Abstract
Stereoelectroencephalography (SEEG) is a method for invasive study of patients with refractory epilepsy. Localization of the epileptogenic zone in SEEG relied on the hypothesis of anatomo-electro-clinical analysis limited by X-ray, analog electroencephalography (EEG), and seizure semiology in the 1950s. Modern neuroimaging studies and digital video-EEG have developed the hypothesis aiming at more precise localization of the epileptic network. Certain clinical scenarios favor SEEG over subdural EEG (SDEEG). SEEG can cover extensive areas of bilateral hemispheres with highly accurate sampling from sulcal areas and deep brain structures. A hybrid technique of SEEG and subdural strip electrode placement has been reported to overcome the SEEG limitations of poor functional mapping. Technological advances including acquisition of three-dimensional angiography and magnetic resonance image (MRI) in frameless conditions, advanced multimodal planning, and robot-assisted implantation have contributed to the accuracy and safety of electrode implantation in a simplified fashion. A recent meta-analysis of the safety of SEEG concluded the low value of the pooled prevalence for all complications. The complications of SEEG were significantly less than those of SDEEG. The removal of electrodes for SEEG was much simpler than for SDEEG and allowed sufficient time for data analysis, discussion, and consensus for both patients and physicians before the proceeding treatment. Furthermore, SEEG is applicable as a therapeutic alternative for deep-seated lesions, e.g., nodular heterotopia, in nonoperative epilepsies using SEEG-guided radiofrequency thermocoagulation. We review the SEEG method with technological advances for planning and implantation of electrodes. We highlight the indication and efficacy, advantages and disadvantages of SEEG compared with SDEEG.
Keywords: intracranial EEG, stereoelectroencephalography, indication, efficacy, epilepsy surgery
Introduction
The term “stereoelectroencephalography” (SEEG) is referred to by Bancaud and Talairach, who have made extensive use of intracranial recording with stereotactically implanted electrodes. 1–4) The SEEG method was popularized in France during the 1950s. Thereafter, it has been used in France, Italy, and Canada for invasive evaluation of patients with refractory focal epilepsy. 1–7)
The original SEEG technique consisted of a multiphase and complex method, using the Talairach stereotactic frame and the double grid system, while patients were under general anesthesia. The approach was based on traditional anatomical data, i.e., ventriculography and catheter angiography. 1–4) The depth electrodes were inserted most often orthogonally (laterally) with the usual targets of mesial temporal and mesial frontal regions, and deep, extratemporal regions. 4,8)
The original SEEG method needs a tailored and individualized strategy, and it is difficult to establish standardized placements. 1) Despite its clinical use for almost 60 years, the technical complexity, e.g., instruments for the placement of depth electrodes may have limited widespread application of this technique in epilepsy centers outside Europe. 9)
Recently, computer technology has advanced methodology and clinical use of SEEG to reduce such complexity and improve safety in several ways, including the use of implantation devices and multimodal neuroimaging integration. 4,5,9,10)
This literature-based review describes the SEEG method with technological advances for planning and implantation of electrodes. In addition, we highlight the indication and efficacy, advantages and disadvantages of SEEG compared with subdural grid electroencephalography (EEG(SDEEG)).
Definition of the Epileptogenic Zone
The “epileptogenic zone” (EZ) is a theoretical concept for epilepsy surgery. The EZ has been defined as the cortical area that generates seizures and needs to be removed to render the patient seizure free. 11) (Table 1) Besides essential analysis of seizure semiology, video-EEG monitoring and magnetic resonance image (MRI), noninvasive diagnostic tools such as single photon emission computed tomography (SPECT), and fluorodeoxyglucose-positron emission tomography (FDG-PET) are also used for evaluation of EZ. 12) Magnetoencephalography (MEG) has increasingly been used as a method complementary to other tools for localizing the EZ. 13)
Table 1.
Definitions of epileptic zones in SEEG interpretation compared with cortical zones determined by modern noninvasive tools
| Definitions of cortical zones of epileptic abnormality determined by noninvasive toolsa | Definition of epileptic zones in SEEG interpretationb | ||||
|---|---|---|---|---|---|
| Cortical zones | Tools | Epileptic zones | Findings of SEEG | ||
| Epileptogenic lesion | MRI | Lesional zone | Permanent slow background activities | ||
| PET* | |||||
| SPECT* | |||||
| Irritative zone | Video-EEG monitoring, MEG | Irritative zone | Abnormal interictal paroxysmal activities | ||
| Seizure onset zone | Video-EEG monitoring (MEG, ictal SPECT) | Epileptogenic zone | Primary organization of ictal discharge The frequency spectrum as well as the interareal synchronization at seizure onset constitute a pattern of organization that is reproducible from one seizure to the others May be triggered by stimulation. | ||
| Symptomatogenic zone | Video-EEG monitoring (Ictal SPECT) | ||||
| Epileptogenic zone (EZ) | Theoretical concept | ||||
| Functional deficit zone | PET SPECT |
||||
In consideration of the SEEG indication, it should be recognized that the principle of SEEG methodology is based on the anatomo-electro-clinical (AEC) “hypothesis” requiring conceptualization of the 3D spatiotemporal organization of the epileptic discharge within the brain. 14–16) In this hypothesis, the ictal EEG changes must be recorded at the very point where they occur (anatomo-electrical relationships), and their initial or secondary impact on the clinical picture (electro-clinical relationships) can be evaluated as the discharge spread. 14,15)
The SEEG has the own concept of epileptic zones from their findings. 17) (Table 1). The concept of the epileptogenic lesion underlying SEEG methodology was proposed by Talairach and Bancaud in the pre-MRI era, with a different emphasis. 1,14,15,18) The epileptogenic lesion was divided into “three zones; 1, lesional zone; 2, irritative zone; 3, epileptogenic zone” in SEEG interpretation. 17) A “lesional zone” is defined as the site(s) of permanent slow background activity, independent of seizure recurrence. The disturbance of background activity may imply a macroscopic alteration of the neural tissue. 19) This area coincides in many instances with the epileptogenic lesion of Rosenow and Lüders 11) which is most often revealed on MR images. 19) An “irritative zone” is defined as the site(s) of abnormal interictal paroxysmal activities. They rarely appear as the only focal but rather tend to spread within cortico-cortical networks. An “epileptogenic zone” is defined as the site(s) of primary organization of ictal discharge. This means that the frequency spectrum as well as the interareal synchronization at seizure onset constitute a pattern of organization that is reproducible from one seizure to the other and which may be triggered as a whole by stimulation. SEEG is so precise in localization that if these conditions are not met it follows that either the electrodes have not been optimally placed or that there is more than one epileptogenic zone. This definition fundamentally differs from the definition of “seizure onset zone”, which is mainly based on absolute latency between the discharge onset in different sites in one given seizure, whatever the frequency pattern. In this definition, their epileptogenic zone does not equate to the region of cortex that needs to be removed. 14,18)
SEEG Method and Technological Advancement
Depth electrodes for SEEG
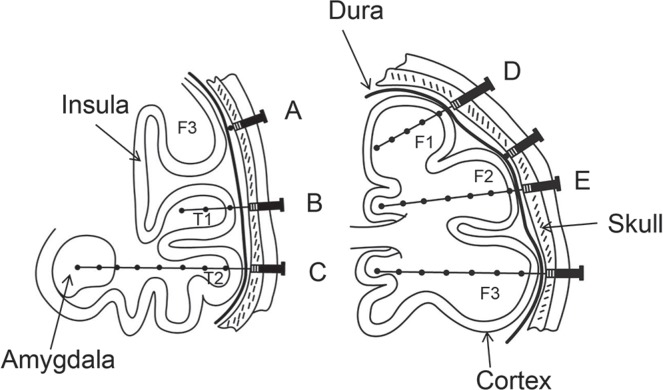
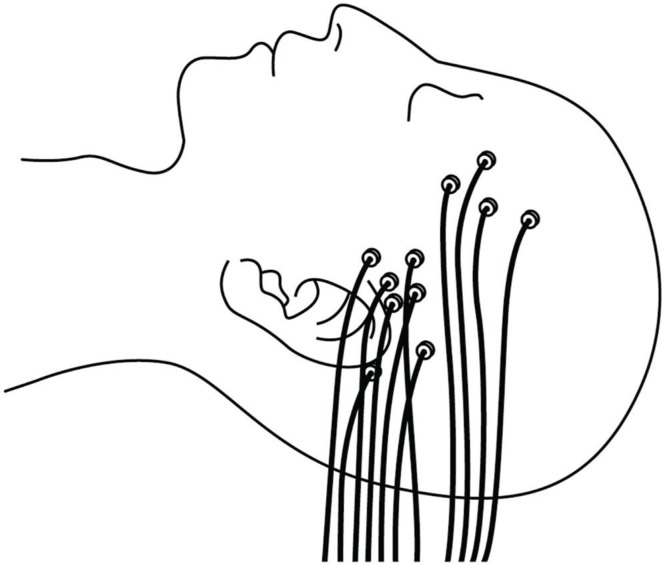
Depth electrodes typically used for SEEG have 4–18 contacts spaced 2–10 mm apart and a diameter of 1 mm or less. The electrodes are either semirigid or flexible with a rigid stylet, which can be removed upon insertion. 14,20) They are most often inserted orthogonally through a standard burr hole or a twist drill hole. 20) Electrodes can be inserted parasagittally. 8) Percutaneous drilling and a screwing bolt device have been developed for convenient fixation of depth electrodes (Fig. 1). This device has the advantage of avoiding the need for a burr hole, which is a time-consuming procedure. 2,4,21) The total number of electrodes inserted varies between epilepsy treatment centers, and has been reported at up to 22 per patient (rarely exceeds 15) 2,8,22) (Fig. 2). Epidural peg electrodes with a mushroom-shaped single contact are also used for cortical discharges via the dura matter 4,20) (Fig. 1). The position of the electrodes is reconstructed using computed tomography (CT) superimposed on MRI, or directly visualized on MRI if the electrodes are MRI compatible. 20)
Fig. 1.
A schema illustrating the concept of SEEG (modified from reference 4). Left, Electrode A is an epidural peg electrode that records from the surface of a gyrus. Electrodes B and C are multicontact electrodes that can be used to record not only from the deep structure (e.g., amygdala), but also from the ribbon of the cortex around the sulci and convexity. Right, A schema illustrating the concept of SEEG showing coverage of the frontal lobe with surface and deep areas (e.g., intragyri, D and cingulate, E gyrus). (F1, superior frontal gyrus; F2, middle frontal gyrus; H3, inferior frontal gyrus; T1, superior temporal gyrus; T2, middle temporal gyrus; SEEG, stereoelectroencephalography).
Fig. 2.
A schema illustrating the final aspect of SEEG implantation. (SEEG, stereoelectroencephalography).
Standard frame-based SEEG technique
A standard frame-based device, e.g., Todd Wells, BRW, or Lexell frame, is mostly used for depth electrode implantation. 2,8,23,24) According to the previous Montreal Neurological Institute method using a stereotactic frame, 4) the procedure for electrode placement is conducted in three stages: Stage I, stereotactic localization, consisting of imaging the brain (angiography and stereotactic MRI with fiducial marker plates in each) with a stereotactic frame affixed to the head; Stage II (usually done the next day), consisting of integrating digital angiography and MRI scans for the selection of multiple target sites; and Stage III, consisting of implantation of electrodes after repositioning of the stereotactic frame.
In modern practice, some centers have introduced a simplified workflow with implantation of depth electrodes based on a three-dimensional (3D) gadolinium-enhanced MRI dataset with or without CT angiography. The vasculature data are needed to avoid electrodes crossing blood vessels. 10,20,23,25) In the Cleveland Clinic Foundation, before their frameless implementation, the planning and implantations are performed in a single stage, where on-table stereotactic CT scans and 3D digital subtraction angiography are performed in a Lexell frame, and the preoperative MR images are fused and used during the implantation procedure. 2)
The advantage of a frame-based technique is the accuracy of electrode delivery to a predefined target with < 3 mm accuracy. 5,10) Cardinale et al. have reported a median target error of 2.69 mm (an interquartile range: 1.89–3.67 mm) with a traditional Talairach frame-based method in 37 SEEG procedures with 517 electrodes. 5)
There are several disadvantages of the frame-based technique, which include potential patient discomfort, additional time for frame placement, restricted access to the surgical field, and a limited ability to define new trajectories in real time during surgery. 10)
Frameless SEEG technique
Neuronavigation is widely used for diagnostic biopsies, implantation of electrodes for deep brain stimulation, and depth electrodes for SEEG. 26,27) Frame-based neuronavigation has recently been replaced by frameless techniques. 4,9,10,21,27,28) The advantages of the frameless techniques are their ease of use, more flexible preoperative planning, and reduced patient discomfort compared with frame-based techniques. 27,29) Dorfer et al. have developed a frameless stereotactic drilling technique, a bone-fixated Guide for Implantation of Depth Electrodes (GIDE), to combine the frameless method with the convenient implantation and fixation of the depth electrodes via a percutaneous bolt. 21)
The quantitative accuracy of the frameless method may reach targeting point errors, and clinical results are comparable with standard frame-based methods. 29) Widmann et al. have reviewed previous studies using frameless methods and reported its high rate (96%–100%) of clinical success (diagnostic yield). 29) The mean targeting errors have been reported as ranging from 2.0–3.2 mm. 29) Three studies of the frameless stereotactic drilling technique reported accuracies of 3.0, 3.5, and 3.6 mm (> 3.0 mm) respectively, which are relatively inferior to those obtained using the conventional, frame-based technique. 10,21,27)
Robot-assisted devices
A robot-assisted device has been applied to both frame-based and frameless SEEG methods. 5,9,30) This technique has even been applied to different areas, e.g., deep brain stimulation and stereotactic surgery. 5,9,30–33) Compared with manual techniques, robotic devices increased the accuracy of the target point to < 2 mm. Cardinale et al. have reported the accuracy after introducing a robot-assisted device (NeuroMate, Reinshaw; Wotton-under-Edge, Glos., United Kingdom) combined with a Talairach frame-based method. 5) The median target error was 1.77 mm with an interquartile range (IQR): 1.25 to 2.51 mm in 81 SEEG procedures with 1050 electrodes. 5) Gonzalez-Martinez et al. reported a similar accuracy (median, 1.7 mm; IQR, 1.2 to 2.3 mm) in their 101 robot-assisted (ROSA, Medtech, Montpellier, France), frameless SEEG procedures. 9) They performed all SEEG procedures guided by the robotic device. The accuracy (1.2 mm) at the entry point was reported to be as high as that (1.1 mm) in frame-based SEEG procedures. 9,34)
The greatest benefit of the robot-assisted, frameless method is a shorter time of implantation. 9,35) Gonzalez-Martinez et al. compared some parameters, i.e., electrode number implanted, accuracy of target point morbidity, and time of implantation, between their former frame-based and robot-assisted frameless SEEG procedures. 9) The robot-assisted implantations were approximately 3.5 h shorter than the fame-based implantations although the other parameters were comparable. 9) Dorfer et al. reported a mean time of 15.7 min for implantation of each electrode (a 20% reduction from their previous manual technique). 35)
An automated planning method using a multitrajectory automatic planner has been developed for SEEG electrode arrangement. 22,36) This method has been applied to robot-assisted frame-based SEEG. 22) The planning software computes the best trajectory configuration as maximizing the distance of the electrode from the vessels and avoiding the sulci and vessels as entry points. 22)
The use of robots, although very costly, is promising, and advantageous for its accuracy, safety, and simplicity without the need for numerous and time-consuming frame adjustments. 9,35)
Indication for SEEG
General indications for intracranial EEG
The overall aims of intracranial EEG are to define the EZ and to determine the location of the eloquent cortex in relation to the EZ, usually by cortical stimulation mapping. 14)
General indications for intracranial EEG have been defined as cases with normal imaging, presumably an extratemporal EZ, discordant findings in noninvasive examinations, proximity of the presumed EZ to the eloquent cortex (requiring cortical functional mapping), and certain findings and syndromes with a tendency to multiple lesions. 14,20)
Patients who have a nonlateralized and nonlocalized EZ based on all or mostly discordant results between seizure semiology and noninvasive tools are unlikely to undergo intracranial EEG. In such cases, particularly seen in MRI-negative cases, intracranial EEG is unlikely to provide additional information and the risks of the procedure outweigh the benefits. 14,19)
Clinical scenarios indicative for SEEG
SEEG can be applied to most general indications for intracranial EEG as with SDEEG. It also allows extensive coverage of both hemispheres without performing a large craniotomy. 19.20) By contrast, because of the limited spatial, noncontiguous, sampling of SEEG electrodes, and although the functional mapping is feasible with SEEG, its accuracy is generally more restricted than SDEEG, especially for mapping atypical representations of the eloquent cortex. 20)
Kovac et al. have outlined clinical scenarios in which either SEEG or SDE are more likely preferred with respect to the results of seizure semiology and noninvasive studies. 14) MRI-negative cases with a presumed EZ involving deep structures, even when not involved, but away from the eloquent cortex, are the most likely candidates for SEEG. Whereas cases with the presumed EZ close to the eloquent area, but not involving deep structures, are the most likely candidates for SDEEG, regardless of the MRI findings. In such cases, if the deep structures are also involved, a few number of depth electrodes can be added with subdural grid electrodes through the same craniotomy. 14,20,37) SEEG is also applicable for cases with deep-seated lesions, away from the eloquent cortex, although intracranial EEG itself may not be needed. Previously failed SDEEG, multilobar epilepsy, or presumed EZs in both hemispheres are also included in scenarios preferred for SEEG. 9,14) However, multilobar epilepsy, lateralized to a single hemisphere following SEEG may be preferred to SDEEG because of the high possibility of eloquent cortex involvement in such cases.
SEEG for temporal lobe
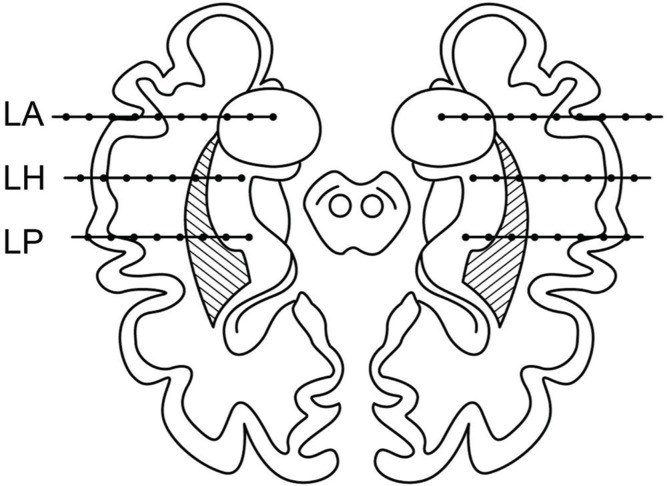
In temporal lobe epilepsy (TLE), intracranial EEG is usually not indicated when presurgical clinical and noninvasive results are concordant with unilateral temporal lobe EZ. 38,39) However, SEEG is indicated if the seizure onset is not lateralized, when bilateral TLE is suspected as a result of incongruent noninvasive data 4,9,40) (Fig. 3). SEEG is also helpful to differentiate mesial TLE from lateral TLE and vice versa, particularly when limited resection is indicated. 41) However, basal temporal regions are more difficult to sample using SEEG than SDEEG or strips. 14) Temporal “plus” epilepsy, previously identified among patients suffering from “atypical” nonlesional TLE, has been well examined by SEEG. 19,42) This term refers to a specific form of multilobar epilepsy, which is characterized by the involvement of a complex epileptogenic network including the temporal lobe and the neighboring structures such as the orbitofrontal cortex, the insula, the frontal and parietal operculum, and the temporoparietooccipital junction. 42) Barba et al. compared the clinical and scalp EEG characteristics of patients proven to have purely TLE (n = 58) with temporal “plus” epilepsy (n = 22). Excellent seizure outcome (Engel’s class I) was found in 51 (88%) patients with TLE and 16 (73%) patients with temporal “plus” epilepsy after resection tailored according to the SEEG results. 42)
Fig. 3.
Typical placement of SEEG electrodes within the limbic structures of both temporal lobes in bitemporal epilepsy (modified from reference 4). Electrode LA passes through the left middle temporal gyrus, and its tip is inserted in the core of the amygdala. Electrode LH passes through the left middle temporal gyrus and is inserted in the anterior part of hippocampus. Electrode LP also passes through the middle temporal gyrus and reaches either the posterior part of the hippocampus or the parahippocampal gyrus. (SEEG, stereoelectroencephalography; LA, left amygdala; LH, left hippocampus, LP, left parahippocampal gyrus).
SEEG for Extratemporal Lobes
Frontal lobe
Patients with frontal lobe epilepsy (FLE) are good candidates for SEEG to lateralize or localize the EZ. 19) Identification of the EZ in patients with FLE may be challenging, particularly in cases without a visible MRI lesion, and sometimes, even in cases when a lesion can be seen on MRI. 43) This may be explained by the large size of the frontal lobe, the complexity of the functional network involved in the generation of frontal lobe seizures, the related different clinical ictal patterns, and the frequent absence of definitely informative ictal and interictal EEG correlates. 44–46) The frontal pole, the mesial premotor cortex, the dorsolateral frontal cortex, the orbitocingulate region, and even outside of the frontal lobe in the temporal lobe structures or in the insula can be adequately sampled by SEEG. 19,47)
Nobili et al. described the clinical, electrophysiological, neuroradiological, and histological findings and the surgical results in 21 patients (10 patients without any relevant abnormality on MRI) who underwent surgery for nocturnal FLE. 43) Eighteen of the 21 patients underwent SEEG evaluation. Sixteen of the 21 patients were completely seizure-free since surgery with > 1 year postoperative follow-up. 43)
Posterior quadrant region
As with FLE, seizures from the posterior neocortex (posterior temporal junction and parietal and occipital lobe structures) have a heterogeneous semiology because of their high connectivity facilitating rapid and widespread propagation of an epileptic discharge even beyond the areas of the posterior quadrant. 19,48)
Typical targets for depth electrode insertions are the calcarine and pericalcarine cortices (in occipital lobe epilepsy); the inferior and superior parietal lobules and the posterior cingulate cortex (in parietal lobe epilepsy), so-called junction territories, such as the lingual lobule and fusiform gyrus, the angular and supramarginal gyri, and the precuneus and posterior insular cortex, can also be targets. 19,48) Occipital pole regions may not be well sampled by SEEG because of its inherently limited spatial sampling. 49) One article described MEG spike-locked SEEG analysis during simultaneous MEG and SEEG recording. 49) MEG demonstrated spike sources (reconstructed based on a moving dipole approach) within the occipital pole, located posteriorly to the SEEG electrodes presenting the maximal number of spikes, where not explored by the SEEG.
In studies of occipital lobe epilepsy, the success rate of surgery is lower compared with that of TLE and may range from 25% to 90% depending on the report. 48,50) Marchi et al. studied 29 patients with occipital and occipital “plus” epilepsies to aim at delineating the organization of neural networks of seizures using methods for SEEG quantification. Surgery was undertaken in 18 of 29 patients, leading to seizure freedom in 55%. SEEG revealed the majority of patients had a widespread EZ organization beyond the occipital lobe and a high prevalence of bilateral EZ organization. 48) In a series of 62 pediatric surgical cases in the posterior cortex, 58 patients (94%) were MRI-positive and 24 of 62 patients (39%) underwent subsequent SEEG for further EZ localization. After surgery, 53 patients (86%) remained seizure-free, and among those who underwent a SEEG procedure, 75% achieved seizure freedom. 51)
Insular lobe
The insula is one of the best targets for SEEG. 9,20) This structure is buried and localized deep in the brain and inaccessible to subdural grid or even strip electrodes. Electrodes can be safely inserted using a lateral orthogonal trajectory through the frontoparietal and temporal operculum, 52) or to obtain a larger sampling of the insula, using oblique trajectories through the frontal or parietal cortices. 53–55)
Insular seizures may mimic or coexist with seizures of temporal, frontal, or perisylvian epilepsy. 47,52,56,57) Furthermore, Aghakhani et al. suspected the contribution of the insula in 6 patients with parietal lobe- and/or temporal lobe-like epilepsies who failed parietal lobe and temporal lobe surgeries. 58) This may be explained by the localization of the insula and its dense connectivity with the surrounding regions.
Insular cortex surgery has previously been considered to be less feasible because of the difficulty in its EZ localization and the surgical risk because of its deep location and dense vascularization. Recently, several studies using SEEG revealed successful EZ localization and resection with satisfactory results in insular-related epilepsy. 59,60) Dylgjeri et al. evaluated 10 children with insular and insulo-opercular epilepsy who had undergone SEEG, followed by individually tailored resection that included part of the insula in all cases. In 8 patients, the tailored resection included a lesion. In 7 patients, an Engel class I outcome was obtained. 59) The EZ typically included the adjacent neocortex, and resection of these areas is required to achieve good seizure outcome. 59) Combined depth and subdural grid electrodes can be also used safely to investigate the widespread EZ in complex insular–opercular or perisylvian epilepsies with a comparable seizure outcome. 60–62)
SEEG for MRI-negative partial epilepsy
Surgical treatment for MRI-negative epilepsy is a challenge. Recently, multimodal neuroimaging tools such as MEG or FDG-PET or ictal SPECT are complementally used to assist in the presurgical localization of the EZ, before performing intracranial EEG. 13,63,64) The current integration technique of multimodal neuroimaging has narrowed down the hypothesized EZ for SEEG. Jung et al. have conducted an SEEG investigation in which sampling was guided by FDG-PET and MEG data in 21 patients with noncontributive MRI. 65) They used Volumetric Imaging of Epileptic Spikes (VIES), a new source modeling procedure for MEG analysis, which identifies spiking volume as the 3D region where sources of the high frequency activities (> 20 Hz) associated with epileptic spikes. For patients with a focal spiking volume on MEG analysis, the seizure-onset zone defined by SEEG was clearly localized in all cases and most patients (6/7, 86%) had a good surgical outcome. Conversely, SEEG failed to delineate a seizure-onset zone in the majority of patients with a lateralized or bilateral spiking volume. None of the patients who had nonfocal spiking volume and underwent operation became seizure-free. Most recently, Murakami et al. have studied correlations between MEG and SEEG findings in 50 patients, of whom a majority (n = 45) had completely negative or noncontributory MRI studies. 66) The strategy for SEEG implantation was based on a variety of diagnostic modalities including MRI, FDG-PET, subtraction ictal SPECT coregistered with MRI (SISCOM), and MEG. Among them, they tried to define the positive and negative predictors based on MEG dipole cluster characteristics (tightness and orientation) pertaining to seizure-freedom. 66) According to their results, patients with complete resection of the MEG clusters had a much higher chance of seizure freedom compared with those with only partial or no resection. Furthermore, patients had a significantly higher chance of being seizure-free when SEEG completely sampled the area identified by MEG compared with those with incomplete or no sampling of MEG results. MEG tight cluster and stable orientation were positive predictors of a good seizure outcome after resective surgery. Thus, MEG clusters can be good landmarks when planning the SEEG strategy.
Comparison of SEEG and SDE
Advantages of SEEG
Table 2 compares the advantages, disadvantages, and complications of SEEG and SDEEG. SEEG mainly targets deep structures with anatomical accuracy. The main advantage of SEEG is that it can sample all cortical areas without a large craniotomy as required for SDEEG, which adds to morbidity. 9,14,19,20) SEEG also allows extensive coverage of both hemispheres with less surgical risk. Exceptionally, certain locations such as basal temporal regions and probably occipital poles, are more difficult to sample using SEEG than SDEEG, which can add strip electrodes to cover the convexity of the frontal/occipital pole. 14,49) Reoperations requiring implants are safer with SEEG than SDEEG. 9,14)
Table 2.
Comparison of advantages, disadvantages, and complication rates between SEEG and SDEEG
| SEEG | SDEEG | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Complications (pooled prevalence from 2 meta-analysis 71, 74)) |
|
|
SEEG: stereoelectroencephalography, SDEEG: subdural grid EEG.
The SEEG electrodes can be inserted via a burr hole or percutaneous bolt and do not require a second operation for removal of the electrode as is the case with SDEEG. This means there is the capability of planning the craniotomy for the EZ resection after all data are analyzed. The resections are typically performed several months later. 14) In SDEEG, the removal of the electrode is usually combined with the resection of the presumed EZ. This approach necessitates quick interpretation of the SDEEG data, which is sometimes difficult in complicated epilepsies with a mixture of seizure semiologies.
SEEG is best suited to record all deep structures. Particularly, subcortical lesions such as nodular heterotopia poorly accessible to surgical resection are well sampled by SEEG for EZ investigation. SEEG-guided radiofrequency thermocoagulation (RF-TC) has been applied as a therapeutic alternative for patients with deep-seated lesions, e.g., nodular heterotopia or insular lesions in nonoperable drug-resistant partial epilepsies. 67–69) Among 89 patients who underwent SEEG-guided RF-TC in the EZ because of different pathologies, more favorable seizure outcomes were obtained in patients with nodular heterotopia. 67)
This procedure can be performed at the same time as the SEEG electrodes are removed with less risk, without additional bleeding or unnecessary anesthesia. 67,69)
Disadvantages of SEEG
SEEG recording can be more difficult to perform in very young children, less than 2–3 years old for technical reasons (e.g., thickness of the skull); 20,70,71) whereas SDEEG can be used safely in young children and is generally well tolerated, even in infancy. 20,70,72)
The greatest disadvantage of SEEG is the poor functional mapping because of its limited ability to record contiguous cortical regions. SDEEG can achieve meticulous contiguous functional mapping of the cortex because of the continuity of its high-density electrode-contacts. We note that a large number of SEEG electrode-contacts are not attached to the cortical surface. Although functional mapping using electrodes within the cortex is feasible with SEEG, its accuracy is generally more restricted than that of SDEEG, especially for mapping atypical representations of the eloquent cortex. 20) Several studies with a small number of cases have made an effort to reduce these limitations. 73,74) Enatsu et al. have reported a hybrid technique of SEEG and subdural strip electrode placement. 73) In this approach, SEEG electrodes were inserted with a robotic stereotactic system, and a skin incision and small craniectomy were performed at the entry point of the strip electrode trajectory. The strip electrodes were slid into the subdural space under real-time fluoroscopic guidance. Munyon et al. have proposed another new methodology called a 3D grid, which consists of a dense array of SEEG parallel electrodes in a rectangular pattern with 1 cm between entry sites as seen with subdural grids. 74) The 3D grid successfully identifies the location and extent of epileptic and eloquent areas, although this technique cannot be used as a “fishing expedition” because of limited targeting areas.
SDEEG can better delineate the interface between EZ and the surrounding cortex (non-EZ) than SEEG. In SEEG, borders of resection are determined by interpolation of most involved depth electrodes and anatomical borders. 14) Thus, the resection area theoretically may be larger than that determined by SDEEG. 14)
SEEG requires a more complicated workflow, i.e., data acquisition, instruments, and surgical procedures than SDEEG. Detailed imaging of the cerebral vasculature is required to avoid the electrode insertion-related complications, i.e., intracerebral hemorrhage. 10,23,25) This workflow may still include digital subtraction angiography as the gold standard to plan SEEG electrode trajectory in many centers. 14,23) Finally, there are still a limited number of centers where highly advanced techniques, such as frame-less neuronavigation or robot devices, are available to simplify the workflow in SEEG methodology.
Complications
In a recent meta-analysis of SEEG 75) , the pooled prevalence for all complications was low (1.3%). The major complications of SEEG were intracerebral hemorrhages (pooled prevalence 1.0%) and infections (pooled prevalence 0.8%). When compared with a recent meta-analysis of SDEEG related hemorrhagic complications (pooled prevalence 4.0%) 71) or (3.2%) in another review article, 76) the safety advantage of the SEEG methodology is significant compared with SDEEG. The morbidity reported using SEEG may vary from 0 to 7.5%, and is mostly related to hemorrhagic or infectious complications. 19,20,,75) Permanent neurological deficits accounted for 0.6% (pooled prevalence), a rate similar to that reported following SDEEG. 72,75) Mortality related directly to the procedure is rare, but can occur. 77) Serletis et al. reported mortality of 0.5% (1 per 200 patients) because of intracerebral hematoma directly ensuing from SEEG electrode placement. 77)
In one series of 215 SEEG implantations in 211 patients, morbidity related to electrode implantation occurred in 12 (5.6%) procedures, with severe permanent deficits from intracerebral hemorrhage in 2 (1%) patients. 25)
The major complications in SDEEG were intracranial hemorrhage (pooled prevalence 4.0%), infections (pooled prevalence 3.0%), and elevated intracranial pressure (pooled prevalence 2.4%). 72) In one series of 198 SDEEG monitoring sessions on 187 patients, one death (mortality, 0.5%) and 3 cases (morbidity, 1.5%) of permanent neurological deficits occurred. 78)
Conclusion
The literature-based review presented here attempts to describe optimal indications for the current SEEG in certain clinical scenarios compared with SDEEG. Both SEEG and SDEEG were widely used and can be combined. 14,20,37,73,79) There is no position of insisting on one particular intracranial EEG modality in all cases. The advantages and disadvantages of both modalities have prompted many epilepsy centers to use both as complementary approaches in individual patients. The current integration technique of multimodal neuroimaging has narrowed down the hypothesized EZ compared with that based on traditional AEC hypothesis. The role of SEEG has also changed from a kind of “fishing expedition” to more precise localization of the EZ. The low complication rate of the SEEG procedure has benefited for many patients particularly those with nonlesional MRI and bilateral epileptic networks. In such cases, SDEEG implantation as a second operation after obtaining lateralization with SEEG may be optional for further resection based on the precise functional mapping and delineation of the extent of resection.
Acknowledgments
We thank Dr. Rei Enatsu for his scientific advice.
Footnotes
Conflicts of Interest Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices mentioned in this article.
References
- 1).Bancaud J, Angelergues R, Bernouilli C, et al. : Functional stereotaxic exploration (SEEG) of epilepsy. Electroencephalogr Clin Neurophysiol 28: 85–86, 1970 [PubMed] [Google Scholar]
- 2).Gonzalez-Martinez J, Mullin J, Vadera S, et al. : Stereotactic placement of depth electrodes in medically intractable epilepsy. J Neurosurg 120: 639–644, 2014 [DOI] [PubMed] [Google Scholar]
- 3).Talairach J, Bancaud J, Bonis A, et al. : Surgical therapy for frontal epilepsies. Adv Neurol 57: 707–732, 1992 [PubMed] [Google Scholar]
- 4).Oliver A, Boling W: Stereotactic intracranial recording (stereoelectroencephalography). In Schmidek HH. (ed): Operative Neurosurgical Techniques, ed 4 Philadelphia, WB Saunders, 2000, pp. 1511–1528 [Google Scholar]
- 5).Cardinale F, Cossu M, Castana L, et al. : Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery 72: 353–366, 2013 [DOI] [PubMed] [Google Scholar]
- 6).Devaux B, Chassoux F, Guenot M, et al. Epilepsy surgery in France. Neurochirurgie 54: 453–465, 2008 [DOI] [PubMed] [Google Scholar]
- 7).Reif PS, Strzelczyk A, Rosenow F: The history of invasive EEG evaluation in epilepsy patients. Seizure 41: 191–195, 2016 [DOI] [PubMed] [Google Scholar]
- 8).Spencer SS, Sperling MR, Shewmon A, Kahane P: Intracranial electrodes. In Engel J, Jr, Pedley TA. (eds): Epilepsy: A Comprehensive Textbook ed 2 Philadelphia, Wolters Kluwer/Lippincott Williams & Wilkins, 2008, pp. 1791–1815 [Google Scholar]
- 9).González-Martínez J, Bulacio J, Thompson S, et al. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery 78: 169–180, 2016 [DOI] [PubMed] [Google Scholar]
- 10).Nowell M, Rodionov R, Diehl B, et al. A novel method for implementation of frameless stereoEEG in epilepsy surgery. Neurosurgery 10 Suppl 4: 525–534, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Rosenow F, Lüders H: Presurgical evaluation of epilepsy. Brain 124: 1683–1700, 2001 [DOI] [PubMed] [Google Scholar]
- 12).Iwasaki M, Jin K, Nakasato N, Tominaga T: Non-invasive evaluation for epilepsy surgery. Neurol Med Chir 6: 632–640, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Iida K, Hashizume A, Otsubo H: MEG and magnetic source imaging in MRI-negative refractory partial epilepsy. In So EL, Ryvlin P. (eds): MRI-negative Epilepsy: Evaluation and Surgical Management. Cambridge, Cambridge University Press, 2015, pp. 47–55 [Google Scholar]
- 14).Kovac S, Vakharia VN, Scott C, Diehl B: Invasive epilepsy surgery evaluation. Seizure 44: 125–136, 2017 [DOI] [PubMed] [Google Scholar]
- 15).Talairach J, Bancaud J: Stereotaxic approach to epilepsy methodology of anatomo-functional stereotaxic investigations. Progress in Neurological Surgery Basel, Karger; 1973, pp. 297–354 [Google Scholar]
- 16).González-Martínez J: Convergence of Stereotactic Surgery and Epilepsy: The Stereoelectroencephalography Method. Neurosurgery 62 Suppl 1: 117–122, 2015 [DOI] [PubMed] [Google Scholar]
- 17).Bulacio JC, Chauvel P, McGonigal A: Stereoelectroencephalography: Interpretation. J Clin Neurophysiol 33: 503–510, 2016 [DOI] [PubMed] [Google Scholar]
- 18).Kahane P, Landre E, Minotti L, Fransione S, Ryvlin P: The Bancaud and Talairach view on the epileptogenic zone: a working hypothesis. Epileptic Disord 8 Suppl 2: S16–S26, 2006 [PubMed] [Google Scholar]
- 19).Kahane P, Dubeau F: Intracerebral depth electrodes electroencephalography (stereoencephalography). In: Eversol JS, Husain AM, Nordli DR. (eds): Current Practice of Clinical Electroencephalography, ed 4 Philadelphia, PA: Wolters Kluwer, 2014. pp. 393–441 [Google Scholar]
- 20).Jayakar P, Gotman J, Harvey AS, et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia 57: 1735–1747, 2016 [DOI] [PubMed] [Google Scholar]
- 21).Dorfer C, Stefanits H, Pataraia E, et al. Frameless stereotactic drilling for placement of depth electrodes in refractory epilepsy: operative technique and initial experience. Neurosurgery 10 Suppl 4: 582–591, 2014 [DOI] [PubMed] [Google Scholar]
- 22).De Momi E, Caborni C, Cardinale F, et al. Multi-trajectories automatic planner for StereoElectroEncephaloGraphy (SEEG). Int J Comput Assist Radiol Surg 9: 1087–1097, 2014 [DOI] [PubMed] [Google Scholar]
- 23).Gilard V, Prust F, Gerardin E, et al. Usefulness of multidetector-row computerized tomographic angiography for the surgical planning in stereoencephalography. Diag Interv Imaging 97: 331–351, 2016 [DOI] [PubMed] [Google Scholar]
- 24).Merriam MA, Bronen RA, Spencer DD, McCarthy G: MR findings after depth electrode implantation for medically refractory epilepsy. Am N Neuroradiol 14: 1343–1346, 1993 [PMC free article] [PubMed] [Google Scholar]
- 25).Cossu M, Cardinale F, Castana L, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery 57: 706–718, 2005 [PubMed] [Google Scholar]
- 26).Holloway KL, Gaede SE, Starr PA, Rosenow JM, Ramakrishnan V, Henderson JM: Frameless stereotaxy using bone fiducial markers for deep brain stimulation. J Neurosurg 103: 404–413, 2005 [DOI] [PubMed] [Google Scholar]
- 27).Verburg N, Baayen JC, Idema S, et al. In Vivo Accuracy of a Frameless Stereotactic Drilling Technique for Diagnostic Biopsies and Stereoelectroencephalography Depth Electrodes. World Neurosurg 87: 392–398, 2016 [DOI] [PubMed] [Google Scholar]
- 28).Linskey ME: The changing role of stereotaxis in surgical neuro-oncology. J Neurooncol 69: 35–54, 2004 [DOI] [PubMed] [Google Scholar]
- 29).Widmann G, Schullian P, Ortler M, Bale R: Frameless stereotactic targeting devices: technical features, targeting errors and clinical results. Int J Med Robot 8: 1–16, 2012 [DOI] [PubMed] [Google Scholar]
- 30).Chátillon CE, Mok K, Hall J, Olivier A: Comparative study of manual versus robot-assisted frameless stereotaxy for intracranial electrode implantation. Poster displayed at: AES; 2011; Baltimore, MD, http://www.medtechsurgical.com/Press-room/Peer-review. Accessed on 2012 June 6 [Google Scholar]
- 31).Lefranc M, Capel C, Pruvot AS, et al. The impact of the reference imaging modality, registration method and intraoperative flat-panel computed tomography on the accuracy of the ROSA® stereotactic robot. Stereotact Funct Neurosurg 92: 242–250, 2014 [DOI] [PubMed] [Google Scholar]
- 32).Sutherland GR, Maddahi Y, Gan LS, Lama S, Zareinia K: Robotics in the neurosurgical treatment of glioma. Surg Neurol Int 6: S1–S8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).von Langsdorff D, Paquis P, Fontaine D: In vivo measurement of the frame-based application accuracy of the Neuromate neurosurgical robot. J Neurosurg 122: 191–194, 2015 [DOI] [PubMed] [Google Scholar]
- 34).Gonzalez-Martinez J, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Najm I: Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia 54: 323–330, 2013 [DOI] [PubMed] [Google Scholar]
- 35).Dorfer C, Minchev G, Czech T, et al. A novel miniature robotic device for frameless implantation of depth electrodes in refractory epilepsy. J Neurosurg 5: 1–7, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36).Nowell M, Sparks R, Zombori G, et al. Comparison of computer-assisted planning and manual planning for depth electrode implantations in epilepsy. J Neurosurg 124: 1820–1828, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Major P, Rakowski S, Simon MV, et al. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia 50: 147–154, 2009 [DOI] [PubMed] [Google Scholar]
- 38).de Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378: 1388–1395, 2011 [DOI] [PubMed] [Google Scholar]
- 39).Wiebe S: Epilepsy: outcome patterns in epilepsy surgery—the long-term view. Nat Rev Neurol 8: 123–124, 2012 [DOI] [PubMed] [Google Scholar]
- 40).Munari C, Musolino A, Blond S, et al. Stereo-EEG exploration in patients with intractable epilepsy: topographic relations between a lesion and epileptogenic areas. In Schimdt D, Morselli PL. (eds): Intractable Epilepsy: Experimental and Clinical Aspects. New York, Raven Press, 1986, pp. 129–146 [Google Scholar]
- 41).Bartolomei F, Wendling F, Vignal JP, et al. Seizures of temporal lobe epilepsy: identification of subtypes by coherence analysis using stereo-electro-encephalography. Clin Neurophysiol 110: 1741–1754, 1999 [DOI] [PubMed] [Google Scholar]
- 42).Barba C, Barbati G, Minotti L, Hoffmann D, Kahane P: Ictal clinical and scalp-EEG findings differentiating temporal lobe epilepsies from temporal ‘plus’ epilepsies. Brain 130: 1957–1967, 2007 [DOI] [PubMed] [Google Scholar]
- 43).Nobili L, Francione S, Mai R, et al. Surgical treatment of drug-resistant nocturnal frontal lobe epilepsy. Brain 130: 561–573, 2007 [DOI] [PubMed] [Google Scholar]
- 44).Bartolomei F, Chauvel P: Seizure symptoms and cerebral localization: frontal and rolandic seizures. In Oxbury JM, Polkey CE, Duchowny M, (eds): Intractable Focal Epilepsy. London, Saunders, 2000, pp. 55–62 [Google Scholar]
- 45).Munari C, Tassi L, Di Leo M, et al. Video-stereo-electroencephalographic investigation of orbitofrontal cortex. Ictal electroclinical patterns. Adv Neurol 66: 273–295, 1995 [PubMed] [Google Scholar]
- 46).Williamson PD, Jobst BC: Frontal lobe epilepsy. Adv Neurol 84: 215–242, 2000 [PubMed] [Google Scholar]
- 47).Ryvlin P, Minotti L, Demarquay G, et al. Nocturnal hypermotor seizures, suggesting frontal lobe epilepsy, can originate in the insula. Epilepsia 47: 755–765, 2006 [DOI] [PubMed] [Google Scholar]
- 48).Marchi A, Bonini F, Lagarde S, et al. Occipital and occipital “plus” epilepsies: A study of involved epileptogenic networks through SEEG quantification. Epilepsy Behav 62: 104–114, 2016 [DOI] [PubMed] [Google Scholar]
- 49).Gavaret M, Dubarry AS, Carron R, Bartolomei F, Trébuchon A, Bénar CG: Simultaneous SEEG-MEG-EEG recordings overcome the SEEG limited spatial sampling. Epilepsy Res 128: 68–72, 2016 [DOI] [PubMed] [Google Scholar]
- 50).Yang PF, Jia YZ, Lin Q, et al. Intractable occipital lobe epilepsy: clinical characteristics, surgical treatment, and a systematic review of the literature. Acta Neurochir (Wien) 157: 63–75, 2015 [DOI] [PubMed] [Google Scholar]
- 51).Liava A, Mai R, Tassi L, et al. Paediatric epilepsy surgery in the posterior cortex: a study of 62 cases. Epileptic Disord 16: 141–164, 2014 [DOI] [PubMed] [Google Scholar]
- 52).Isnard J, Guénot M, Sindou M, Mauguière F: Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia 45: 1079–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 53).Afif A, Chabardes S, Minotti L, Kahane P, Hoffmann D: Safety and usefulness of insular depth electrodes implanted via an oblique approach in patients with epilepsy. Neurosurgery 62: 471–479, 2008 [DOI] [PubMed] [Google Scholar]
- 54).Desai A, Jobst BC, Thadani VM, et al. Stereotactic depth electrode investigation of the insula in the evaluation of medically intractable epilepsy. J Neurosurg 114: 1176–1186, 2011 [DOI] [PubMed] [Google Scholar]
- 55).Robles SG, Gelisse P, El Fertit H, et al. Parasagittal transinsular electrodes for stereo-EEG in temporal and insular lobe epilepsies. Stereotact Funct Neurosurg 87: 368–378, 2009 [DOI] [PubMed] [Google Scholar]
- 56).Nguyen DK, Nguyen DB, Malak R, et al. Revisiting the role of the insula in refractory partial epilepsy. Epilepsia 50: 510–520, 2009 [DOI] [PubMed] [Google Scholar]
- 57).Ryvlin P, Kahane P: The hidden causes of surgery-resistant temporal lobe epilepsy: extratemporal or temporal plus? Curr Opin Neurol 18: 125–127, 2005 [DOI] [PubMed] [Google Scholar]
- 58).Aghakhani Y, Rosati A, Dubeau F, Olivier A, Andermann F: Patients with temporoparietal ictal symptoms and inferomesial EEG do not benefit from anterior temporal resection. Epilepsia 45: 230–236, 2004 [DOI] [PubMed] [Google Scholar]
- 59).Dylgjeri S, Taussig D, Chipaux M, et al. Insular and insulo-opercular epilepsy in childhood: an SEEG study. Seizure 23: 300–308, 2014 [DOI] [PubMed] [Google Scholar]
- 60).Malak R, Bouthillier A, Carmant L, et al. Microsurgery of epileptic foci in the insular region. J Neurosurgery 110: 1153–1163, 2009 [DOI] [PubMed] [Google Scholar]
- 61).Weil AG, Fallah A, Lewis EC, Bhatia S: Medically resistant pediatric insular-opercular/perisylvian epilepsy. Part 1: invasive monitoring using the parasagittal transinsular apex depth electrode. J Neurosurg Pediatr 18: 511–522, 2016 [DOI] [PubMed] [Google Scholar]
- 62).Weil AG, Le MN, Jayakar P, et al. Medically resistant pediatric insular-opercular/sylvian epilepsy. Part 2: outcome following resective surgery. J Neurosurg 18: 523–535, 2016 [DOI] [PubMed] [Google Scholar]
- 63).Seo JH, Holland K, Rose D, et al. Multimodality imaging in the surgical treatment of children with nonlesional epilepsy. Neurology 76: 41–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Widjaja E, Shammas A, Vali R, et al. FDG-PET and magnetoencephalography in presurgical workup of children with localization-related nonlesional epilepsy. Epilepsia 54: 691–699, 2013 [DOI] [PubMed] [Google Scholar]
- 65).Jung J, Bouet R, Delpuech C, et al. The value of magnetoencephalography for seizure-onset zone localization in magnetic resonance imaging-negative partial epilepsy. Brain 136: 3176–3186, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Murakami H, Wang ZI, Marashly A, et al. Correlating magnetoencephalography to stereo-electroencephalography in patients undergoing epilepsy surgery. Brain 139: 2935–2947, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Cossu M, Fuschillo D, Casaceli G, et al. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: a retrospective study on 89 cases. J Neurosurg 123: 1358–1367, 2015 [DOI] [PubMed] [Google Scholar]
- 68).Ferrari-Marinho T, Perucca P, Mok K, et al. Pathologic substrates of focal epilepsy influence the generation of high-frequency oscillations. Epilepsia 56: 592–598, 2015 [DOI] [PubMed] [Google Scholar]
- 69).Catenoix H, Mauguière F, Montavont A, Ryvlin P, Guénot M, Isnard J: Seizures Outcome After Stereoelectroencephalography-Guided Thermocoagulations in Malformations of Cortical Development Poorly Accessible to Surgical Resection. Neurosurgery 77: 9–15, 2015 [DOI] [PubMed] [Google Scholar]
- 70).Taussig D, Dorfmüller G, Fohlen M, et al. Invasive explorations in children younger than 3 years. Seizure 21: 631–638, 2012 [DOI] [PubMed] [Google Scholar]
- 71).Dorfmüller G, Ferrand-Sorbets S, Fohlen M, et al. Outcome of surgery in children with focal cortical dysplasia younger than 5 years explored by stereo-electroencephalography. Childs Nerv Syst 30: 1875–1883, 2014 [DOI] [PubMed] [Google Scholar]
- 72).Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA: Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia 54: 828–839, 2013 [DOI] [PubMed] [Google Scholar]
- 73).Enatsu R, Bulacio J, Najm I, et al. Combining stereo-electroencephalography and subdural electrodes in the diagnosis and treatment of medically intractable epilepsy. J Clin Neurosci 21: 1441–1445, 2014 [DOI] [PubMed] [Google Scholar]
- 74).Munyon C, Sweet J, Luders H, Lhatoo S, Miller J: The 3-dimensional grid: a novel approach to stereoelectroencephalography. Neurosurgery 11 Suppl 2: 127–134, 2015 [DOI] [PubMed] [Google Scholar]
- 75).Mullin JP, Shriver M, Alomar S, et al. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia 57: 386–401, 2016 [DOI] [PubMed] [Google Scholar]
- 76).Tebo CC, Evins AI, Christos PJ, Kwon J, Schwartz TH: Evolution of cranial epilepsy surgery complication rates: a 32-year systematic review and meta-analysis. J Neurosurg 120: 1415–1427, 2014 [DOI] [PubMed] [Google Scholar]
- 77).Serletis D, Bulacio J, Bingaman W, Najm I, González-Martínez J: The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. J Neurosurg 121: 1239–1246, 2014 [DOI] [PubMed] [Google Scholar]
- 78).Hamer HM, Morris HH, Mascha EJ, et al. Complications of invasive video-EEG monitoring with subdural grid electrodes. Neurology 58: 97–103, 2002 [DOI] [PubMed] [Google Scholar]
- 79).Munyon CN, Koubeissi MZ, Syed TU, Lüders HO, Miller JP: Accuracy of frame-based stereotactic depth electrode implantation during craniotomy for subdural grid placement. Stereotact Funct Neurosurg 91: 399–403, 2013 [DOI] [PubMed] [Google Scholar]