins such as tumor necrosis factor, interleukin-1β, and interferon gamma [12,13]. Both developing and mature neurons and glia possess numerous pro-inflammatory cytokine receptors, which make them susceptible to inflammation-assocaited damage [14]. The neuroinflammatory response was reported to occur with weight loss [15,16], neurodegeneration [17], white matter damage [18], and cognitive and affective disorder [19]. Since LPS can reproduce many of the neuroinflammatory complications, it has been considered as the most established animal model for investigating the impact of bacterial-induced neuroinflammation [12,17,20]. Demyelination induced by bacteria can be studied by analyzing the molecular changes in the unique myelin axonal proteins like proteolipid protein, myelin basic protein, myelin-associated glycoprotein, and myelin oligodendrocyte glycoprotein (MOG) [7].
Thus, in order to understand the demyelination effects of bacteria, we developed a rat bacterial infection model by exposing the neonatal rats with LPS, a common bacteria-associated molecular pattern and experimentally determined its impact on the myelination in young, adult, and senile rats. Specifically, we aimed to unravel whether LPS-infected animals differ in their myelin content along their life span, if so, whether it would affect their motor ability. Since, no long-term study on the behavioral impairments caused by LPS-induced demyelination has been reported so far, this work would help us to understand the long-term pathological implications of bacterial infection-assiciated demyelination.
Methods
Experimental Animals
The Sprague Dawley rats were procured from the in-house animal stock of the departmental animal house and maintianed on standard conditions viz., 12:12-h light-dark cycle, controlled temperature of 23 ± 2°C, and 50–65% humidity. The standard rat pellet feed and water ad libitum were given to all animals. The experiments were pre-approved by the Institutional Animal Ethics Committee of Jiwaji University, Gwalior, and animal sufferings were restricted to minimal level during the experimental procedures. The animals were put for breeding with 2 females and 1 male per cage, overnight. The mating was confirmed next morning by vaginal smear test and the presence of sperms in the vaginal smear confirmed the fertilization and the day was considered as gestation day 0. The female dams found pregnant were separated and housed individually in a cage for providing extra comfort/care. The day when pups were born was designated as PND 0. After parturition, litter size and weight was recorded, and pups were routinely monitored.
Intraperitoneal Infusion of LPS
An in vivo bacterial infection model was created by injecting LPS at a dosage of 0.3 mg/kg body weight intraperitoneally on PND 3 followed by a booster dose at PND 5. For the purpose, 1 mg of LPS procured from Sigma Aldrich (E. coli, serotype O111:B4) was dissolved in 2 mL of sterilized phosphate-buffered saline (PBS) and injected to the pups using a Hamilton microsyringe and a Stoelting Nanoinjector for precise volume and constant flow rate. This group comprised the “LPS Group,” while the age-matched pups injected with equal amount of PBS served as “Control group.”
Perfusion and Tissue Preparation
For the histochemical studies, brains were perfusion fixed at various age points, that is, 3, 6, 12, 18, and 24 months, from both the groups. The rats were deeply anesthetized with diethyl ether and perfused transcardially with chilled PBS (0.01 M; pH 7.4) followed by 2% paraformaldehyde prepared in PBS. The brains were dissected out and occipitotemporal region was sectioned and fixed in the same fixative at 4°C overnight. Next day, the tissues were washed with phosphate buffer to remove excess fixative and cryoprotected with 10, 20, and 30% sucrose gradients prepared in phosphate buffer at 4°C. Fifteen micrometer thick coronal sections were cut using Leica Cryostat CM1900. The sections were collected on chrome alum gelatin-coated slides and stored at −20°C for histochemical studies.
Histochemical Studies
Luxol Fast Blue Staining
Randomly selected cryosections from each group and age (3, 6, 12, 18, and 24 months, n = 3) were washed in distilled water for 5 min and dehydrated through ascending series of ethanol, that is, 30, 50, 70, and 90%, 5 min each. The sections were then stained in preheated (57°C) luxol fast blue stain (LFB; 0.1 g LFB in 100 mL of 90% ethanol and 0.5 mL of 10% glacial acetic acid) overnight. Sections were washed in 95% alcohol and differentiated with lithium carbonate solution (Himedia; 0.25 g lithium carbonate in 500 mL distilled water) followed by differentiation in 70% alcohol, 3–4 dips. These LFB-stained sections were counterstained with 0.1% Cresyl Violet (pH 3.5, Sigma) for 10 min. The sections were rinsed in distilled water, air dried at 37°C for 2 h, dehydrated in n-butanol, and cleared in xylene for 10 min. The sections were finally mounted with DPX and viewed under the microscope.
Anti-MOG Immunohistochemistry
Cryosections from each time point (i.e., 3, 6, 12, 18, and 24 months, n = 3) and groups were washed thrice in PBS for 5 min each and then treated with 0.5% Triton X-100 (Sigma) for 30 min for membrane permeabilization. The sections were again washed thrice with PBST (0.1% Tween 20 added to PBS), for 5 min each, and incubated with 10% normal goat serum (Sigma) diluted in PBS for 2 h in a humid chamber to block non-specific proteins. Subsequent to this, the sections were incubated with rabbit polyclonal anti-MOG antibody (1:100; MOG, Abcam, ab32760) diluted in 5% bovine serum albumin in PBS with 0.5% Tween 20, overnight at 4°C. Next day, the sections after washing thrice with PBST were incubated with anti-rabbit tetramethylrhodamine labelled secondary antibody (1:200; Sigma) diluted with 5% BSA and 0.5% Tween 20 in PBS (Sigma) for 2 h in dark at room temperature. The sections were then washed well in PBS for 5 × 10 min and mounted with Vector hardset with DAPI, stored at 4°C for imaging.
Image Acquisition and Analysis
Image acquisition and analysis was done by using Fluorescence microscope (Leica DM6000) equipped with LAS version 4.2 (Leica Application Suite) software for LFB bright field imaging and LAS AF for fluorescent (MOG) imaging.
Neurobehavioral Studies
Grip Strength Test
Forelimb neuromuscular strength of control and LPS-treated animals (n = 12) was assessed by using Grip strength meter (Columbus, OH, USA) as described by Naik et al. [21]. The animal was handled with its tail and placed carefully over the pull bar assembly/metallic grid (76 × 250 mm) of the instrument and allowed to hold the grid through its forelimbs. The animal was then gently pulled back in a straight horizontal line until the grip was released. The peak force was recorded automatically via computer interface, RS-232, and software 1.19. For analysis, 7 readings per animal were recorded and mean value was calculated.
Rotarod Test
Motor coordination of both control and treated animals (n = 12) was analyzed using a Rotarod instrument procured from Columbus Instruments, USA. The instrument consists of a semi-enclosed chamber with a series of 32 infrared beams and a rotating rod suspended at a height of 35 cm above the floor. All the experiments were done at the consistent room conditions (light and temperature) and timing to avoid any variations. Prior to final recordings, the animals were acclimatized for 3 consecutive days, 4 trials/day. First 3 trials were given at the speed of 10 rpm for a total duration of 100 s and the fourth trial was given at 40 rpm for 420 s to ensure better acclimatization. The final readings were acquired by running the animals at a speed of 40 rpm for 420 s and the total time spent by the animals on the accelerating wheel of rotarod was automatically recorded by the photocells as the falling latency time using Rotamex 5 software. Average of 4 readings was taken as a single mean value for each animal [22].
Statistical Analysis
Data were statistically analyzed using SigmaStat 3.5 software and expressed as mean ± SEM. Unpaired t test for inter group comparisons followed by Holm-Sidak test was done. p value of <0.05 was considered as significant and <0.001 as highly significant.
Results
Neonatal LPS Exposure Leads to Reduced White Matter Density and Demyelination at Adulthood and Senility
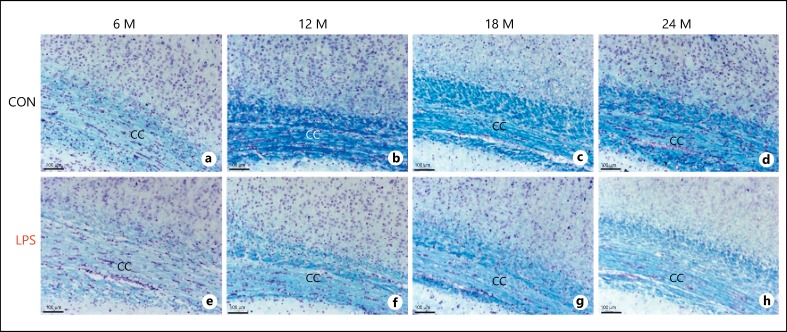
The forebrain consists of most of the white matter including the fiber tracts of cerebral cortex, corpus callosum, the largest white matter structure, which connects 2 hemispheres and subcortical tracts of limbic system. First, the effect of neonatal infection of LPS was studied in 3, 6, 12, 18, and 24 months old rat brains by staining the white matter (WM) with luxol fast blue. From the mild staining of WM with luxol fast blue in the LPS group rats, it was evident that there was a consistent reduction in WM density in all age groups studied, specifically from 6 months onwards with gradual amplification of axonal damage. The corpus callosum of 3 month rats of both groups was still developing with less bundle thickness and was fully developed with huge fibrous WM by 6 months. But the corpus callosum of 6 months LPS group rats (Fig. 1e) was less dense with diffused margin fibers when compared to the age-matched control (Fig. 1a). Several small oblique lesions with cellular infiltrations revealed that LPS infection damages the cytoarchitecture of corpus callosum in developing rat brain. The corpus callosum of 12, 18, and 24 months adult control group was densely packed with fibers having high myelin content and thickness (Fig. 1b, 1b, 1d). In contrast, the LPS group rats (Fig. 1f, 1g, 1h) showed cellular atrophy and significant loss of myelin with less dense, dispersed, fragmented thinner fibers with poor margins. It was shown that irrespective of the increased myelination in young control rats, LPS induced chronic demylination, triggering the intiation of early onset of aging and degeneration. Further, in senile 24-month-old rat group, the progressive WM damage with disorted structural symmetry was obrserved in the LPS group (Fig. 1h) as compared to the control (Fig. 1d).
Fig. 1.
a-h Light microscopic images of the luxol fast blue stained sections from both the control and LPS-treated groups at various age points showing the myelin degeneration following neonatal LPS exposure (n = 3). LPS, lipopolysaccharide; CC, corpus callosum; M, months; scale bar = 100 μm.
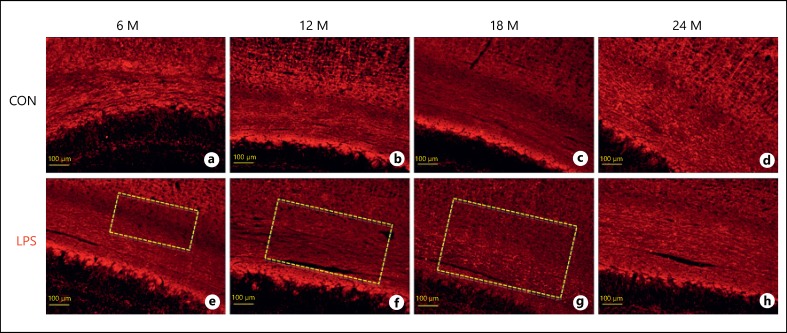
The demyelinating effects of LPS were further confirmed and elucidated with anti-MOG immunolabeling. MOG is the surface protein found on the outermost lamellae of axonal myelin sheath and mature oligodendrocytes and is actively involved in myelination of central nervous system (CNS). By targeting this myelin marker protein using an anti-MOG antibody, we can effectively visualize the specific morphological changes in the myelination of WM tracts. Our results show a weak immunolabeling with anti-MOG following neonatal LPS exposure at all the age points, that is, 3, 6, 12, 18, and 24 months (Fig. 2), as compared to their age-matched controls. Neonatally exposed animals presented significant demyelination at the age of 12 months as evident from the loss of compaction and weak labeling of the corpus callosum fiber bundles (Fig. 2d) with respect to the 12-month-old controls (Fig. 2c). At 18 months of age, fiber bundles were disorganized with demyelinating lesions (Fig. 2f). The aging effects were prominent in control animals as well when compared within groups (Fig. 2a, c, d, g). However, at 24 months of age, not much difference was seen in the neonatally LPS exposed group and the respective control as far as the intensity of immunostaining is concerned, except the diffuse staining of the fibers (Fig. 2g, 2h).
Fig. 2.
a-h Immunofluorescence images of the corpus callosum of the rat brain sections immunolabeled with anti-myelin oligodendrocyte glycoprotein antibody and visualized with tetramethylrhodamine at various age points depicting the loss of myelinated fibers following neonatal LPS exposure (n = 3). The demyelinating lesions are indicated with dotted rectangle. Scale bar = 100 μm.
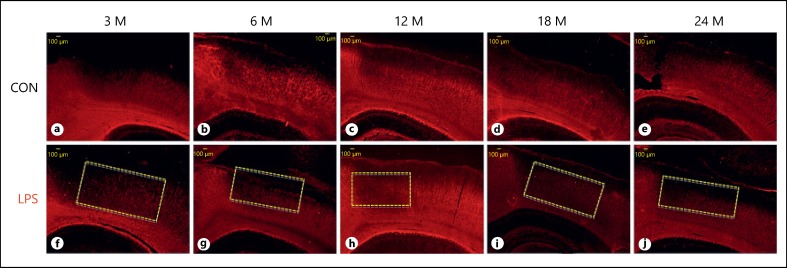
When compared with the huge fibrous corpus callosum, less dense cortical WM was found to be the severe target of LPS infection. The LPS sections showed well-defined demyelinated lesions in the cortex of all age groups, that is, 3, 6, 12, 18, and 24 months, as compared to their age-matched control (Fig. 3). In control, with increasing age, there was reduction in the cortical fibers myelination, but in LPS group, the cortex was severely demyelinated with only very few fibers labeled with anti-MOG antibody (Fig. 3a, 3b, 3c, 3d, 3e, 3f, 3g, 3h, 3i, 3j). This may suggest that the normal aging symptoms were futher amplified and accelerated by the LPS infection, which could lead to several neurodegerative problems characterized with motor abnormalities.
Fig. 3.
a-j Immunofluorescence images of the cortical region of the rat forebrain, immunolabeled with anti-myelin oligodendrocyte glycoprotein antibody and visualized with tetramethylrhodamine at various age points depicting the loss of myelinated fibers following neonatal lipopolysaccharide exposure as compared to the age-matched controls (n = 3). The demyelinating lesions are indicated with dotted rectangle. Scale bar = 100 μm.
Lowered Neuromuscular Strength Following Neonatal LPS Treatment
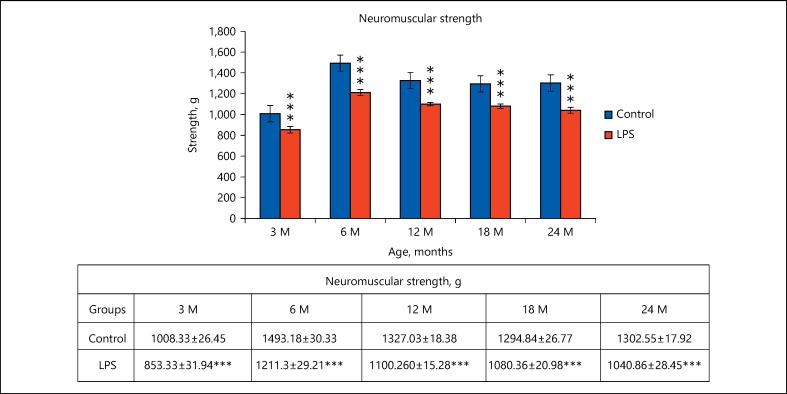
Significant reduction in grip strength was recorded in the LPS-treated animals as compared to the control animals, at all age points examined, that is, 3 months (t(12) = 3.737, p ≤ 0.001), 6 months (t(12) = 6.693, p ≤ 0.001), 12 months (t(12) = 9.486, p ≤ 0.001), 18 months (t(12) = 7.839, p ≤ 0.001), 24 months (t(12) = 7.782, p ≤ 0.001). Increase in neuromuscular activity of control animals from 3 to 6 months suggests the duration of fast development and decline from 6 to 24 months loss of strength due to normal aging. However, a consistent and significant reduced neuromuscular strength in neonatally LPS challenged rats at all age points from 3 to 24 months of age clearly indicates the impaired muscular ability (Fig. 4).
Fig. 4.
Graph showing the grip strength performance of the fore-limbs of control and neonatally lipopolysaccharide (LPS)-exposed animals at various age groups. The values are presented as mean ± SE of the neuromuscular strength. *** p < 0.001 for LPS vs. control (n = 12; t test followed by post hoc Mann-Whitney rank sum test).
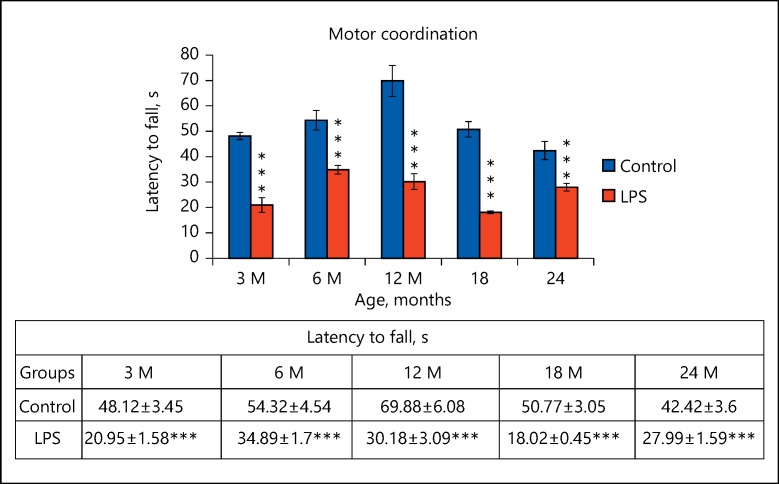
Poor Motor Coordination Following Neonatal LPS Treatment
In addition to the poor neuromuscular strength, the LPS exposed rats also performed significantly poor on the rotating rod of the rotarod at all age points. That is, 3 months (t(12) = 7.153, p ≤ 0.001), 6 months (t(12) = 4.002, p ≤ 0.001), 12 months (t(12) = 5.82, p ≤ 0.001), 18 months (t(12) = 10.597, p ≤ 0.001), 24 months (t(12) = 3.666, p ≤ 0.001) than their respective control animals. This suggests that LPS exposure led to poor motor coordination which persisted throughout their life span. Although the age-associated impact was clearly evident in terms of increased duration of keeping balance on rotarod treadmill or the latency time to fall from 3 to 12 months and subsequent decrease till 24 months, there was a highly significant reduction in latency of neonatally LPS exposed rats all age points studied (Fig. 5).
Fig. 5.
Graph showing the performance of the animals as total time spent on the accelerating wheel of the rotarod in both control and neonatally lipopolysaccharide (LPS)-exposed animals at various age groups. Data are presented as latency to fall (mean ± SE). *** p < 0.001 for LPS vs. control (n = 12; t test followed by post hoc Mann-Whitney rank sum test).
Discussion
We tested our hypothesis that LPS-induced inflammation differentially impacts WM density, compactness, and myelination leading to neurobehavioral deficits. Early postnatal exposure of rats to LPS mimics the septicemia in human infants [23,24] that can negatively influence the process of brain development. The developing brain has several vulnerable and overlapping windows representing major brain developmental events in structural formation and maturation. The rat brain develop rapidly during its early postnatal life with oligodendrogenesis and initiation of myelination by PND 10-PND 12 in cerebellum with a peak at PND 20 [7]. A comparison of rat vs. human brain development was projected earlier from our group [25].
LPS exposure has been variously reported to cause astrocytic and microglial activation. Prolonged over-expression of pro-inflammatory cytokines has been extensively reported to cause neuronal loss [12,13,17,26,27,28]. The present study makes a novel attempt to answer the questions concerning the long-term impact of early life bacterial infection on myelination and behavior. Demyelinating changes observed in the 3- and 6-month-old rats and increased WM damage in the corpus callosum and cortex at 12, 18, and 24 months of age validates the neuronal and WM degeneration, simulating the cystic lesions and other severe changes following sepsis or prenatal brain injury in human septicemia [29,30]. The preferential loss of MOG in the cortical region at all the time points are comparable with inflammatory demyelinating lesions of MS, as presented by Lucchinetti et al. [31,32]. The histological data validating the loss of myelin by neonatal LPS exposure very well exemplifies the decline in motor behavior. The rotarod task [22,33,34], the notched balance beam test [35], and the grip strength test [21,36] have been useful in study of motor impairments following CNS injury. We have also assessed the impact of demyelination on the motor activity of rats using grip strength and rotarod test. Poor neuromuscular strength and impaired motor coordination observed in the young rats and further decline with age in adult and senile rats (12 to 24 months) proves that the loss of myelin caused motor deficits. Our results also explained the demyelinating lesions in the WM. Cerebral cortex governs motor activity, and lesions or disorganization in the WM may promote the occurrence of motor disorders. The WM like corpus callosum is composed of nerve tracts consisting of myelinated axons, which link different parts of the brain and transmit nervous influx between neurons [37]. WM lesions also hinder the neurotransmission between cerebral structures [38]. The internal capsule is a link between the cortex and other cerebral structures like the thalamus and basal ganglia in both rodents and humans. In the latter, these regions have been significantly correlated with general movement disorders in children who suffered perinatal brain injury [39]. Direct neonatal exposure of LPS to brain showed irreversible decline in motor and cognitive activities in rats [12]. In contrast to this, there were other reports showing the motor dysfunction in neonates following maternal LPS exposure but functional recovery in the adult rats. This shows that the first order bacterial infections had more severe impact on the brain due to chronic demyelination and neurodenegerative changes. Demyelination and less efficient remyelination of demyelinated axons with age were also reported in the CNS. Such effects were also seen in demyelinating diseases like MS [40,41]. In 2005, Altmann-Schneider et al. [42] using magnetization transfer imaging techniques reported a linear decrease in cortical gray and WM with increasing chronological age. A progressive myelin breakdown in the senile brain has been variously reported. This is considered to be largely due to the lipid rich feature of myelin sheath that renders the myelin more vulnerable to environmental challenges, infection, malnutrition, etc. At the same time, influence of such infections on the ultrastructural organization and the integrity of the myelin sheath and their impact on myelin protein expression remain to be explored in details [43,44]. In the present study, the downregulation of MOG has been recorded with advanceing age, following early life exposure to LPS. MOG being the highly expressed myelin protein in the mature WM, its reduction in the corpus callosum and cortex indicates that the neonatal LPS infection may have chronic effects by downregulating such myelin proteins, leading to the loss of myelin compaction, subsequently causing demyelinating lesions during adulthood and senility. Thus, it can be inferred that early life infection may have a strong bearing on the motor ability of an individual during later life and may be a serious threat toward the development of age-related degenerative disorders.
Conclusions
Thus, it was concluded from the present study that early life bacterial infections can cause demyelinating changes at adulthhod and senilty by altering the expression of myelinating proteins. Cortical fibers were found to be more susceptible to demyelination than the thick axonal bundle fibers of corpus callosum, and the rate and extent of demyelination increased linearly with age with relative decline in the motor activity. Further perspective studies have to be carried out to understand the relative impact of demyelination damages in the cognitive, sensory, and behavioral deficits at different stages of the organism.
Author Contributions
I.P. conceptualized the idea and designed the study, N.P. executed the experiments and did the analysis, K.S. and P.M. performed the experiments and wrote the manuscript.
Disclosure Statement
The authors have no conflicts of interest to declare. This article receieved grants from Department of Biotechnology, Govt. of India (grant No. BT/PR3908/MED/97/8/2011).
Acknowledgments
The authors are thankful to Department of Biotechnology, Goverment of India for financial assistance through a project grant (BT/PR3908/MED/97/8/2011). Facilities developed through the DBT-Human Resource Development and Bioinformatics Infrastructural facilities from Department of Biotechnology used in this study are also thankfully acknowledged.
References
- 1.Wingerchuk DM, Weinshenker BG. Multiple sclerosis: epidemiology, genetics, classification, natural history, and clinical outcome measures. Neuroimaging Clin N Am. 2000;10:611–624. vii. [PubMed] [Google Scholar]
- 2.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32:1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 5.Bear MF, Connors BW, Paradiso M. Neuroscience-Exploring the Brain. ed 4. Lippincott Williams & Wilkins; 2016. pp. 103–107. [Google Scholar]
- 6.Felts PA, Woolston AM, Fernando HB, Asquith S, Gregson NA, Mizzi OJ, Smith KJ. Inflammation and primary demyelination induced by the intraspinal injection of lipopolysaccharide. Brain. 2005;128((pt 7)):1649–1666. doi: 10.1093/brain/awh516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady S, Siegel G, Albers RW, Price D. Basic Neurochemistry: Principles of Molecular, Cellular, and Medical Neurobiology. ed 8. Academic Press; 2011. pp. 691–704. [Google Scholar]
- 8.Fernández O, Fernández VE, Guerrero M. Demyelinating diseases of the central nervous system. Medicine. 2015;11:4601–4609. [Google Scholar]
- 9.Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10:701–706. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love S. Demyelinating diseases. J Clin Pathol. 2006;59:1151–1159. doi: 10.1136/jcp.2005.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang KC, Fan LW, Kaizaki A, Pang Y, Cai Z, Tien LT. Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience. 2013;234:146–157. doi: 10.1016/j.neuroscience.2012.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Patro N, Patro IK. Lipopolysaccharide-induced apoptosis of astrocytes: therapeutic intervention by minocycline. Cell Mol Neurobiol. 2016;36:577–592. doi: 10.1007/s10571-015-0238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brites D, Fernandes A. Bilirubin-induced neural impairment: a special focus on myelination, age-related windows of susceptibility and associated co-morbidities. Semin Fetal Neonatal Med. 2015;20:14–19. doi: 10.1016/j.siny.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock - a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 16.Nemzek JA, Hugunin KM, Opp MR. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med. 2008;58:120–128. [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso FL, Herz J, Fernandes A, Rocha J, Sepodes B, Brito MA, McGavern DB, Brites D. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J Neuroinflammation. 2015;12:82. doi: 10.1186/s12974-015-0299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousset CI, Chalon S, Cantagrel S, Bodard S, Andres C, Gressens P, Saliba E. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–433. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- 19.Lazosky A, Young GB, Zirul S, Phillips R. Quality of life after septic illness. J Crit Care. 2010;25:406–412. doi: 10.1016/j.jcrc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Steiner AA, Chakravarty S, Robbins JR, Dragic AS, Pan J, Herkenham M, Romanovsky AA. Thermoregulatory responses of rats to conventional preparations of lipopolysaccharide are caused by lipopolysaccharide per se - not by lipoprotein contaminants. Am J Physiol Regul Integr Comp Physiol. 2005;289:R348–R352. doi: 10.1152/ajpregu.00223.2005. [DOI] [PubMed] [Google Scholar]
- 21.Naik AA, Patro IK, Patro N. Slow physical growth, delayed reflex ontogeny, and permanent behavioral as well as cognitive impairments in rats following intra-generational protein malnutrition. Front Neurosci. 2015;9:446. doi: 10.3389/fnins.2015.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar K, Patro N, Patro I. Impaired structural and functional development of cerebellum following gestational exposure of deltamethrin in rats: role of reelin. Cell Mol Neurobiol. 2013;33:731–746. doi: 10.1007/s10571-013-9942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallard C, Wang X. Infection-induced vulnerability of perinatal brain injury. Neurol Res Int. 2012;2012:102153. doi: 10.1155/2012/102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tissières P, Ochoda A, Dunn-Siegrist I, Drifte G, Morales M, Pfister R, Berner M, Pugin J. Innate immune deficiency of extremely premature neonates can be reversed by interferon-γ. PLoS One. 2012;7:e32863. doi: 10.1371/journal.pone.0032863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morell P. Myelin. ed 2. New York: Plenum Press; 1984. [Google Scholar]
- 26.Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, Berger M, Schäfers M, Heneka MT. NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci. 2009;29:14177–14184. doi: 10.1523/JNEUROSCI.3238-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossù P, Cutuli D, Palladino I, Caporali P, Angelucci F, Laricchiuta D, Gelfo F, De Bartolo P, Caltagirone C, Petrosini L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J Neuroinflammation. 2012;9:101. doi: 10.1186/1742-2094-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson ST, Commins S, Moynagh PN, Coogan AN. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav Immun. 2015;43:98–109. doi: 10.1016/j.bbi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30:473–478. doi: 10.1016/j.tins.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis. 2014;14:751–762. doi: 10.1016/S1473-3099(14)70710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Brück W, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Boehm SL, 2nd, Schafer GL, Phillips TJ, Browman KE, Crabbe JC. Sensitivity to ethanol-induced motor incoordination in 5-HT(1B) receptor null mutant mice is task-dependent: implications for behavioral assessment of genetically altered mice. Behav Neurosci. 2000;114:401–409. [PubMed] [Google Scholar]
- 34.Rustay NR, Wahlsten D, Crabbe JC. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav Brain Res. 2003;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- 35.Lipp HP, Wahlsten D. Genetically Defined Animal Models of Neurobehavioral Dysfunctions. ed 1. Boston: Birkhäuser; 1992. Absence of the Corpus Callosum. pp. 217–252. [Google Scholar]
- 36.Nagayach A, Patro N, Patro I. Experimentally induced diabetes causes glial activation, glutamate toxicity and cellular damage leading to changes in motor function. Front cell Neurosci. 2014;8:355. doi: 10.3389/fncel.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serra-de-Oliveira N, Boilesen SN, Prado de França Carvalho C, LeSueur-Maluf L, Zollner Rde L, Spadari RC, Medalha CC, Monteiro de Castro G. Behavioural changes observed in demyelination model shares similarities with white matter abnormalities in humans. Behav Brain Res. 2015;287:265–275. doi: 10.1016/j.bbr.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari F, Todeschini A, Guidotti I, Martinez-Biarge M, Roversi MF, Berardi A, Ranzi A, Cowan FM, Rutherford MA. General movements in full-term infants with perinatal asphyxia are related to basal ganglia and thalamic lesions. J Pediatr. 2011;158:904–911. doi: 10.1016/j.jpeds.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Franklin RJ, Zhao C, Sim FJ. Ageing and CNS remyelination. Neuroreport. 2002;13:923–928. doi: 10.1097/00001756-200205240-00001. [DOI] [PubMed] [Google Scholar]
- 41.Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altmann-Schneider I, de Craen AJ, van den Berg-Huysmans AA, Slagboom P, Westendorp RG, van Buchem MA, van der Grond J. An in vivo study on brain microstructure in biological and chronological ageing. PLoS One. 2015;10:e0120778. doi: 10.1371/journal.pone.0120778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherin JE, Bartzokis G. Human brain myelination trajectories across the life span: implications for CNS function and dysfunction. In: Masoro EJ, Austa SN, editors. Handbook of the Biology of Aging. ed 7. San Diego: Academic Press; 2011. pp. 333–346. [Google Scholar]
- 44.Xie F, Zhang JC, Fu H, Chen J. Age-related decline of myelin proteins is highly correlated with activation of astrocytes and microglia in the rat CNS. Int J Mol Med. 2013;32:1021–1028. doi: 10.3892/ijmm.2013.1486. [DOI] [PubMed] [Google Scholar]
- 45.Franco-Pons N, Torrente M, Colomina MT, Vilella E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett. 2007;169:205–213. doi: 10.1016/j.toxlet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Torkildsen Ø, Brunborg LA, Myhr KM, Bø L. The cuprizone model for demyelination. Acta Neurol Scand Suppl. 2008;188:72–76. doi: 10.1111/j.1600-0404.2008.01036.x. [DOI] [PubMed] [Google Scholar]