Abstract
Despite being the largest part of the human gastrointestinal (GI) tract, the small intestine accounts for only 1–1.4% of all GI malignancies. Adenocarcinoma is the most common primary small bowel malignancy, with the most common site being the duodenum. On the other hand, squamous cell carcinoma (SCC) of the duodenum is extremely uncommon. We report the first case of mixed adenocarcinoma and SCC occurring in the third part of duodenum (D3). Our patient, a 64-year-old female with history of GERD, hypertension, and IDDM presented with 4 weeks of nausea, vomiting, and abdominal pain. Tomographic imaging of her abdomen demonstrated a distended stomach and a proximal duodenum with narrow caliber changes at the level of D3. An EGD revealed a tight stricture at D3 that could not be traversed. Stricture biopsies revealed duodenal mucosa with two small foci of SCC (positive for p63 and CK5/6) and adenocarcinoma (positive for CK7 and Moc31). Peritoneal metastases were detected on exploratory laparotomy, making the tumor surgically incurable. As she progressively declined and with worsening liver enzymes and general debility, she was not a candidate for chemotherapy and was eventually discharged on home hospice. Small bowel SCC/adenocarcinoma is an exceedingly uncommon cancer, making further case reports such as ours important to understand the nature of this entity and establish management guidelines.
Keywords: Adenocarcinoma of duodenum, Squamous cell carcinoma of duodenum, Small bowel squamous cell carcinoma/adenocarcinoma
Introduction
The small intestine is the largest part of the human gastrointestinal (GI) tract, encompassing nearly 90% of its mucosal surface. Interestingly, it only contributes minimally to the total tumor burden from the GI tract [1]. Only 1–2% of GI malignancies originate from the small intestine; however, the incidence of these malignancies is trending upwards, partly due to increased tumor detection via advanced diagnostic endoscopic and radiographic modalities [1]. The ileum carries the majority of the small intestinal tumor burden, followed by the duodenum, and lastly the jejunum [1]. The majority of duodenal cancers originate from the descending duodenal segment (D2), followed by the horizontal (D3) and ascending (D4) segments, and rarely from the proximal horizontal segment (D3) [2]. More than 40 histological subtypes of small intestinal malignancies have been described, the most common being adenocarcinoma, sarcoma, lymphoma, and neuroendocrine tumors [3]. Rare cases of squamous cell carcinoma (SCC) and mixed tumors like adenosquamous carcinoma (ASC) and adenoneuroendocrine tumors have been reported as isolated case reports [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. ASC of the duodenum is an exceedingly rare neoplasm, with only a few cases described in the medical literature. In the majority of these cases, the tumor originates from the ampulla of Vater. Presentations of patients with duodenal cancer are highly variable and include nonspecific symptoms like abdominal pain, anemia, nausea, and vomiting [16]. We report a patient who presented with altered mental status and persistent nausea and vomiting. She was found to have ASC of the third segment of the duodenum (D3). To the best of our knowledge, this is the first reported case of duodenal ASC arising from the third duodenal segment (D3).
Case Summary
A 64-year-old woman with a history of poorly controlled diabetes mellitus (hemoglobin A1c of 9.3%) and suspected diabetic gastroparesis presented with a 4-week history of nausea, vomiting, bloating, epigastric pain, and a 2-day history of altered mental status. Laboratory testing revealed a blood glucose level of 756 mg/dl, anion gap of 21, β-hydroxybutyrate of 2.8 mmol/L, and positive urine ketones. She was diagnosed with diabetic ketoacidosis and managed accordingly with intravenous fluids and insulin infusion. Despite correction of the ketoacidosis, her symptoms persisted. Further history indicated that the nausea and vomiting had been progressively worsening over the past year. This was thought to be related to underlying gastroparesis, given her long–standing history of uncontrolled diabetes mellitus. An upper endoscopic evaluation performed a year ago was unremarkable. She was started on Metoclopramide with no symptomatic benefit. Contrast computed tomography demonstrated a distended stomach and proximal duodenum, with caliber change at the level of the third portion of the duodenum and minimal adjacent fat stranding (Fig. 1). The dilatation was further assessed with an upper GI barium series demonstrating a segmental constriction of D3/D4, causing high-grade obstruction (Fig. 2). An upper endoscopic evaluation demonstrated a stricture and mucosal abnormality in the third segment of the duodenum (D3), which was biopsied. At that time, a decision was made to initiate total parenteral nutrition to meet her nutrition needs. Pathology from the duodenum revealed duodenal mucosa with two small foci of SCC and adenocarcinoma (Fig. 3). Immunohistochemical staining of the SCC stained positive for p63, weakly CK5/6, and was focally positive for CDX2, and negative for CK20 and Moc31. The adenocarcinoma cells stained positive for CK7, focally positive for CK20, weakly positive for CDX2, and negative for p63 and CK5/6 (Fig. 4). Tumor cells from both foci stained negative for TTF-1 and ER. CD34 and D2–40 stains were positive in the endothelial lining of the lymph-vascular spaces, and no lymphovascular invasion of the tumor cells was seen. About 50% of the tumor cells of the adenocarcinoma and 8% of the tumor cells of the SCC stained positive for Ki67/Mib-1. These findings were consistent with a mixed adenocarcinoma/SCC. Tumor staging with computed tomography of the chest, abdomen, and pelvis revealed no evidence of metastatic disease or other primary malignancy. Locoregional staging with endoscopic ultrasound revealed three enlarged lymph nodes in the peripancreatic and periduodenal region. She underwent an exploratory laparotomy and was found to have peritoneal metastasis with encasement of the middle colic vein and superior mesenteric vein. Tumor staging was upgraded to unresectable advanced stage IV cancer. Curative intent was aborted, and a gastrojejunostomy with gastrostomy for decompression and a feeding jejunostomy were performed. Her postoperative course was prolonged and complicated by intractable vomiting resulting in worsening renal function and progressive malnutrition, severe obstructive jaundice (total bilirubin 8.8 mg/dL, conjugated bilirubin 6.4 mg/dL, and alkaline phosphatase 549 units/L) secondary to a localized stricture in the left hepatic duct. This was identified on endoscopic retrograde cholangio-pancreatography and treated by placement of one plastic biliary stent across the left hepatic duct and well into the left intrahepatic system. Despite improvement of liver enzymes, her debility worsened and she progressively declined. She was deemed to be a poor candidate for chemotherapy and was eventually discharged on home hospice.
Fig. 1.
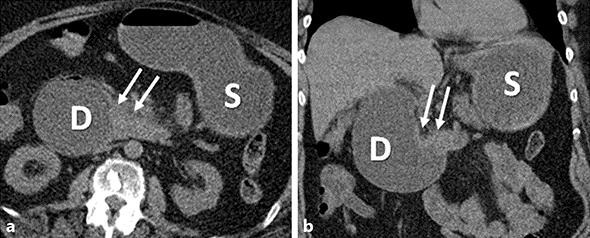
Axial (a) and coronal (b) noncontrast CT images demonstrate asymmetric thickening of the wall of the third part of the duodenum (arrows), resulting in obstruction and marked distention of the stomach (S) and the proximal duodenum (D).
Fig. 2.
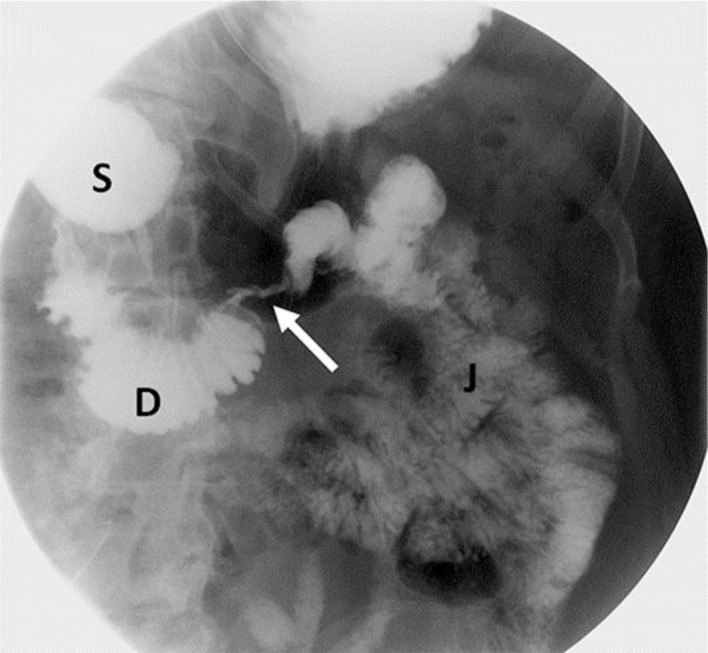
A spot image of an upper GI examination demonstrating severe constriction (arrow) at the third/fourth part of the duodenum, showing opacification of an irregular and narrowed lumen. The stomach (S) and the portion of the duodenum (D) proximal to the stricture are distended. The portion of duodenum distal to the stricture and jejulum (J) are normal.
Fig. 3.
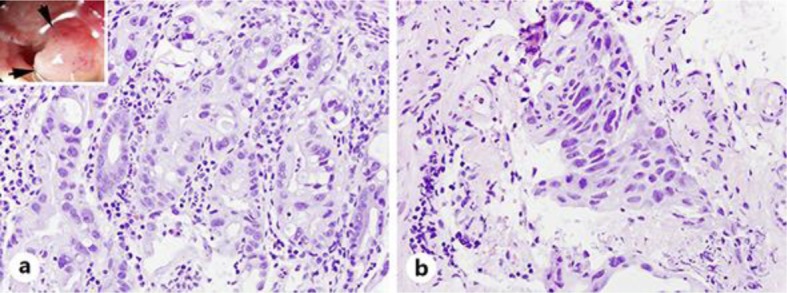
Microscopically, the tumor was composed of adenocarcinoma (a) and squamous cell carcinoma (b). HE. ×400.
Fig. 4.
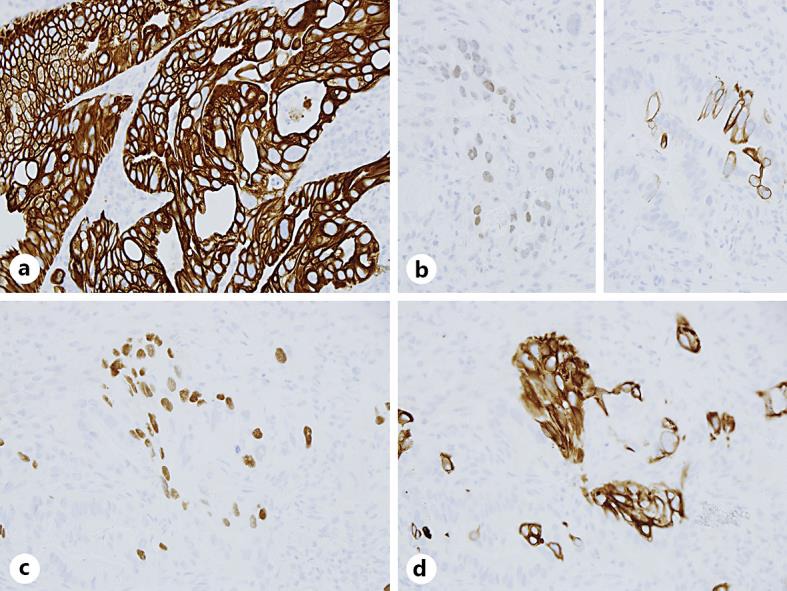
Immunohistochemistry of the adenocarcinoma (AdCa) and squamous cell carcinoma (SCC) components. The tumor cells of AdCa are strongly and diffusely positive for CK7 (a), and focally positive for CDX2 (b, left) and CK20 (b, right). The SCC cells are positive for P63 (c) and CK5/6 (d). a–d ×400.
Discussion
ASC of the duodenum is an exceptionally rare tumor. Histologically, it is composed of a variable combination of glandular architecture in the form of acini/papillae (adenocarcinoma) and squamous architecture in the form of keratinization and intercellular bridges (SCC). Published literature suggests that duodenal adenocarcinomas are largely positive for gastro-pancreatobiliary markers (CK7), and less commonly positive for intestinal markers (CK20, CDX2) [17], similar to the findings in our case. Typical markers to characterize SCC are p63 and CK5/6 [18]. Our patient's tumor cells stained positive for these markers, confirming the SCC component. This, beyond doubt, proves the mixed ASC nature of the tumor described in our case report.
The origin of SCC in the duodenum is debatable, especially since the duodenal mucosa is composed of glandular epithelium with no squamous tissue. There are a few hypotheses to explain this observation: (1) presence of pluripotent stem cells in the mucosa capable of transforming into both adenocarcinoma and SCC components, (2) squamous metaplasia in the intestinal mucosa, and (3) squamous transformation of the adenocarcinoma cells [19]. Bile has been postulated as a potential carcinogen, provoking malignant transformation in the duodenal mucosa, as about 57% of all adenocarcinoma of the small intestine is found in the D2 segment, which is less than 1% of the length of small intestine [20]. Furthermore, evidence from previous studies suggests that the SCC components grow more aggressively than their adenocarcinoma counterparts, as demonstrated by the short doubling time of SCC cells [4]. Thus, the extent of SCC presence in the ASC tumor may be related to the overall tumor progression and can have prognostic implications.
Only 7 cases of primary ASC of the ampulla of Vater [4, 5, 6, 19], 8 cases of primary SCC of the duodenum [7, 8, 9, 10, 11, 12, 13], and 2 cases of primary SCC of the ampulla of Vater [14, 15] have been reported in the medical literature. Clinical characteristics, treatment, and prognosis of all these cases are summarized in Table 1. Based on the reported cases, it appears that ASC and pure SCC of the duodenal region affect both males and females, but the reported number of male cases exceeds female cases by about 2: 1. The majority of these tumors originated from the ampulla of Vater; thus, the most common clinical presentation includes abdominal pain, jaundice, nausea, and vomiting. Surgical resection of the tumor was the predominant treatment modality, but unfortunately the mortality remained very high, approaching 50% within 12 months and 75% within 24 months of diagnosis.
Table 1.
Clinical characteristics, treatment, and prognosis of all reported cases of ASC and SCC of the small bowel
| First author [Ref]., year | Age, years/gender | Symptoms | Pathology | Treatment | Prognosis, months |
|---|---|---|---|---|---|
| Ueno [10], 2002 | 47/man | Fatigue, jaundice | ASC of AmV | PD | 10 (dead) |
| Yang [7], 2013 | 82/man | Jaundice | ASC of AmV | Ampullectomy | 14 (dead) |
| Yang [7], 2013 | 68/man | RUQ pain, jaundice | ASC of AmV | PD | 7 (dead) |
| Yang [7], 2013 | 34/woman | RUQ pain, jaundice | ASC of AmV | PD | 10 (dead) |
| Yang [7], 2013 | 77/man | RUQ pain, jaundice | ASC of AmV | PD | 6 (dead) |
| Kshirsagar [11], 2014 | 58/man | Abdominal pain, jaundice, vomiting, anorexia | ASC of AmV | PD | Not mentioned |
| Hoshimoto [9], 2015 | 81/woman | Asymptomatic elevation of liver enzymes | ASC of AmV | Pylorus-preserving PD | 20 (alive) |
| Friedman [12], 1986 | 61/man | Abdominal pain, weight loss | SCC of D3 | Partial duodenectomy and duodeno jejunostomy | 4 (alive) |
| Delius [13], 2006 | 75/woman | Upper GI bleeding | SCC of D1 | Not mentioned | Not mentioned |
| Diffaa [14], 2012 | 60/woman | Epigastric pain, melena, weight loss | SCC of D3 | Palliative chemotherapy | 1 (dead) |
| Battal [15], 2015 | 39/man | Epigastric pain, weakness, vomiting | SCC of D3 | Surgical resection of duodenal diverticulum harboring SCC | 10 (alive) |
| Terada [16], 2010 | 75/man | Vomiting, weakness | SCC of D2 | Chemoradiation | 17 (died) |
| Terada [17], 2009 | 58/woman | Abdominal pain | SCC of D2 | Chemoradiation | 21 (died) |
| Terada [17], 2009 | 54/man | Abdominal pain | SCC of D2 | Not mentioned | Not mentioned |
| Pahl [18], 2012 | 65/man | Epigastric pain, weakness | SCC of D3 | PD | 60 (died) |
| Gupta [19], 2009 | 28/woman | Abdominal pain, jaundice, vomiting | SCC of AmV | PD | Not mentioned |
| Bolanaki [20], 2014 | 68/man | Jaundice, fatigue | SCC of AmV | PD | 5 (died) |
PD, pancreaticoduodenectomy; AmV, ampulla of Vater.
However, no reports of primary ASC originating from the duodenal mucosa were found. Also, we describe the first case of a primary mixed ASC of the third part of the duodenum (D3) presenting with nonspecific symptoms of nausea and vomiting and unfortunately with high metastatic potential and a dismal prognosis. Little is known about the pathogenesis and natural history of this disease, given the rarity of this malignancy. Approach and treatment guidelines remain unestablished. The majority of patients reported presented with jaundice and abdominal pain with survival of about 12 months after diagnosis. General consensus regarding the treatment of these infrequent cancers is surgical resection of the tumor with negative margins, irrespective of the histology. Postoperative chemotherapy and radiotherapy should also be considered especially in tumors with a squamous component, as it confers a worse prognosis. Unfortunately, specific details of the management remain unelucidated given the rarity of these tumors.
In summary, we present the first case of a primary mixed ASC of the duodenum specifically originating from segment D3. Given the rareness of the pure SCC and mixed ASC of the duodenum, very limited information exists in the medical literature regarding the clinicopathological features and ideal management strategies. Further cases of these uncommon cancers need to be identified and reported for better pathological and clinical understanding of these tumors, as well as to gain more insight into different treatment strategies and their overall outcome on the prognosis.
Statement of Ethics
Consent for publication was obtained from the patient.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, Longo WE. Small-bowel tumors: epidemiologic and clinical characteristics of 1,260 cases from the Connecticut Tumor Registry. Arch Surg. 2007;142:229–235. doi: 10.1001/archsurg.142.3.229. [DOI] [PubMed] [Google Scholar]
- 2.Goldner B, Stabile BE. Duodenal adenocarcinoma: why the extreme rarity of duodenal bulb primary tumors? Am Surg. 2014;80:956–959. [PubMed] [Google Scholar]
- 3.Reynolds I, Healy P, Mcnamara DA. Malignant tumours of the small intestine. Surgeon. 2014;12:263–270. doi: 10.1016/j.surge.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Hoshimoto S, Aiura K, Shito M, Kakefuda T, Sugiura H. Adenosquamous carcinoma of the ampulla of Vater: a case report and literature review. World J Surg Oncol. 2015;13:287. doi: 10.1186/s12957-015-0709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno N, Sano T, Kanamaru T, Tanaka K, Nishihara T, Idei Y, Yamamoto M, Okuno T, Kawaguchi K. Adenosquamous cell carcinoma arising from the papilla major. Oncol Rep. 2002;9:317–320. [PubMed] [Google Scholar]
- 6.Kshirsagar AY, Nangare NR, Vekariya MA, Gupta V, Pednekar AS, Wader JV, Mahna A. Primary adenosquamous carcinoma of ampulla of Vater – a rare case report. Int J Surg Case Rep. 2014;5:393–395. doi: 10.1016/j.ijscr.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman E, Kwan MR, Cummins L. Squamous cell carcinoma of the transverse duodenum. Gastrointest Endosc. 1986;32:99–101. doi: 10.1016/s0016-5107(86)71766-5. [DOI] [PubMed] [Google Scholar]
- 8.von Delius S, Lersch C, Neu B, Huber W, Eckel F, Pitzl H, Fend F, Gaa J, Schmid RM. Squamous-cell carcinoma of the duodenum as a rare cause of upper gastrointestinal bleeding. Endoscopy. 2006;38:956. doi: 10.1055/s-2006-925161. [DOI] [PubMed] [Google Scholar]
- 9.Diffaa A, Samlani Z, Narjis Y, et al. The primitive squamous cell carcinoma of the third duodenum. Gastroenterol Insights. 2012;4:22–23. [Google Scholar]
- 10.Battal M, Bostancı O, Basak T, Kartal K, Ekiz F. Pure squamous cell carcinoma of the duodenum. Case Rep Surg. 2015;2015:714640. doi: 10.1155/2015/714640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terada T. Primary pure squamous cell carcinoma of the duodenum: a case report. Gastroenterol Res. 2010;3:39–40. doi: 10.4021/gr2010.01.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terada T. Primary pure squamous cell carcinoma of the duodenum: report of three cases. Endoscopy. 2009;41((suppl 2)):E329–E330. doi: 10.1055/s-0029-1214940. [DOI] [PubMed] [Google Scholar]
- 13.Pahl KS, Blount AL, Bedolla GM, Moore CR, Eichhorn MG, Paulson JE. Squamous cell carcinoma of the duodenum: an exceedingly rare diagnosis. Am Surg. 2012;78:E498–E500. [PubMed] [Google Scholar]
- 14.Gupta A, Kumar S, Gupta S, Makhija M, Singh B. Primary squamous cell carcinoma of the ampulla of Vater – a rare entity. Internet J Surg. 2009;22 [Google Scholar]
- 15.Bolanaki H, Giatromanolaki A, Sivridis E, Karayiannakis AJ. Primary squamous cell carcinoma of the ampulla of Vater. JOP. 2014;15:42–45. doi: 10.6092/1590-8577/1649. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Cui Y, Zhong B, Xiao W, Gong X, Chao K, Chen M. Clinicopathological characteristics and survival analysis of primary duodenal cancers: a 14-year experience in a tertiary centre in South China. Int J Colorectal Dis. 2011;26:219–226. doi: 10.1007/s00384-010-1063-x. [DOI] [PubMed] [Google Scholar]
- 17.Xue Y, Vanoli A, Balci S, Reid MM, Saka B, Bagci P, Memis B, Choi H, Ohike N, Tajiri T, Muraki T, Quigley B, El-Rayes BF, Shaib W, Kooby D, Sarmiento J, Maithel SK, Knight JH, Goodman M, Krasinskas AM, Adsay V. Non-ampullary-duodenal carcinomas: clinicopathologic analysis of 47 cases and comparison with ampullary and pancreatic adenocarcinomas. Mod Pathol. 2017;30:255–266. doi: 10.1038/modpathol.2016.174. [DOI] [PubMed] [Google Scholar]
- 18.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24:1348–1359. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 19.Yang SJ, Ooyang CH, Wang SY, Liu YY, Kuo IM, Liao CH, Wu TJ. Adenosquamous carcinoma of the ampulla of Vater – a rare disease at unusual location. World J Surg Oncol. 2013;11:124. doi: 10.1186/1477-7819-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross RK, Hartnett NM, Bernstein L, Henderson BE. Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Br J Cancer. 1991;63:143–145. doi: 10.1038/bjc.1991.29. [DOI] [PMC free article] [PubMed] [Google Scholar]


