Abstract
As the world population becomes progressively older, the overall incidence of chronic subdural hematoma (CSDH) is increasing. Peak age of onset for CSDH has also increased, and recently the 80-year-old level has a peak. Many patients with CSDH have had prior treatment with anticoagulants and antiplatelet drugs, which have an accompanying risk of CSDH. In elderly patients with CSDH, symptoms of cognitive change (memory disturbance, urinary incontinence, and decreased activity) and disturbance of consciousness at admission were more frequent compared to younger patients with CSDH. The literature actually offers conflicting advice regarding CSDH treatment; however, burr hole surgery with drainage under local anesthesia is the most common surgical procedure, even in elderly patients. The recurrence rate of CSDH has not decreased over recent decades, and it has ranged from 0.36–33.3%. Outcomes in patients over 75 years old was significantly worse than for those younger than 75. Moreover, long-term outcomes for elderly patients with CSDH are poor. CSDH in the elderly is no longer a benign disease. In the future, it will be important for us to understand the mechanisms of onset and recurrence of CSDH and to develop more effective medical treatments and noninvasive surgical techniques for elderly patients.
Keywords: chronic subdural hematoma, epidemiology, treatment, recurrence rate, long-term outcome
Introduction
Chronic subdural hematoma (CSDH) is the most common neurosurgical disease, and it affects mainly elderly patients. As the world population becomes progressively older, the overall incidence is increasing. Moreover, Japan is one of the fastest aging countries in which people older than 65 years comprised 26.8% of the overall population in 2015, and they are expected to increase to 40% in 2060. Therefore, the number of elderly patients with CSDH will explosively increase in Japan and all over the world.
There are many papers about CSDH; however, papers focusing on CSDH in elderly patients are rare. The aim of this review is to analyze the recent literature about CSDH in elderly patients and to discuss the epidemiology, symptoms, treatment, and short- and long-term outcomes. Although it is difficult to make the definition of elderly patients in the review article, we mainly review the papers among the patients older than 65 years old.
Epidemiology
According to the Helsinki study from Foelholm et al. in 1975, the incidence of CSDH was 1.7/100,000/year in the general population. 1) However, recently, the incidence has been increasing. In 1992, Kudo et al. reported the incidence in the local area of Awagishima, Japan was 13.1/100,000/year, 2) and Karibe et al. reported in 2011 incidence in the Miyagi prefecture in Japan was 20.6/100000/year. 3) The incidence of CSDH increases with age. 3) In North Wales, the incidence in people over 65 years old was 8.2/100,000/year. 4) Kudo et al. reported the incidence in those over 65, was 58.1/100,000/year 2) , and Karibe et al. reported it was 80.1/100,000/year in people over 65 years of age and was 127.1/100,000/year in those over 80 years of age in 2011. 3)
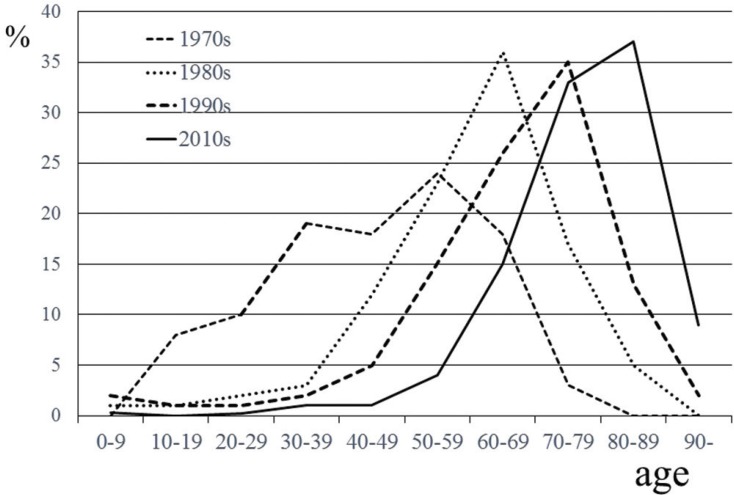
Peak age of onset of CSDH has also increased. In the 1970s, the age of onset was most commonly 50 years old, 5) in the 1980s, it was 60 years old, 6) and from 1990 to 2010, it was more common to have an age of onset of 70 years. More recently, the peak age of onset was shown to be 80 years old according to data from a Japanese national administrative database (Fig. 1). 7)
Fig. 1.
Chronological change in age distribution of chronic subdural hematoma. Previous studies have reported the etiology of chronic subdural hematoma (CSDH) in Japan. In 1970s, 1980s, and 1990s, most patients with CSDH were in their 50s (- - -), in their 60s (.......), and in their 70s (- - -)) respectively. One most recent study showed that most patients with CSDH were in their 80s (—).
These days, the sex ratio of CSDH has also changed. Elderly women are increasingly being diagnosed 7) . In the 1970s, the ratio of women with CSDH was only 9%, and in the 1980s, it was 22.4%. Moreover, in the 2010s, it was 31.6%. Thus, male preponderance diminishes with age. 7,8)
We expect that the incidence of CSDH in 2016 in Japan, according to the Karibe’s incidence ratio, 3) will be 26,146/year in the entire Japanese population and 13,066/year in those over 80 years old. 9) In the future, the incidence will very likely increase, especially in elderly women.
Traumatic Associated with CSDH
In elderly CSDH patients, a history of trauma is less frequent than in younger CSDH patients. The cause of trauma was fallen in elderly patients and traffic accident was less than younger patients 3,8) . With increasing age, brain weight declines, which leads to an increase in extracerebral volume. Thus, an arachnoid tear might easily appear after mild head trauma in elderly patients, and subdural hygromas develop into CSDH in about one-quarter of them 10) .
Risk Factors in Elderly Patients with CSDHs
Anticoagulants and antiplatelet drugs
Many patients with CSDH had prior treatment with anticoagulants and antiplatelet drugs. The rate of patients treated with antiplatelet drugs was between 4.5% and 44%, and that of anticoagulants was between 2.5% and 40.6% 4,8,11–19) . In Austria, 41% of patients had either anticoagulant or antithrombotic drug treatment, and elderly patients (over 65 year old) were significantly more likely to have taken anticoagulant or antithrombotic therapy 8) . In Germany, 50.3% of patients had anticoagulant therapy, and the ratio of this treatment was increasing with increasing age 12) . On the other hand, treatment of antithrombotic therapy was only 12.2% in Spain 20) and 4.5% of antiplatelet and 2.8% of anticoagulants (2.6% warfarin and 0.2% non-vitamin K antagonist oral anticoagulants [NOACs]) in Japan 7) . There are some differences in each country.
There have been many reports that treatment with antithrombotic drugs increases the incidence of CSDH 11,13,21) . Patients treated with warfarin had 42.5-fold increase in incidence of CSDH 13) . Vitamin K usually provides a gradual normalization of the international normalized ratio, and fresh frozen plasma (FFP), prothrombin complex concentrate (PCC), or recombinant factor VIIa can provide more rapid normalization. However, FFP can promote fluid overload in elderly patients, and it is necessary to use it carefully. 22)
There are no evidence-based reports or guidelines about the treatment for CSDH patients with NOACs. FFP, PCC, and recombinant factor VIIa should be considered as possibly treatment options, and reversal with PRADAXA (dabigatran etexilate) (Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA) is also helpful. However, most elderly patients can wait for surgery for approximately 12 hours after admission; then surgery can be performed after the half-life of the NOAC has passed.
Hemodialysis
It is well known that SDH (there was no definition of acute or chronic in these papers) is an important risk for patients on long-term hemodialysis 23) . Forty years ago, the incidence of SDH was 3.3% in dialysis patients 24) . However, the incidence of SDH in them has increased to 188.6–191/100,000 dialysis patients/year 23,25) , and has double compared to 10 years ago 25) . This rate is 10 times that of the general population, and mortality rate in dialysis patients experiencing SDH is 39% 25) . It seems that volume-overloaded long-term dialysis patients may have venous hypertension, and if the patient’s coagulation status is abnormal, then small venous tears of the dural bridging veins may easily expand and cause SDH 25) . Moreover, dialysis patients are older and have complex comorbidities and are more often disabled. They also fall more easily, which may cause SDH. Cook et al. showed that the incidence of falls in hemodialysis patients who were over 65 years of age was about 1.6 falls/patients-year and fall-related morbidity was high 26) .
Symptoms of CSDH in Elderly Patients
Elderly patients over 80 years of age who experienced CSDH without a history of head trauma were more frequently seen than younger patients 27) . In elderly patients with CSDH, symptoms associated with cognitive change (memory disturbance, urinary incontinence, and decreased activity) and disturbance of consciousness at admission were more frequent compared to younger patients who commonly presented without a disturbance of consciousness 3,4,20,27) . These symptoms were more often seen in patients over 80 years of age 3,7) . However, elderly patients with CSDH do not typically present in a comatose state 16,18) .
Bilateral CSDH was seen in 9.7–34.8% of the patients studied 8,15,19,20,28–30) . Bilateral CSDH tends to occur more commonly in elderly patients 8,28,29) . These patients had symptoms associated with increased intracranial pressure such as headache, vomiting, and rapid disturbance of consciousness 28,29) . Agawa et al. reported that bilateral hematoma was a risk factor for poor outcomes 28) ; however, Leroy et al. reported that there was no significant difference with regard to outcomes between bilateral and unilateral cases 15) . Huang et al. cautioned that diagnosis of bilateral CSDH could be delayed by lack of brain shift symptoms. 30)
In some patients with disturbance of consciousness, acute subdural hematoma may overlap with CSDH. In these patients, computed tomography (CT) shows a fresh hematoma within the CSDH, and the fresh hematoma has both a higher and of relatively more uniform density in comparison to the surrounding CSDH. This condition is termed acute-on-chronic SDH, a type of SDH with unique characteristics 31–33) . Patients with acute-on-chronic SDH have more hemostatic complications and more unfavorable outcomes.
Treatment
The literature actually offers conflicting advice regarding CSDH treatment, and these lead to considerable variation in actual clinical prospect. In Japan, most symptomatic patients underwent surgical treatment, and conservative treatment was rare 3,7) . However, in the US and UK, the rate of conservative treatment of CSDH in elderly patients was higher 4,18,19) . Even though watchful waiting has been the usual course of action in elderly patients with mild symptoms or in asymptomatic patients, there are no evidence-based guidelines about the treatment or the waiting period or about the frequency of serial CT imaging 21) . Kageyama et al. reported that tranexamic acid could treat CSDH patients without surgery 34) and Muramatu et al. and Miyagami and Kagawa reported Chinese Gore-San was a useful option in the conservative treatment of elderly CSDH patients 35–37) . In the future, medical treatment for CSDH in elderly patients will increase. Thotakura and Marabathina reported that factors related to successful conservative treatment were female sex, less midline shift, lesser hematoma thickness, and lesser attenuation values on CT imaging 37) .
There have been no evidence-based reports or guidelines about the timing of surgery in the treatment of CSDH in elderly patients; however, it is better to operate as soon as possible after a neurological deficit appears to increase the likelihood of good operative outcomes 38) .
Burr hole surgery (BHS) with drainage under local anesthesia is the most common surgical procedure, even in elderly patients 16,39–41) . Weigel et al. reported that twist-drill craniotomy (TDC), BHS, and open craniotomy have approximately the same mortality rate (2–4%); open craniotomy has significantly higher morbidity, and TDC has a higher rate of recurrence, suggesting that BHS is the preferred technique 39,40) (Table 1). However, a recent systematic review about the recurrence rate of BHS and TDC showed no significant differences; therefore, in the future, a minimally invasive and cost saving approach like TDC would be favored for elderly patients 39,42,43) .
Table 1.
Average of mortality, morbidity, cure rate, and recurrence rate for the three surgical methods for treatment of chronic subdural hematoma. We modified the original figure from Weigel et al. in 2003 to fit this table
| TDC | BHC | Craniotomy | |
|---|---|---|---|
| Mortality | 2.9% (0–7.9%) | 2.7% (0–32%) | 4.6% (0–11%) |
| Morbidity | 3.0% (0–7.6%) | 3.8% (0–9%) | 12.3%* (0–25%) |
| Cure rate | 88.2% (77–100%) | 79.1% (52–98%) | 67.8% (40–94%) |
| Recurrence | 33.0%* (3–76%) | 12.1% (0–28.8%) | 10.8% (0–44%) |
TCD: twist-drill craniotomy, BHC: burr hole surgery craniotomy: open craniotomy, Data adapted from Weigel R et al: J Neurol Neurosurg Psychiatry 74: 937–943, 2003.
The duration of drainage after BHS is usually within 48 hours after operation 16,44) , and there are no reports about BHS with or without a drain in relationship to duration in elderly CSDH patients. Few studies have compared results for CSDH between BHS drainage alone and BHS drainage with irrigation. In previous reports, the application of intraoperative irrigation during BHC is associated with fewer recurrences than without (2.9% vs. 10.3%, respectively) and is also associated with better outcomes 44,45) .
There is also contradictory advice about the relative risk of bed rest and rehabilitation 38,46,47) . A recent prospective study demonstrated that recurrence rates after BHC were independent of patients’ head position 46) , and in another report, patients with a few days of bed rest after BHC demonstrated a significantly lower recurrence rate. If early elevation of the head and mobilization after CSDH operation in elderly patients does not impact the recurrence rate, early elevation of the head and mobilization should be employed to help prevent pneumonia and deep vein thrombosis.
Recurrence Rate of CSDH
The recurrence rate of CSDH has not decreased in recent decades, and it has ranged from 0.36 to 33.3% 7,8,12,15,16,18,20,30,47–49) ; however, most studies have shown that the recurrence rate was around 10–15% 7,8,15,16,30,50,51) . In general, the definition of recurrence used by most authors is that of postoperative symptomatic re-collection of hematoma that requires reoperation of any kind. Elderly patients with CSDH had a higher recurrence rate compared to younger patients 15,20) . Ooba et al. reported that there was no significant difference with regard to the recurrence rate between patients over 80 years old and those younger than 80 years of age 27) . On the other hand, Borger et al. demonstrated that the recurrence rate in patients over 85 years of age was lower than that of those under 85 years of age 12) . Goto et al. reported that the overall recurrence rate was 9.3% and that it was the highest in those who were 70–79 years of age (14.4%) compared to those who were ≥80 years old (8.4%) 52) . Toi et al. reported that the recurrence rate in those who were 75–80 years of age was the highest and that of those older than 80 years of age was decreased 7) .
The impact of antithrombotic drugs on recurrence following surgical intervention for CSDH, however, is controversial 13,52,53) . Goto et al. and Fujitani et al. reported that there was no significant difference in recurrence between patients treated with antithrombotic drugs and those without treatment 52,54) . On the other hand, Wada et al. reported that the recurrence rate of CSDH in patients receiving antiplatelet agents was significantly associated with recurrence 55) . However, they demonstrated that they could achieve a major reduction in the impact of antiplatelet agents by delaying the initial surgery by just 1 day 55) .
Protection from Recurrence
Medical treatments such as carbazochrome, the Chinese herb Gore-san, and steroids have been used to protect patients from recurrence of CSDH. Several reports have demonstrated a reduction in the recurrence of CSDH; however, most studies were retrospective and could not show the significant effects 36,56,57).
For patients with repeated recurrence of CSDH, use of a neuroendoscope during surgery to cut the trabecula and to separate the membrane of the intrahematoma cavity has been reported 14,58) . Majovsky et al. reported on the use of flexible endoscope-assisted evacuation of CSDH, and this technique could reduce the recurrence rate 14) .
Embolization of the middle meningeal artery (MMA) for protection from recurrence of CSDH was first reported by Mandai et al. in 2000 59) . The diameter of the MMA on one side of the CSDH is larger than that of the opposite side 60) , thus, the MMA may be related to the bleeding mechanism. Although the access route to the MMA in elderly patients is not very easy, the technique is simple; hence, this treatment is acceptable for repeated recurrences in elderly patients 61,62) .
To protect from or reduce the risk of recurrence of CSDH, use of artificial cerebral spinal fluid, has been reported; however, there is no scientific evidence for the effectiveness of this technique 63) .
Resumption of antithrombotic drugs after evacuation surgery for CSDH is highly variable, and so far there have been no guidelines regarding the timing of resumption 38) . Kawamata et al. recommended that anticoagulants may be resumed 3 days after surgery in patients with a high thromboembolic risk 64) . Recently, MoGuha et al. reported that resumption of antithrombotic drugs following surgical evacuation of CSDH should be at 3 days postoperatively because patients with a history of preoperative antithrombotic drugs experienced thromboembolic complications significantly earlier than those patients without antithrombotic drugs, which peaks at 3 days postoperatively, with no increased hemorrhage risk when antithrombotic drugs were restarted 65) . On the other hand, Kamenova et al. reported that there was no significant increase in the recurrence rate of CSDH after evacuation surgery between patients with early resumption of aspirin and those with late resumption 17) . Resumption of antithrombotic drugs should be started as soon as possible if a patient has a high risk of thrombosis.
Outcomes in Elderly Patients with CSDH
Surgical treatment can bring about rapid clinical improvement with favorable outcomes in 70–90% of patients, and there is no significant difference with age. 1,12,20,66) However, outcomes in patients over 75 years of age were significantly worse than those younger than 75 years of age 12,15,67) . Ooba et al. reported that outcome improvement in patients over 80 years was lower than that under 80 years (86% vs. 95%, P < 0.05) 27) . Moreover, Karibe et al. reported that outcomes in patients over 70 years old were significantly worse than in those under 70 years old 3) . Toi et al. reported that age-specific frequencies of poor outcomes at discharge were 11.7%, 20.4%, 37.4%, and 56.8% for patients younger than 70 years old, or in their 70s, 80s, or 90s, respectively 7) .
Mortality rate of CSDH in the hospital has been shown to be between 0.21% and 27.5% 3,4,7,12,15,16,18,20,51,68) . Borger et al. demonstrated that mortality was still low in elderly patients; however, that in patients over 75 years old was significantly higher than that in patients less than 75 years old (75–84 years, 3.6%; older than 85 years, 3.8%; younger than 75 years, 1.7%) 12) . Weigel et al. reviewed 48 publications about CSDH and indicated a modest average mortality rate of 2.8%. However, most publications have reported the short-term outcome and mortality rate. Miranda et al. demonstrated the long-term outcomes of elderly patients (average age 80.6 years) with CSDH and reported that the mortality rate in the hospital was 16.7%, and only 21.1% of patients returned home 18) . Six-month and 1-year mortality rates after discharge were 26.3% and 32%, respectively 18) . They concluded that CSDH in the elderly is no longer a benign disease 18) (Table 2). Several reports have also demonstrated a high mortality rate both in the hospital and in long-term follow-up 4,19,48,68) . Mean survival periods was significantly short according to the age of onset and average survival was 1.5 ± 0.6 years in patients over 85 years old 68) . Causes of death in elderly patients with CSDH were disturbance of consciousness at onset and pneumonia after surgery 48) .
Table 2.
Mortality rate at discharge and over the long term
| In hospital | 6 months | 1 year | |
|---|---|---|---|
| Jones and Kafels (1999) (n = 43, 83.8 y.o ) | 4.7% | 31% | – |
| Asghar et al (2002) (n = 40, 79 y.o. ) | 27.5% | 33% | – |
| Santarius et al (2009) (n = 215, 76.8 y.o ) | 5.7% | 13.3% | – |
| Miranda et al (2011) (n = 209, 80.6 y.o ) | 16.7% | 26.3% | 32% |
| Dumont et al (2013) (n = 287, 75y.o ) | 8% | – | 30% |
| Manickam et al (2016) (n = 155, 69.3 y.o. ) | 8.39% | 14.19% | 20.35% |
n: number of patients, Ages shown are average ages during the study.
In recent reports from the US and Europe, discharge destination has been discussed. Most reports demonstrated that discharge directly home was significantly associated with increased long-term survival and discharge to a nursing home was associated with decreased long-term survival 18,19,51,68) (Table 3). In Japan, although more than 90% of patients younger than 70 years old were discharged home, a tendency toward lower rates was seen with increasing age of the patient. Approximately 30% of patients in their 80s and 40% of patients in their 90s could not be discharged home and were transferred to a rehabilitation hospital or nursing home 7) . Stipper et al. also reported that in patients over 90 years old with CSDH, only 24% of them returned home 69) .
Table 3.
Discharge destination and average long-term survival periods. Lengths in years shown in first column represent average survival periods for each report. Lengths in years in the Dumont study represent the average survival period for each destination, respectively
| Home or self-care | Acute rehabilitation | Nursing home | |||
|---|---|---|---|---|---|
| Miranda et al (2013) (n = 174, 4.4 years ) | 21.1% | 62.2% | |||
| Dumont et al (2013) (n = 267, 4.0 years ) | 55% 6.7 years | 16% 3.5 years | 29% 1.5 years | ||
| Stipper et al (2013) (n = 21*, NA ) | 24% | 14% | 38% | ||
| Manickam et al (2016) (n = 152, 5.29 years ) | 60.7% | 6.45% | 6.45% | ||
All patients were aged 90 years and older.
CSDH is an important cause of morbidity and mortality, and it has been described as a “sentinel health event” indicating underlying systemic pathology 68) . CSDH is associated with reduced long-term survival.
Future Issues and Problems of CSDH in the Elderly
According to the recent literature, the incidence of CSDH has been progressively increasing, and the long-term outcomes of CSDH patients are poor in spite of recent various medical and surgical treatments 18,70) . However, useful primary prevention has not been established, and none of these treatments are supported by Class I evidence 40) . So far, it is very difficult to establish an experimental animal model of CSDH. Therefore, we cannot understand the exact mechanisms of the increase in CSDH. In the future, it will be important to find the mechanisms of onset and recurrence of CSDH and more effective medical treatments and noninvasive surgical techniques for elderly patients should be developed.
Footnotes
Conflicts of Interest Disclosure
The authors report no conflicts of interest.
References
- 1).Foelholm R, Waltimo O: Epidemiology of chronic subdural haematoma. Acta Neurochir (Wien) 32: 247–250, 1975 [DOI] [PubMed] [Google Scholar]
- 2).Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N: Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo) 32: 207–209, 1992 [DOI] [PubMed] [Google Scholar]
- 3).Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T: [Epidemiology of chronic subdural hematomas]. No Shinkei Geka 39: 1149–1153, 2011. (Japanese) [PubMed] [Google Scholar]
- 4).Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A: Chronic subdural haematoma in the elderly—a North Wales experience. J R Soc Med 95: 290–292, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Hirakawa K, Hashizume K, Fuchinoue T, Takahashi H, Nomura K: Statistical analysis of chronic subdural hematoma in 309 adult cases. Neurol Med Chir (Tokyo) 12: 71–83, 1972 [DOI] [PubMed] [Google Scholar]
- 6).Fujioka S, Matsukado Y, Kaku M, Sakurama N, Nonaka N, Miura G: [CT analysis of 100 cases with chronic subdural hematoma with respect to clinical manifestation and the enlarging process of the hematoma (author’s transl)]. Neurol Med Chir (Tokyo) 21: 1153–1160, 1981 [DOI] [PubMed] [Google Scholar]
- 7).Toi H, Kinoshita K, Hirai S, et al. : Present epidemiology of chronic subdural hematoma in Japan: analysis of 63,358 cases recorded in a national administrative database. J Neurosurg 2017 [DOI] [PubMed] [Google Scholar]
- 8).Baechli H, Nordmann A, Bucher HC, Gratzl O: Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev 27: 263–266, 2004 [DOI] [PubMed] [Google Scholar]
- 9).Statistics Bureau MoIAaC : Japan Annual Report of Population Estimates 2016
- 10).Ishibashi A, Yokokura Y, Miyagi J: Clinical analysis of nineteen patients with traumatic subdural hygromas. Kurume Med J 41: 81–85, 1994 [DOI] [PubMed] [Google Scholar]
- 11).De Bonis P, Trevisi G, de Waure C, et al. : Antiplatelet/anticoagulant agents and chronic subdural hematoma in the elderly. PLoS One 8: e68732, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Borger V, Vatter H, Oszvald Á, Marquardt G, Seifert V, Güresir E: Chronic subdural haematoma in elderly patients: a retrospective analysis of 322 patients between the ages of 65-94 years. Acta Neurochir (Wien) 154: 1549–1554, 2012 [DOI] [PubMed] [Google Scholar]
- 13).Rust T, Kiemer N, Erasmus A: Chronic subdural haematomas and anticoagulation or anti-thrombotic therapy. J Clin Neurosci 13: 823–827, 2006 [DOI] [PubMed] [Google Scholar]
- 14).Májovský M, Masopust V, Netuka D, Beneš V: Flexible endoscope-assisted evacuation of chronic subdural hematomas. Acta Neurochir (Wien) 158: 1987–1992, 2016 [DOI] [PubMed] [Google Scholar]
- 15).Leroy HA, Aboukaïs R, Reyns N, et al. : Predictors of functional outcomes and recurrence of chronic subdural hematomas. J Clin Neurosci 22: 1895–1900, 2015 [DOI] [PubMed] [Google Scholar]
- 16).Santarius T, Kirkpatrick PJ, Ganesan D, et al. : Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet 26: 1067–1073, 2009 [DOI] [PubMed] [Google Scholar]
- 17).Kamenova M, Lutz K, Schaedelin S, Fandino J, Mariani L, Soleman J: Does Early Resumption of Low-Dose Aspirin After Evacuation of Chronic Subdural Hematoma With Burr-Hole Drainage Lead to Higher Recurrence Rates? Neurosurgery 79: 715–721, 2016 [DOI] [PubMed] [Google Scholar]
- 18).Miranda LB, Braxton E, Hobbs J, Quigley MR: Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg 114: 72–76, 2011 [DOI] [PubMed] [Google Scholar]
- 19).Jones S, Kafetz K: A prospective study of chronic subdural haematomas in elderly patients. Age Ageing 28: 519–521, 1999 [DOI] [PubMed] [Google Scholar]
- 20).Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R: Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg 107: 223–229, 2005 [DOI] [PubMed] [Google Scholar]
- 21).Shapey J, Glancz LJ, Brennan PM: Chronic Subdural Haematoma in the Elderly: Is It Time for a New Paradigm in Management? Curr Geriatr Rep 5: 71–77, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Ducruet AF, Grobelny BT, Zacharia BE, et al. : The surgical management of chronic subdural hematoma. Neurosurg Rev 35: 155–169, 2012 [DOI] [PubMed] [Google Scholar]
- 23).Power A, Hamady M, Singh S, Ashby D, Taube D, Duncan N: High but stable incidence of subdural haematoma in haemodialysis–a single-centre study. Nephrol Dial Transplant 25: 2272–2275, 2010 [DOI] [PubMed] [Google Scholar]
- 24).Leonard A, Shapiro FL: Subdural hematoma in regularly hemodialyzed patients. Ann Intern Med 82: 650–658, 1975 [DOI] [PubMed] [Google Scholar]
- 25).Sood P, Sinson GP, Cohen EP: Subdural hematomas in chronic dialysis patients: significant and increasing. Clin J Am Soc Nephrol 2: 956–959, 2007 [DOI] [PubMed] [Google Scholar]
- 26).Cook WL, Tomlinson G, Donaldson M, et al. : Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol 1: 1197–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 27).Ooba S, Shiomi N, Shigemori M: [Clinical features and surgical results of chronic subdural hematoma in the extremely aged patients]. No Shinkei Geka 34: 273–278, 2006. (Japanese) [PubMed] [Google Scholar]
- 28).Agawa Y, Mineharu Y, Tani S, Adachi H, Imamura H, Sakai N: Bilateral Chronic Subdural Hematoma is Associated with Rapid Progression and Poor Clinical Outcome. Neurol Med Chir (Tokyo) 56: 198–203, 2016. (Japanese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF: A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma 68: 571–575, 2010 [DOI] [PubMed] [Google Scholar]
- 30).Huang YH, Yang KY, Lee TC, Liao CC: Bilateral chronic subdural hematoma: what is the clinical significance? Int J Surg 11: 544–548, 2013 [DOI] [PubMed] [Google Scholar]
- 31).Honda Y, Sorimachi T, Momose H, Takizawa K, Inokuchi S, Matsumae M: Chronic subdural haematoma associated with disturbance of consciousness: significance of acute-on-chronic subdural haematoma. Neurol Res 37: 985–992, 2015 [DOI] [PubMed] [Google Scholar]
- 32).Lee KS, Shim JJ, Yoon SM, Doh JW, Yun IG, Bae HG: Acute-on-Chronic Subdural Hematoma: Not Uncommon Events. J Korean Neurosurg Soc 50: 512–516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Kloss BT, Lagace RE: Acute-on-chronic subdural hematoma. Int J Emerg Med 3: 511–512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Kageyama H, Toyooka T, Tsuzuki N, Oka K: Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg 119: 332–337, 2013. (Japanese) [DOI] [PubMed] [Google Scholar]
- 35).Miyagami M, Kagawa Y: [Effectiveness of Kampo medicine Gorei-San for chronic subdural hematoma]. No Shinkei Geka 37: 765–770, 2009. (Japanese) [PubMed] [Google Scholar]
- 36).Muramatsu M, Yoshikawa T, Hanabusa K: [Effectiveness of kampo medicine gorei-san-ryo for chronic subdural hematoma in very elderly patients]. No Shinkei Geka 33: 965–969, 2005. (Japanese) [PubMed] [Google Scholar]
- 37).Thotakura AK, Marabathina NR: Nonsurgical treatment of chronic subdural hematoma with steroids. World Neurosurg 84: 1968–1972, 2015 [DOI] [PubMed] [Google Scholar]
- 38).Shofty B, Grossman R: Treatment options for chronic subdural hematoma. World Neurosurg 87: 529–530, 2016 [DOI] [PubMed] [Google Scholar]
- 39).Almenawer SA, Farrokhyar F, Hong C, et al. : Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg 259: 449–457, 2014 [DOI] [PubMed] [Google Scholar]
- 40).Weigel R, Schmiedek P, Krauss JK: Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatr 74: 937–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ: The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg 22: 529–534, 2008 [DOI] [PubMed] [Google Scholar]
- 42).Liu W, Bakker NA, Groen RJ: Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg 121: 665–673, 2014 [DOI] [PubMed] [Google Scholar]
- 43).Lee J, Park JH: Clinical Characteristics of Bilateral versus Unilateral Chronic Subdural Hematoma. Korean J Neurotrauma 10: 49–54, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Tahsim-Oglou Y, Beseoglu K, Hänggi D, Stummer W, Steiger HJ: Factors predicting recurrence of chronic subdural haematoma: the influence of intraoperative irrigation and low-molecular-weight heparin thromboprophylaxis. Acta Neurochir (Wien) 154: 1063–1068, 2012 [DOI] [PubMed] [Google Scholar]
- 45).Ishibashi A, Yokokura Y, Adachi H: A comparative study of treatments for chronic subdural hematoma: burr hole drainage versus burr hole drainage with irrigation. Kurume Med J 58: 35–39, 2011 [DOI] [PubMed] [Google Scholar]
- 46).Ivamoto HS, Lemos HP, Atallah AN: Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg 86: 399–418, 2016 [DOI] [PubMed] [Google Scholar]
- 47).Abouzari M, Rashidi A, Rezaii J, et al. : The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery 61: 794–797, 2007 [DOI] [PubMed] [Google Scholar]
- 48).Rohde V, Graf G, Hassler W: Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg Rev 25: 89–94, 2002 [DOI] [PubMed] [Google Scholar]
- 49).Abe Y, Maruyama K, Yokoya S, et al. : Outcomes of chronic subdural hematoma with preexisting comorbidities causing disturbed consciousness. J Neurosurg 27: 1–5, 2016 [DOI] [PubMed] [Google Scholar]
- 50).Mori K, Maeda M: Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 41: 371–381, 2001 [DOI] [PubMed] [Google Scholar]
- 51).Manickam A, Marshman LA, Johnston R: Long-term survival after chronic subdural haematoma. J Clin Neurosci 34: 100–104, 2016 [DOI] [PubMed] [Google Scholar]
- 52).Goto H, Ishikawa O, Nomura M, Tanaka K, Nomura S, Maeda K: Magnetic resonance imaging findings predict the recurrence of chronic subdural hematoma. Neurol Med Chir (Tokyo) 55: 173–178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S: Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery 63: 1125–1129, 2008 [DOI] [PubMed] [Google Scholar]
- 54).Fujitani S, Ishikawa O, Miura K, Takeda Y, Goto H, Maeda K: Factors predicting contralateral hematoma growth after unilateral drainage of bilateral chronic subdural hematoma. J Neurosurg 126: 755–759, 2017 [DOI] [PubMed] [Google Scholar]
- 55).Wada M, Yamakami I, Higuchi Y, et al. : Influence of antiplatelet therapy on postoperative recurrence of chronic subdural hematoma: a multicenter retrospective study in 719 patients. Clin Neurol Neurosurg 120: 49–54, 2014 [DOI] [PubMed] [Google Scholar]
- 56).Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R: The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol 2012: 1397–1403, 2012 [DOI] [PubMed] [Google Scholar]
- 57).Wakabayashi Y, Yamashita M, Asano T, et al. : [Effect of Gorei-san with tranexamic acid for preventing recurrence of chronic subdural hematoma]. No Shinkei Geka 40: 967–971, 2012. (Japanese) [PubMed] [Google Scholar]
- 58).Mobbs R, Khong P: Endoscopic-assisted evacuation of subdural collections. J Clin Neurosci 16: 701–704, 2009 [DOI] [PubMed] [Google Scholar]
- 59).Mandai S, Sakurai M, Matsumoto Y: Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg 93: 686–688, 2000 [DOI] [PubMed] [Google Scholar]
- 60).Takizawa K, Sorimachi T, Ishizaka H, et al. : Enlargement of the middle meningeal artery on MR angiography in chronic subdural hematoma. J Neurosurg 124: 1679–1683, 2016 [DOI] [PubMed] [Google Scholar]
- 61).Tempaku A, Yamauchi S, Ikeda H, et al. : Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: Five cases and a review of the literature. Interv Neuroradiol 21: 366–371, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Hashimoto T, Ohashi T, Watanabe D, et al. : Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int 4: 104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Adachi A, Higuchi Y, Fujikawa A, et al. : comparison of irrigation with artificial cerebrospinal fluid and normal saline in a cohort analysis. PLoS ONE 9: e103703, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Kawamata T, Takeshita M, Kubo O, Izawa M, Kagawa M, Takakura K: Management of intracranial hemorrhage associated with anticoagulant therapy. Surg Neurol 44: 438–443, 1995 [DOI] [PubMed] [Google Scholar]
- 65).Guha D, Coyne S, Macdonald RL: Timing of the resumption of antithrombotic agents following surgical evacuation of chronic subdural hematomas: a retrospective cohort study. J Neurosurg 124: 750–759, 2016 [DOI] [PubMed] [Google Scholar]
- 66).Fukui S: [Evaluation of surgical treatment for chronic subdural hematoma in extremely aged (over 80 years old) patients]. No To Shinkei 45: 449–453, 1993. (Japanese) [PubMed] [Google Scholar]
- 67).Ro HW, Park SK, Jang DK, Yoon WS, Jang KS, Han YM: Preoperative predictive factors for surgical and functional outcomes in chronic subdural hematoma. Acta Neurochir (Wien) 158: 135–139, 2016 [DOI] [PubMed] [Google Scholar]
- 68).Dumont TM, Rughani AI, Goeckes T, Tranmer BI: Chronic subdural hematoma: a sentinel health event. World Neurosurg 80: 889–892, 2013 [DOI] [PubMed] [Google Scholar]
- 69).Stippler M, Ramirez P, Berti A, Macindoe C, Villalobos N, Murray-Krezan C: Chronic subdural hematoma patients aged 90 years and older. Neurol Res 35: 243–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Balser D, Farooq S, Mehmood T, Reyes M, Samadani U: Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg 123: 1209–1215, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]