Abstract
Traumatic cerebrovascular injury (TCVI) is an uncommon clinical entity in traumatic brain injury (TBI), yet it may cause devastating brain injury with high morbidity and mortality. Early recognition and prioritized strategic treatment are of paramount importance. A total of 1966 TBI patients admitted between 1999 and 2015 in our tertiary critical care center were reviewed. Screening of TCVI was based on the Guidelines for the Management of Severe Head Injury in Japan. TCVI was confirmed in 33 (1.7%) patients; 29 blunt and 4 penetrating injuries. The primary location of the injury included 16 cervical, 6 craniofacial, and 11 intracranial lesions. On arrival, 15 patients presented with hemorrhage, 5 of these arrived in shock status with massive hemorrhage. Ten presented with ischemic symptoms. Sixteen patients underwent surgical or endovascular intervention, 13 of whom required immediate treatment upon arrival. Surgical procedures included clipping or trapping for traumatic aneurysms, superficial temporal artery - middle cerebral artery bypass, carotid endarterectomy, and direct suture of the injured vessels. Endovascular intervention was undertaken in 7 patients; embolization with Gelfoam (Pharmacia and Upjohn Company, Kalamazoo, MI, USA) or coil for 6 hemorrhagic lesions and stent placement for 1 lesion causing ischemia. Patients’ outcome assessed by the Glasgow Outcome Scale at 3 months were good recovery in 8, moderate disability in 3, severe disability in 9, persistent vegetative state in 1, and death in 12, respectively. In order to rescue potentially salvageable TCVI patients, neurosurgeons in charge should be aware of TCVI and master basic skills of cerebrovascular surgical and endovascular procedures to utilize in an emergency setting.
Keywords: neurotrauma, traumatic brain injury, traumatic cerebrovascular injury, microneurosurgery, emergency medicine
Introduction
Traumatic cerebrovascular injury (TCVI) is defined as an extracranial or intracranial cerebrovascular structural defect caused by a known injury to the head, neck, and/or chest. 1) TCVI is classified into either blunt or penetrating according to the mechanism of injury. Blunt TCVI is uncommon, accounting for approximately 1% of all blunt traumatic brain injury (TBI), 2,3) yet it may cause devastating brain injury with high morbidity and mortality. 1–4) Screening guidelines for blunt TCVI serve to establish early recognition with better diagnostic accuracy, 3,5) however in real practice throughout the course of management, emergency neurosurgical or endovascular intervention is occasionally required to salvage patients from ongoing ischemic or hemorrhagic events. Diagnosis of penetrating TCVI may be self-evident but the decision to initiate appropriate emergent testing or intervention is often needed to control life-threatening hemorrhage or ischemia. Furthermore, systemic injury and/or brain parenchymal injury should be simultaneously evaluated and managed in accordance with treatment priority. 6) Intervention in TCVI is challenging and the outcome is often time-dependent. Our recent experience with TCVI is presented in order to share these rare but life-threatening entities of TBI and to hopefully prevent serious pitfalls.
Patients and Methods
TBI patients admitted between 1999 and 2015 in our tertiary critical care center were retrospectively assessed. Medical records, charts, and radiological data of eligible patients were reviewed to identify patients with potential TCVI. Screening of TCVI was based on the following criteria from the Guidelines for the Management of Severe Head Injury in Japan: 1) neurological conditions difficult to be explained based only on the TBI; 2) delayed and newly-developed neurological symptoms; 3) newly developed onset of cerebral hemorrhage or infarction on computed tomography (CT) or magnetic resonance image (MRI); 4) neck injuries (fractures in the cervical vertebrae, hyperextension, or hyper-rotation of the neck, etc.); 5) cranial base fractures (bleeding from the nasal or external auditory meatus, etc.; 6) thick, diffuse, severe subarachnoid hemorrhage or localized intense subarachnoid hemorrhage. 6,7)
All patients at risk of TCVI underwent MR angiography (MRA) or 3 dimensional CT angiography (3D-CTA) or both for screening as early as possible. Digital subtraction angiography (DSA) was added as needed to confirm the diagnosis or to treat the lesion directly. Patients presenting acutely with hemorrhagic shock underwent emergency DSA without MRA or 3D-CTA.
Vascular injuries were classified according to their DSA appearance using the Denver grading scale: Grade I injuries were defined as a vessel lumen stenosis of less than 25%; Grade II injuries as stenosis of vessel lumen between 25% and 50%; Grade III injuries as stenosis of the vessel greater than 50% or the development of a pseudoaneurysm; Grade IV injuries as complete vessel occlusion, and Grade V injuries as complete transection of the artery. 5)
Results
During the study period, a total of 1966 TBI patents were admitted to our hospital. Patient demographics are presented in Table 1. A total of 33 (1.7%) patients had confirmed TCVI, 29 blunt and 4 penetrating injuries. Sixteen blunt injuries were caused by traffic accidents. The mean age of patients was 42.6 years (range 2–85), mean Glasgow Coma Scale (GCS) score was 7.9, mean Injury Severity Score (ISS) was 20.5. Nine patients suffered from multiple compound trauma with 2 or more lesions correlating with an Abbreviated Injury Score (AIS) ≥3. Primary location of injury included 16 cervical, 6 craniofacial, and 11 intracranial lesions. On arrival, 15 patients presented with hemorrhage: 5 of these arrived in hemodynamic shock due to massive bleeding from the oronasal cavities. Ischemic symptoms were seen in 10 patients, of whom 9 presented in the acute phase and 1 developed after admission. Nine patients had asymptomatic vascular injury.
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| Age (mean ± SD) (range) (yrs) | 42.6 ± 2.7 (2–85) |
| Sex (male/female) (n) | 22/11 |
| Glasgow Coma Scale (mean ± SD) (range) | 7.9 ± 5.1 (3–15) |
| Injury severity score (mean ± SD) (range) | 20.5 ± 9.3 (4–38) |
| Mechanism (n) | |
| blunt | 29 |
| penetrating | 4 |
| Primary location (n) | |
| cervical | 16 |
| craniofacial | 6 |
| intracranial | 11 |
| Symptom (n) | |
| hemorrhage (shock status) | 15 (5) |
| ischemia | 9 |
| asymptomatic | 9 |
including carotid cavernous fistula, 2 followed from hemorrhage, 1 followed form ischemia.
Table 2 presents the incidence of blunt TCVI by screening criteria. 6,7) TCVI was detected in 29 patients (42%) who met the overall criteria. A diagnosis of vascular injury was confirmed using DSA in 18 patients. Other patients were diagnosed using 3D-CTA or MRA or both. Traumatic aneurysms were detected in three patients, one of the distal anterior cerebral artery (ACA), one of the internal carotid artery (ICA), and one of the basilar artery (BA). Carotid cavernous fistula (CCF) was diagnosed in three patients, two in the subacute phase following occlusion of the ICA and one in acute phase with massive hemorrhage. Of the 14 blunt common carotid artery (CCA) or ICA injuries, lesions were classified into grade I in 1, grade II in 6, grade III in 2, and grade IV in 5, respectively. Six blunt vertebral injuries were classified into grade II in 2, grade III in 3, and grade IV in 1, respectively. There were no grade V injuries.
Table 2.
Incidence of blunt cerebrovascular injury by screening criteria
| Screening criteria | Incidence (number of cases) | |
|---|---|---|
| 1 | Neurological conditions difficult to be explained based only on the TBI | 100 (3) |
| 2 | Delayed and newly-developed neurological symptoms | 100 (1) |
| 3 | Newly developed onset of cerebral hemorrhage or infarction on CT or MRI | 100 (1) |
| 4 | Neck injuries (fractures in the cervical vertebrae, hyperextension, or hyper-rotation of the neck, etc.) | 18.9 (11) |
| 5 | Cranial base fractures (bleeding from the nasal or external auditory meatus, etc.) | 87.5 (7)* |
| 6 | Thick, diffuse, severe subarachnoid hemorrhage or localized intense subarachnoid hemorrhage | 40 (6) |
100% (5/5) for patients presenting with massive oronasal bleeding.
Table 3 shows the type of treatment procedure. Sixteen patients underwent surgical or endovascular intervention, 13 of whom required immediate treatment upon hospital arrival. Embolization with Gelfoam (Pharmacia and Upjohn Company, Kalamazoo, MI, USA) was achieved in three patients presenting with massive hemorrhage from the superior maxillary artery and its branches. Coil embolization for hemorrhage was performed in two arteries, the ICA and posterior cerebral artery, because of massive hemorrhage in both vascular distributions. One patient with massive oronasal hemorrhage in whom endovascular approach was unavailable underwent ligation of the external carotid artery (ECA) but hemostasis was not obtained. Traumatic aneurysms were treated by clipping in one, coil embolized in one, and trapping in one. Superficial temporal artery (STA) - middle cerebral artery (MCA) bypass was performed in two patients, one for intracranial ICA occlusion and one for laceration of an ICA injury in combination with trapping. Injured vessels were directly sutured in three patients, one with a lacerated ACA, one with a penetrating injury of the common carotid artery, and one with a lacerated sagittal sinus. Emergency carotid endarterectomy (CEA) was performed for traumatic CCA occlusion in one patient. In another patient with CCA injury causing by cerebral embolism, stent placement was performed. Seventeen patients underwent conservative treatment with antithrombotic medication only. Patient outcomes assessed by the Glasgow Outcome Scale at 3 months were good recovery in 8, moderate disability in 3, severe disability in 9, persistent vegetative state in 1, and death in 12, respectively.
Table 3.
Treatment
| Procedure | Number of cases |
|---|---|
| Arterial embolization | |
| with Gelfoam | 3 |
| with coil | 2 |
| Coil embolization of aneurysm | 1 |
| Clipping of aneurysm | 1 |
| Trapping of the aneurysm | 1 |
| STA-MCA* bypass | 2 |
| Direct vascular suturing | 3 |
| Ligation of external carotid artery | 1 |
| Stent placement | 1 |
| Carotid endoarterectomy | 1 |
| Conservative | 17 |
MCA: middle cerebral artery,
STA: superficial temporal artery.
Illustrative Cases
Case 1. Cervical blunt injury with ischemia (Fig. 1).
Fig. 1.
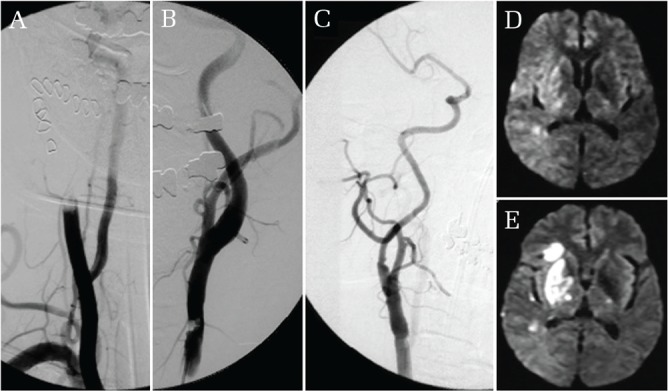
Cervical blunt injury with ischemia. Cerebral angiograpm showing occlusion of the right common carotid artery (CCA) (Grade IV) (A) and an intimal flap in the left CCA (Grade II) (B). Diffusion weighted image (DWI) showing faint hyperintensity change in the right cerebral hemisphere (D). Angiogram after carotid endarterectomy demonstrates a patent left CCA (C). Follow-up DWI shows infarction primarily in the right basal ganglia not including cerebral cortex (E).
A 69-year-old woman suffered blunt cervical injury caused after being caught in a cargo elevator door. She presented with a GCS score of 6 (E1V1M4) and left hemiparesis when she arrived 37 minutes after injury. Brain CT showed no obvious evidence of hemorrhage or infarction. 3D-CTA showed Grade IV injury in the right and Grade II injury in the left CCA. Diffusion-weighted image (DWI) showed a faint hyperintensity change in the right cerebral hemisphere. DSA was initiated 48 minutes after arrival and confirmed the bilateral CCA injury. An emergency CEA was performed for the right CCA occlusion 222 minutes after arrival. Intraoperative findings revealed a calcified vascular intima with intraluminal distortion forming mechanical occlusion of the vascular lumen. Post-operative DSA demonstrated a patent left CCA. DWI showed an acute cerebral infarction in the right basal ganglia not extending to the cerebral cortex. She was discharged to a rehabilitation hospital because of residual left hemiparesis with no other neurological deficits.
Case 2. Cervical penetrating injury with hemorrhage (Fig. 2).
Fig. 2.
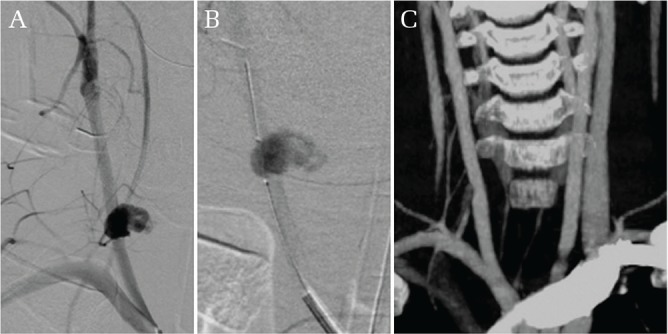
Cervical penetrating injury with hemorrhage. Angiogram showing extravasation of contrast media at the right common carotid artery (A). Balloon occlusion catheter was placed to prepare for intraoperative rerupture (B). Postoperative computed tomographic angiogram showed slight stenosis at the anastomosis site (C).
A 9-year-old boy fell and suffered a penetrating injury to his neck by a pencil held in his hand. He was transferred from a nearby hospital where he was intubated and sedated. Severe hematoma formation was noted on the right side of his neck. Deviation of the trachea was seen on the initial chest X-ray. DSA showed extravasation at the right CCA. Fifty-seven minutes after arrival, a balloon occlusion catheter was placed endovascularly in the right CCA to prepare for possible intraoperative rerupture prior to surgery. He was transferred to the operating room 147 minutes after arrival. Rerupture occurred soon after surgical manipulation and consequently the CCA was temporally occluded using the balloon, followed by direct occlusion via hemostatic forceps. The penetrated segment of the CCA was resected and end to end anastomosis was performed. Postoperative 3D-CTA showed slight stenosis at the anastomosis site. The boy was discharged without neurological deficits.
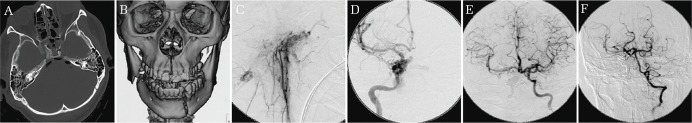
Case 3. Craniofacial blunt injury with hemorrhage (Fig. 3).
Fig. 3.
Craniofacial blunt injury with hemorrhage. CT showing skull base fracture involving the right carotid canal and the left petrous bone (A). Reconstruction CT image shows fractures in the maxilla and mandibula (B). External carotid angiogram shows multiple extravasation at branches of the internal maxillary artery (C), which was embolized with Gelfoam®. Angiogram of the internal carotid artery (ICA) showing carotid cavernous fistula and contrast media leaking into the oral cavity (D). Post coil embolization angiogram of right ICA shows confirmation of good collateral (E and F).
A 20-year-old man was involved in a traffic accident while riding a motorcycle. The patient was transported to our hospital within 10 minutes of injury and on arrival was in a state of hemodynamic shock from massive oronasal bleeding. Blood pressure was not measurable, however very weak radial artery pulsation was palpable with a rate of 170/min. His GCS score was 10 (E3V2M5). Seventeen minutes after arrival following endotracheal intubation, a catheter sheath was inserted into the femoral artery immediately prior to brain CT for the preparation of emergency endovascular embolization. DSA was initiated 46 minutes after arrival. The external carotid angiogram showed multiple extravasation in the branches of the internal maxillary artery, which were subsequently embolized using Gelfoam. However, persistent oronasal bleeding was observed and internal carotid angiogram showed a carotid cavernous fistula with contrast media leaking into the oral cavity. An attempt to occlude the shunt point via the arterial side failed which was followed by successful complete coil embolization of the right ICA. Good collateral blood flow was then confirmed. Oronasal bleeding was reduced yet persisted despite these endovascular procedures, and tight gauze packing of the oral cavity was performed to ensure hemostasis. Finally, the patient’s hemodynamic status stabilized. Perfusion CT the next day showed no remarkable decrease of blood flow. On postoperative day 3, he developed a high fever, and despite the removal of the gauze, the patient died of septic shock. The gauze was dark brown in color and noted to have a foul odor. Bacterial culture detected oral indigenous bacteria. Anaerobic bacterial culture was not conducted.
Case 4. Intracranial blunt injury with hemorrhage (Fig. 4).
Fig. 4.
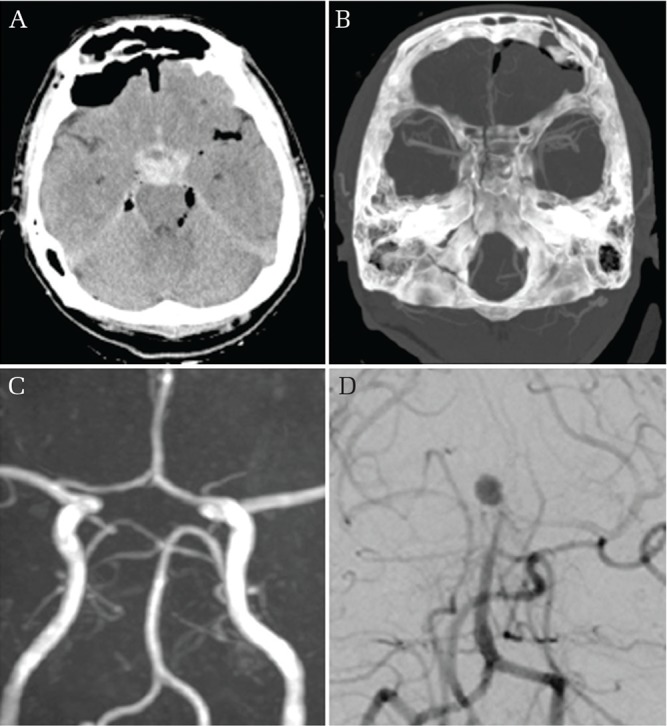
Intracranial blunt injury with hemorrhage. Initial Brain CT showing dense subarachnoid hemorrhage in the prepontine cistern (A) as well as intracranial air and clival fracture (B) and magnetic resonance angiography showing no vascular injury (D). On hospital day 10 a bleeding event was observed and vertebral angiogram shows the presence of a de novo basilar top aneurysm (D).
A 31-year-old man suffered head trauma from a power shovel and was transported to our institute with a GCS score of 8 (E1V2M5). Brain CT after resuscitation revealed dense subarachnoid hemorrhage (SAH) in the prepontine cistern as well as intracranial air and a clival fracture. 3D-CTA on arrival and MRA the next day showed no evidence of vascular injury. Intracranial pressure (ICP) monitoring was performed using Camino Ventrix catheter (Integra Neuroscience, San Diego, CA, USA) catheter inserted into the left lateral ventricle. On the 10th day after admission, bloody cerebrospinal fluid (CSF) drainage and ICP elevation greater than 80 mmHg was observed. The patient’s GCS score declined to 3 and a brain CT revealed recurrent SAH. A basilar top aneurysm was detected using 3D-CTA and DSA. Despite treatment of the aneurysm by coil embolization, the patient’s neurological condition did not improve and he was discharged in a vegetative state.
Case 5. Intracranial blunt injury with hemorrhage (Fig. 5).
Fig. 5.
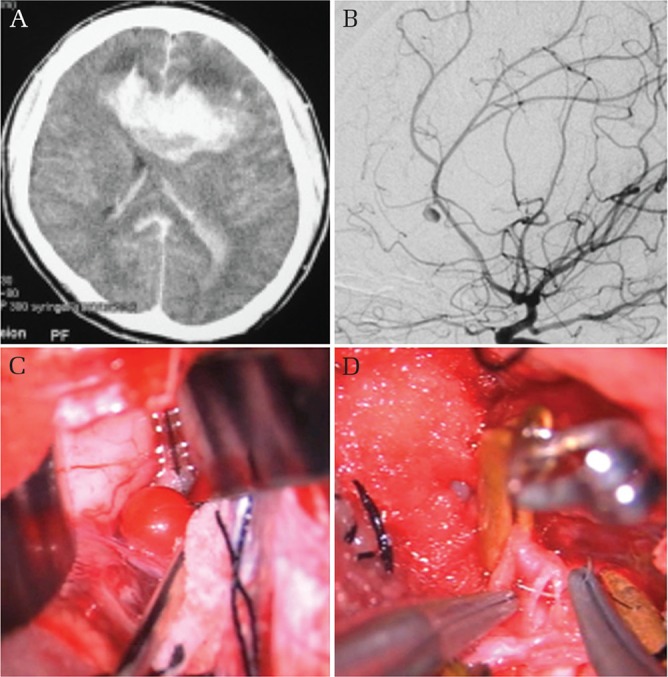
Intracranial blunt injury with hemorrhage. Brain CT on admission showing deep bifrontal, left intraventricular, and subarachnoid hemorrhages (A). Left carotid angiogram shows an aneurysm-like lesion arising from the left pericallosal artery (B). Intraoperative picture of a pulsating red neck-less dome projecting from the pericallosal artery (D), which abruptly bursted during the procedure, leaving a laceration defect at the pericallosal artery without aneurysm wall. A picture shows uneventful suturing of the laceration (D).
A 34-year-old man was admitted in coma following a motor vehicle accident. Brain CT revealed SAH with a deep bifrontal cerebral hematoma and bilateral intraventricular hemorrhage. 3D-CTA and DSA revealed an aneurysm-like lesion arising from the left pericallosal artery. The formation of massive intracerebral hematoma prompted emergency surgical intervention. After hematoma evacuation via the interhemispheric approach, a pulsating red sphere projecting from the pericallsal artery was encountered. The lesion ruptured almost immediately intraoperatively, leaving a defect in the pericallosal artery with no aneurysm wall. The defect was promptly sutured uneventfully. Postoperative DSA confirmed patency of the pericallosal artery. The patient was discharged to a rehabilitation facility with a severe functional disability.
Case 6. Intracranial blunt injury with hemorrhage (Fig. 6).
Fig. 6.
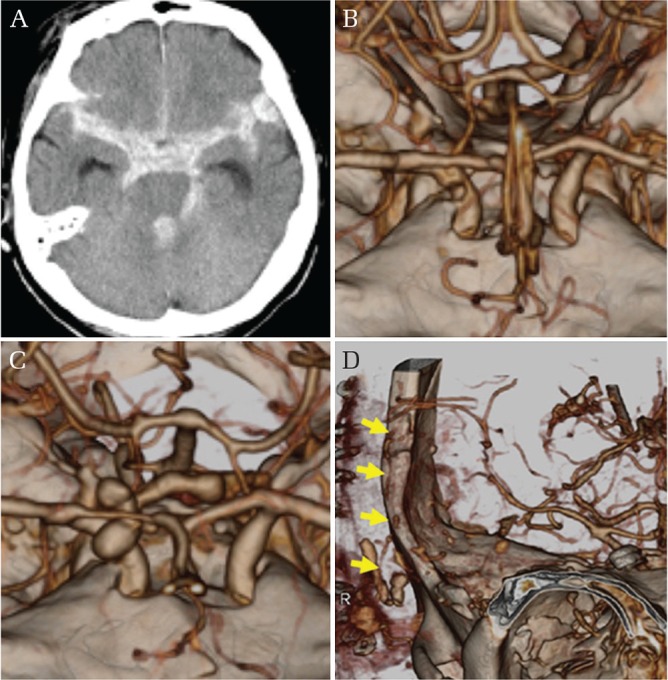
Intracranial blunt injury with hemorrhage. Initial brain CT scan demonstrating diffuse SAH in the basal cisterns (A). Three-dimensional computed tomographic angiogram (3D-CTA) shows a small dimple at the anterior wall of the supraclinoid segment of the right ICA (B). 3D-CTA after rebleeding episode shows bizarre configuration contrast media indicating extravasation (C). Postoperative 3D-CTA showing patent superficial temporal artery-middle cerebral artery bypass (arrows) (D).
A 78-year-old woman was found in coma after falling from a 5-meter-high embankment. She regained consciousness during ambulance transport. On admission, she complained of severe headache. Her GCS score was 14 (E3V5M6). A brain CT scan demonstrated diffuse SAH in the basal cisterns. 3D-CTA revealed a small dimple at the anterior wall of the supraclinoid segment of the right ICA adjacent to the anterior clinoid process. A diagnosis of the right ICA injury was made and trapping of the ICA with double STA-MCA bypass was planned. Because her personal identification was obtainable on admission, emergency surgical treatment was deferred. She was intubated and treated with deep sedation using propofol and blood pressure control to prevent rebleeding. Contact with her family was made at midnight and informed consent for next day surgery was obtained. Several hours later, bilateral pupil dilation with blood pressure elevation was observed. 3D-CTA showed evidence of contrast extravasation. An emergency craniotomy followed by trapping of the right ICA and STA-MCA bypass was performed. During the surgical procedure, bleeding and brain swelling continued despite completion of the procedure. A second bypass was abandoned and external decompression was performed. Despite patency of the bypass observed on post-operative 3D-CTA, massive brain swelling lead to death on the 4th postoperative day.
Discussion
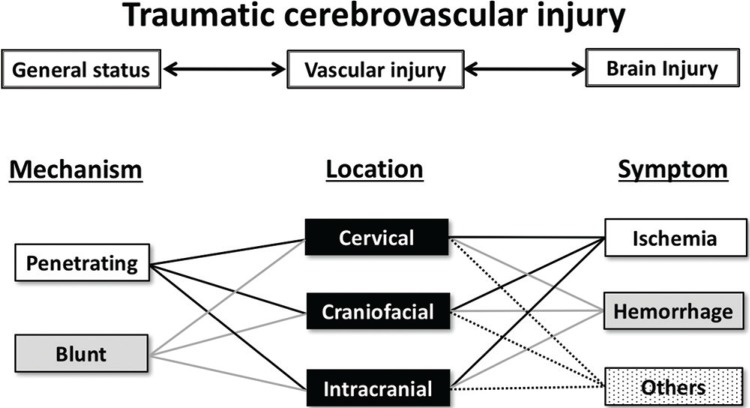
Trauma patients may present with a wide range of complex problems. On initial resuscitation, rapid and accurate assessment of the patient’s systemic condition is indispensable. 6,7) TCVI patients occasionally present with massive bleeding causing airway and circulatory crisis. 8) In our series, 27% (9/33) of patients had multiple injuries and 15% (5/33) arrived in hemorrhagic shock status. Furthermore, TCVI patients may have a concomitant life-threatening intracranial injury requiring immediate surgery. Early screening and antithrombotic therapy are recommended for patients at risk of embolic complications, 9–11) however initiation of therapy should be considered with caution and postponed for patients with hemorrhage. The physicians in charge of TCVI treatment must prioritize treatment with attention to time management in decision-making. Screening criteria for blunt TCVI 6,7) was useful, accounting for 42% of the true positive rate. This was slightly higher than results from a previous report (31.3%) by a major emergency critical care center. 8) TCVI is categorized based on a consideration of a combination of parameters including mechanism of injury, lesion location, and clinical symptoms (Fig. 7). In addition, it is important to search for possible multiple lesion locations or multiple symptoms which can develop over time. The following addresses the specific points of caution for each TCVI category based on patient examples from our series.
Fig. 7.
Schematic drawing showing relationship between vascular injury, general status and brain injury. Traumatic cerebrovascular injury is categorized by mechanism of injury, affected location, and clinical symptom.
In our series, two cervical injuries required emergent surgical intervention: one with blunt ischemic trauma and another with hemorrhagic penetrating injury. Owing to recent advancements in both equipment and technique, these injuries could be treated by less invasive endovascular means. 12) However, fundamental surgical procedures such as CEA and carotid anastomosis should be mastered and carried out in appropriate emergency settings. Systemic antithrombotic therapy following endovascular procedures may not be indicated for patients with intracranial or non-craniocervical hemorrhagic complications.
Craniofacial injuries are often associated with life-threatening hemorrhage and delays in treatment can bring about fatal outcome. 13) We could save only one out of five patients who arrived in hemorrhagic shock. One should bear in mind that time is the essential factor and prolongation can initiate a vicious circle of events: massive blood loss can cause coagulopathy and potential intractable hemostasis. Gauze packing may be required as in case 3. However, gauze should be removed as early as possible after hemostasis with concomitant use of broad spectrum antibiotics covering anaerobic bacteria.
Patients with intracranial vascular lesions associated with hemorrhage may be candidates for microvascular surgery as well as endovascular treatment. Of note, traumatic aneurysms may not be identified on the initial evaluation and can present with delayed enlargement and rupture with a significant mortality rate as high as 50%. 14) Consequently, repeated studies are mandatory if the patient has suspected clinical or radiological findings meeting guideline specified criteria. Bleeding from traumatic aneurysms, a condition known as “delayed” apoplexy, is considered to occur approximately 2–3 weeks following the initial traumatic event. According to the present case experience and other reports, 15) early reevaluation is recommended, preferably within one week. Because traumatic vascular lesions can be extremely fragile during this early time window, treatment should be initiated at the earliest possible opportunity, as rebleeding is usually associated with devastating outcome. Surgical clipping is often impossible and trapping with bypass may be required. 16) Indications and methodology for bypass surgery should be considered prudently keeping in mind that autoregulation in injured brain may be significantly impaired.
This study was limited by the inherent features of a single-center retrospective study and due to selection and treatment biases, results may not be generalizable. In addition, because our institute is a tertiary neurosurgical referral center, long-term follow-up was challenging/difficult. Furthermore, because all patients were screened according to the national guidelines for TBI, retrospective patient selection may have contributed to an underestimation of the number of patients. Despite the benefits of screening by CTA or MRA, these modalities are considered to be less sensitive than DSA. And since most ischemic stroke events due to TCVI are embolic and occur prior to both screening CTA and the initiation of antithrombotic treatment, 17) this underestimation may not be clinically relevant or significant in the setting of emergency care. Although the incidence of TCVI has been reported to be approximately 1% overall, the incidence is reported to be higher in tertiary critical care centers (1.6%) 8) and especially in patients with severe TBI (9.2%).
The results of this study highlight the need for training and simulation of fundamental surgical and endovascular procedures for treating TCVI in an emergency setting. Systemic evaluation and emergent prioritization should guide treatment planning and action. Anticipation of potential lesions with timely evaluations can be crucial for prevention of disastrous outcomes. In order to rescue potentially salvageable TCVI patients, neurosurgeons in charge at emergency care centers should learn and master basic skills in the field of cerebrovascular surgical and endovascular procedures.
Acknowledgment
The author is grateful to Dr. Adam Tucker for preparing the English manuscript.
Footnotes
Conflicts of Interest Disclosure
The author hereby declares no conflict of interest regarding this article.
References
- 1).Fusco MR, Harrigan MR: Cerebrovascular dissections: a review. Part II: blunt cerebrovascular injury. Neurosurgery 68: 517–530; discussion 530, 2011 [DOI] [PubMed] [Google Scholar]
- 2).Biffl WL, Moore EE, Ryu RK, et al. : The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg 228: 462–470, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Miller PR, Fabian TC, Croce MA, et al. : Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg 236: 386–393; discussion 393–395, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Bromberg WJ, Collier BC, Diebel LN, et al. : Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma 68:471–477, 2010 [DOI] [PubMed] [Google Scholar]
- 5).Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM: Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 47: 845–853, 1999 [DOI] [PubMed] [Google Scholar]
- 6).Shigemori M, Abe T, Aruga T, et al. Guidelines Committee on the Management of Severe Head Injury, Japan Society of Neurotraumatology : Guidelines for the Management of Severe Head Injury, 2nd Edition guidelines from the Guidelines Committee on the Management of Severe Head Injury, the Japan Society of Neurotraumatology. Neurol Med Chir (Tokyo) 52: 1–30, 2012 [DOI] [PubMed] [Google Scholar]
- 7).The Guidelines Committee on the Management of Severe Head Injury : The Guidelines for the Management of Severe Head Injury, ed 3 Tokyo, Igakushoin, 2013. (Japanese) [Google Scholar]
- 8).Onda H, Fuse A, Yamaguchi M, et al. : Traumatic cerebrovascular injury following severe head injury: proper diagnostic timetable and examination methods. Neurol Med Chir (Tokyo) 53: 573–579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Biffl WL, Cothren CC, Moore EE, et al. : Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma 67: 1150–1153, 2009 [DOI] [PubMed] [Google Scholar]
- 10).Eastman AL, Muraliraj V, Sperry JL, Minei JP: CTA-based screening reduces time to diagnosis and stroke rate in blunt cervical vascular injury. J Trauma 67: 551–556; discussion 555–556, 2009 [DOI] [PubMed] [Google Scholar]
- 11).Fleck SK, Langner S, Baldauf J, Kirsch M, Kohlmann T, Schroeder HW: Incidence of blunt craniocervical artery injuries: use of whole-body computed tomography trauma imaging with adapted computed tomography angiography. Neurosurgery 69: 615–623; discussion 623–624, 2011 [DOI] [PubMed] [Google Scholar]
- 12).Kansagra AP, Cook DL, English JD, et al. : Current trends in endovascular management of traumatic cerebrovascular injury. J NeuroIntervent Surg 6: 47–50, 2014 [DOI] [PubMed] [Google Scholar]
- 13).Bynoe RP, Kerwin AJ, Parker HH, et al. : Maxillofacial injuries and life-threatening hemorrhage: treatment with transcatheter arterial embolization. J Trauma 55: 74–79, 2003 [DOI] [PubMed] [Google Scholar]
- 14).Cohen JE, Gomori JM, Segal R, et al. : Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery 63: 476–485; discussion 485–486, 2008 [DOI] [PubMed] [Google Scholar]
- 15).Komiyama M, Morikawa T, Nakajima H, Yasui T, Kan M: “Early” apoplexy due to traumatic intracranial aneurysm—Case report. Acta Neurol Med Chir (Tokyo) 41: 264–270, 2001 [DOI] [PubMed] [Google Scholar]
- 16).Inoue T, Tsutsumi K, Iijima A, Shinozaki M, Ishida J, Yako K: Urgent treatment of severe subarachnoid hemorrhage caused by ruptured traumatic aneurysm of the cavernous internal carotid artery using coil embolization followed by superficial temporal artery-middle cerebral artery anastomosis: a case report. Surg Neurol 64: 450–454; discussion 454–455, 2005 [DOI] [PubMed] [Google Scholar]
- 17).Griessenauer CJ, Fleming JB, Richards BF, et al. : Timing and mechanism of ischemic stroke due to extracranial blunt traumatic cerebrovascular injury. J Neurosurg 118: 397–404, 2013 [DOI] [PubMed] [Google Scholar]