Abstract
Background and Aim
Depressive pseudodementia (DPD) is a condition which may develop secondary to depression. The aim of this study was to contribute to the differential diagnosis between Alzheimer disease (AD) and DPD by comparing the neurocognitive tests and hippocampal volume.
Materials and Methods
Patients who met criteria of AD/DPD were enrolled in the study. All patients were assessed using the Wechsler Memory Scale (WMS), clock-drawing test, Stroop test, Benton Facial Recognition Test (BFRT), Boston Naming Test, Mini-Mental State Examination (MMSE), and Geriatric Depression Scale (GDS). Hippocampal volume was measured by importing the coronal T1-weighted magnetic resonance images to the Vitrea 2 workstation.
Results
A significant difference was found between the AD and DPD groups on the WMS test, clock-drawing test, Stroop test, Boston Naming Test, MMSE, GDS, and left hippocampal volume. A significant correlation between BFRT and bilateral hippocampal volumes was found in the AD group. No correlation was found among parameters in DPD patients.
Conclusions
Our results suggest that evaluation of facial recognition and left hippocampal volume may provide more reliable evidence for distinguishing DPD from AD. Further investigations combined with functional imaging techniques including more patients are needed.
Keywords: Alzheimer disease, Depression, Dementia, Cognition, Magnetic resonance imaging
Introduction
The burden on medical and social services is increasing as the world's population grows older. Alzheimer disease (AD) is the most prevalent form of dementia and the most common progressive disease of an aging population [1].
In 1898, Ganser reported some psychiatric cases that masqueraded as dementia, and thereafter the terms DPD and Ganser's syndrome started to be used interchangeably. Later, this syndrome was referred to as “depressive pseudodementia” owing to its association with depression. It has been highlighted that the intellectual loss in these patients might be associated with an unnoticed depression, and that it might be treatable [2]. Attention deficit and memory impairment are common in patients with pseudodementia, but cognitive deficit is more prominent in AD than pseudodementia. In some cases, it is difficult to differentiate DPD from other types of dementia until full recovery from depression is achieved [3].
Although the results of cerebrospinal fluid biomarkers, magnetic resonance imaging (MRI) and positron emission tomography studies can provide valuable clues about illness, clinical findings still provide best diagnostic clues for AD [4, 5].
Patients with AD have a significantly smaller hippocampus (10–50% reduction) than age-matched healthy controls [6]. A study of Dolek et al. [7] suggested a relationship between reduced hippocampal volume and cognitive impairment. A comparative study between AD and late life depression showed that AD is correlated with atrophy of the left anterior hippocampus and bilateral posterior cingulate cortex [8].
The present study investigated the relation between the neurocognitive test scores, which are used to differentiate AD from DPD, and hippocampal volume.
Materials and Methods
Patient Selection
Literate subjects, who were aged over 60 and presented to our neurology outpatient clinic for amnesia, were randomly enrolled in the study. Among these subjects, patients diagnosed with AD and DPD according to the DSM-IV criteria were selected, and then those with available MR images in the Picture Archiving and Communication System of the Department of Radiology were included in the study. The Global Deterioration Scale was administered to the patients diagnosed with AD, and accordingly, those with mild-to-moderate AD (stage 4–5) were included in the study. The patients were required to have had no prior treatment for AD or DPD in the past 6 months. Patients with a history of systemic diseases that may affect cognitive functions (uncontrolled endocrine diseases, etc.), stroke, epilepsy, neuroleptic drug use, and psychiatric diseases were excluded. The study was approved by the local ethics committee (acceptance No: B104ISM4340029/1009/53).
Neurocognitive Tests
A detailed medical history was obtained from patients and their relatives prior to the neurocognitive evaluation. Among the parameters of the Wechsler Memory Scale (WMS), we used WMS-I (personal and actual information), WMS-II (orientation), WMS-V (digit spans, backward and forward), and WMS-VI (visual memory test). The Turkish validation of the WMS was established by Karakas et al. [9]. Furthermore, the scores of the clock-drawing test, Stroop test, Benton Facial Recognition Test (BFRT) and Mini-Mental State Examination (MMSE) calculated for all patients were compared with the scores of the Geriatric Depression Scale (GDS). The Stroop test (color-word naming test) evaluates the executive processes to inhibit prepotent responses and has been validated by Karakas et al. [10]. The BFRT is focused on the topic of visuospatial perception based on prosopagnosia. The Turkish validation has been established by Keskinkilic [11].
The Boston Naming Test measures confrontational word retrieval in patients with neurodegenerative disorders. A short unregistered version of this test developed by the Neuropsychology Laboratory of Istanbul University was used. Participants were asked to name 31 items shown in the pictures. A revised version of the MMSE validated by Keskinoglu et al. [12] was applied to all subjects. GDS is a 30-item self-report assessment used to identify depression in elderly populations. The following cutoff levels are used to identify severity: normal 0–9, mild depression 10–19, and severe depression 20–30. The Turkish validation has been done by Ertan and Eker [13].
MRI and Volumetry
MRI sequences of the patients were obtained from the Picture Archiving and Communication System of the Department of Radiology. All examinations were carried out with a 1.5-T MRI Scanner (Intera, Philips Medical Systems, the Netherlands). Coronal, axial and sagittal sequences of T1- and T2-weighted MR images were obtained for each subject in the groups.
Hippocampal volume measurements were performed by importing the coronal T1-weighted MR images to the Vitrea 2 workstation (Vital Images, USA).
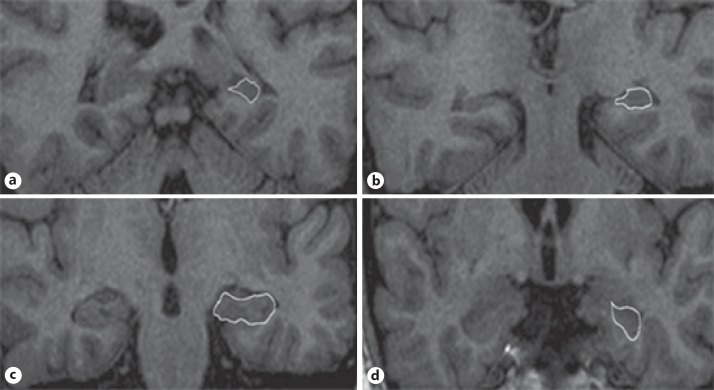
Boundaries of the hippocampus were defined using multiple sources and measured manually according to the method described by Soininen et al. [14]. The identifying method of hippocampal boundaries is illustrated and described, as a sample, in the legend to the selected images of a case of DPD as shown in Figure 1. The hippocampal volume was calculated automatically by a software program, and the results were presented in cubic millimeters. All hippocampal volumes were rated blindly to clinical diagnosis by consensus between two experienced raters (T.O.Ö. and R.Ç.).
Fig. 1.
Selected images of a depressive pseudodementia case illustrating the boundaries of the hippocampal formation from anterior (a) to posterior margins (d). The drawn objects indicate hippocampal formation; Ammon's horn, dentate gyrus, fimbria, and subiculum (b, c). The posterior end slice of the hippocampal tail is defined as the slice in which the crus of fornix is the longest on a coronal section (d).
Statistical Analysis
The Statistical Package for Social Sciences (SPSS for Windows, version 15.0) software was used for the statistical analyses. We used the Mann-Whitney U test to compare the right and left hippocampal volumes of the AD and DPD patients taking into account age, gender, education level and results of the neurocognitive tests. The comparison of the right and left hippocampal volumes with the results of the neurocognitive tests for the AD and DPD groups was performed using the Pearson correlation analysis. A p value less than 0.05 was considered significant.
Results
The present study included 20 patients with AD (13 female, 7 male) and 15 patients with DPD (10 female, 5 male). The age of the subjects in the AD group ranged from 60 to 87 years (mean: 73.9 ± 7.9 years), whereas the age of the subjects in the DPD group ranged from 62 to 86 years (mean: 70.5 ± 8.2 years). The duration of education was between 2 and 15 years (mean: 5.6 ± 3.6 years) in the AD group versus between 1 and 15 years (mean: 9.4 ± 5.3 years) in the DPD group. No statistically significant difference was found between the groups for the duration of education, gender, and age. There were statistically significant differences between the AD and DPD groups in the scores of the MMSE and WMS, clock-drawing test, Stroop test, Boston Naming Test, BFRT, GDS and the left hippocampal volume. Mild depression was found in 4 cases of AD. A descriptive analysis for each subject in the AD and DPD groups is shown in Table 1.
Table 1.
The results of a descriptive analysis of patients with Alzheimer disease (AD) and depressive pseudodementia (DPD)
| Demographic and neurocognitive data of groups | Mean ± SD | Min–Max | p value |
|---|---|---|---|
| Age, years | |||
| AD | 73.9±7.9 | 56–87 | 0.179 |
| DPD | 70.5±8.2 | 62–86 | |
| Education, years | |||
| AD | 5.6±3.6 | 2–15 | 0.059 |
| DPD | 9.4±5.3 | 1–15 | |
| Right hippocampus volume, mm3 | |||
| AD | 1.82±0.62 | 0.88–3.10 | 0.179 |
| DPD | 2.04±0.39 | 1.30–2.80 | |
| Left hippocampus volume, mm3 | |||
| AD | 1.58±0.64 | 0.68–3.20 | 0.019* |
| DPD | 1.90±0.30 | 1.30–2.30 | |
| WMS-I (personal and actual information) | |||
| AD | 3.9±1.3 | 1–6 | <0.0001* |
| DPD | 5.8±0.4 | 5–6 | |
| WMS-II (orientation) | |||
| AD | 3.25±1.4 | 1–5 | <0.0001* |
| DPD | 5±0 | 5–5 | |
| WMS-V (forward digit span) | |||
| AD | 4.3±0.97 | 3–6 | 0.261 |
| DPD | 4.6±0.81 | 3–6 | |
| WMS-V (backward digit span) | |||
| AD | 5.4±2.9 | 0–10 | 0.023* |
| DPD | 8.2±3.34 | 4–15 | |
| WMS-VI (visual memory) | |||
| AD | 1.75±1.25 | 0–4 | 0.009* |
| DPD | 2.93±1.16 | 0–4 | |
| Clock-drawing test | |||
| AD | 1.05±0.94 | 0–3 | 0.001* |
| DPD | 2.33±0.81 | 1–3 | |
| Stroop test | |||
| AD | 20.4±19.46 | 0–51 | <0.0001* |
| DPD | 0.66±0.89 | 0–3 | |
| Boston Naming Test | |||
| AD | 27.9±2.52 | 22–31 | 0.030* |
| DPD | 29.3±3.01 | 22–31 | |
| Benton Facial Recognition Test | |||
| AD | 37.4±3.99 | 29–45 | 0.002* |
| DPD | 39±7.08 | 19–47 | |
| Geriatric Depression Scale | |||
| AD | 8.05±4.65 | 3–19 | 0.026* |
| DPD | 14.06±8.5 | 2–31 | |
| MMSE score | |||
| AD | 22.35±2.36 | 19–26 | <0.0001* |
| DPD | 27.8±1.20 | 25–30 | |
WMS, Wechsler Memory Scale; MMSE, Mini-Mental State Examination.
p value less than 0.05 was considered significant.
When bilateral hippocampal volumes were compared with results of the neurocognitive tests, education level, and age in the AD cases, a positive significant correlation was found between BFRT, MMSE, and left hippocampal volume (Table 2). The linear regression test showed a significant impact of left hippocampal volume on the BFRT in the AD group (Table 3).
Table 2.
The correlation between hippocampal volumes and age, education level and neurocognitive profile of patients with Alzheimer disease
| Demographic and neurocognitive data of the Alzheimer disease group | Right hippocampal volume |
Left hippocampal volume |
||
|---|---|---|---|---|
| correlation coefficient | P | correlation coefficient | P | |
| Age (years) | −0.4 | 0.72 | −0.30 | 0.19 |
| Education (years) | +0.10 | 0.67 | +0.13 | 0.58 |
| WMS-I (personal and actual information) | −0.22 | 0.33 | −0.21 | 0.36 |
| WMS-II (orientation) | +0.13 | 0.58 | +0.19 | 0.41 |
| WMS-V (forward digit span) | +0.15 | 0.52 | +0.15 | 0.50 |
| WMS-V (backward digit span) | −0.18 | 0.44 | −0.20 | 0.39 |
| WMS-VI (visual memory) | +0.15 | 0.51 | +0.05 | 0.83 |
| Clock-drawing test | +0.31 | 0.18 | +0.21 | 0.37 |
| Stroop test | −0.23 | 0.31 | −0.24 | 0.30 |
| Boston Naming Test | +0.03 | 0.88 | +0.26 | 0.91 |
| Benton Facial Recognition Test | +0.65 | 0.002* | +0.706 | 0.001* |
| Geriatric Depression Scale | −0.12 | 0.59 | −0.24 | 0.29 |
| MMSE | +0.43 | 0.054 | +0.51 | 0.02* |
WMS, Wechsler Memory Scale; MMSE, Mini-Mental State Examination.
p value less than 0.05 was considered significant.
Table 3.
A linear regression model for variables which have an effect on the Benton Facial Recognition Test in the Alzheimer disease group
| Beta | t | P | |
|---|---|---|---|
| Left HV | 0.400 | 2.471 | 0.019 |
| Right HV | 0.081 | 0.333 | 0.741 |
| MMSE | 0.195 | 1.066 | 0.781 |
HV, Hippocampal volume; MMSE, Mini-Mental State Examination. p value less than 0.05 was considered significant. Dependent variable: Benton Facial Recognition Test.
No statistically significant correlation was found between right and left hippocampal volumes and other parameters in DPD patients (Table 4).
Table 4.
Correlation between hippocampal volumes and age, education level and neurocognitive profile of patients with depressive pseudodementia
| Demographic and neurocognitive data of pseudodementia group | Right hippocampal volume |
Left hippocampal volume |
||
|---|---|---|---|---|
| correlation coefficient | P | correlation coefficient | P | |
| Age (years) | −0.05 | 0.83 | −0.16 | 0.54 |
| Education (years) | +0.44 | 0.09 | +0.12 | 0.65 |
| WMS-I (personal and actual information) | +0.23 | 0.32 | +0.11 | 0.45 |
| WMS-II (orientation) | −0.27 | 0.31 | +0.26 | 0.33 |
| WMS-V (forward digit span) | +0.04 | 0.98 | −0.08 | 0.75 |
| WMS-V (backward digit span) | +0.02 | 0.93 | −0.28 | 0.30 |
| WMS-VI (visual memory) | −0.05 | 0.85 | +0.13 | 0.66 |
| Clock-drawing test | −0.21 | 0.44 | +0.11 | 0.68 |
| Stroop test | −0.15 | 0.57 | −0.00 | 0.99 |
| Boston Naming Test | +0.30 | 0.91 | −0.03 | 0.89 |
| Benton Facial Recognition Test | −0.24 | 0.38 | −0.41 | 0.12 |
| Geriatric Depression Scale | +0.15 | 0.58 | −0.23 | 0.40 |
| MMSE | +0.18 | 0.26 | −0.30 | 0.45 |
WMS, Wechsler Memory Scale; MMSE, Mini-Mental State Examination. p value less than 0.05 was considered significant.
Discussion
It is usually difficult to differentiate AD from DPD. Neurocognitive tests are quite helpful in the differential diagnosis between DPD and neurodegenerative dementia. Nevertheless, they often do not provide definite results [15].
In the present study, AD and DPD groups were compared on the basis of neurocognitive tests and radiological findings. All test results were significantly impaired in the AD patients compared to the DPD patients. When results of neurocognitive tests were compared on the basis of hippocampal volumes, a significant correlation was found between the left hippocampal volume and the MMSE and BFRT scores in the AD group. If patients with similar MMSE scores had been enrolled to study in order to compare the hippocampal volume, the results of our study might have been more valuable.
Watson et al. [16] conducted a study in healthy volunteers and measured amygdala and hippocampal volumes using high-resolution MR images. They found that the right hippocampal volume was 5.2 ± 0.65 cm3 whereas the left hippocampal volume was 4.9 ± 0.68 cm3. Hsu et al. [17] measured hippocampal volume in healthy older persons and in patients with dementia, both manually and automatically, and compared these two techniques. They found no significant difference between the manual and automatic measurements. In patients with AD, the right hippocampal volume was 1.82 ± 0.59 cm3 while the left hippocampal volume was 1.79 ± 0.54 cm3. Chupin et al. [18] found that the mean volumes of the left and right hippocampus were 2.49 cm3 in healthy older persons and 1.69 cm3 in AD patients. In the present study, the measurements were done manually, and the mean volume was 1.82 cm3 for the right hippocampus and 1.58 cm3 for the left hippocampus of the AD patients. O'Brien [19] reported that temporal lobe imaging has 85–95% specificity and sensitivity in differentiating AD. Bottino et al. [20] demonstrated that the volumes of the left amygdala, hippocampus and parahippocampal gyrus show differences between those with mild cognitive impairment (MCI) and normal subjects. Eckerström et al. [21] found that the left hippocampal volume, in particular, has prognostic value in progression to AD in patients with MCI. In the present study, a comparison of the hippocampal volumes between the AD and DPD groups revealed that the left hippocampal volume was significantly reduced in the AD group compared to the DPD group.
The relationship between AD and depression is complicated. Depression may involve an increased risk for later developing AD, especially at an advanced age [22, 23]. One possible link may be the long-term occurrence of inflammatory processes that may underlie depression and AD. This possibility is intriguing in light of evidence that antidepressants can modify levels of inflammatory cytokines. Also, depression treatment will affect cognitive outcome of AD [24].
In our study, the mean score on the GDS in the AD group was found to be within normal limits, whereas mild depression was found in the DPD group. Follow-up studies of the DPD group may provide more knowledge about the relation between these two disorders. Suzuki et al. [25] found that the left hippocampal volume was negatively correlated with corticolimbic activation when identifying emotional faces in both children with a history of preschool onset of major depressive disorder and healthy children during functional MRI study. In the present study, reduced left hippocampal volume in AD patients, in whom corticolimbic connections are known to be impaired, was consistent with the result of the above-mentioned study.
In AD, cognitive disorders develop in three main stages. Episodic memory impairment is prominent in the first stage. In the next stage, impairment in mental functions has an impact on daily life. In this stage, which is defined as the early clinical stage, visuospatial perception, verbal fluency and naming are significantly influenced in addition to episodic memory. Impairment in semantic memory, which is the basis of knowledge and language, becomes more prominent in this stage. Impairment in knowledge recall and knowledge access begins to appear [26, 27]. We evaluated semantic memory using neurocognitive tests including the Boston Naming Test and MMSE. These tests revealed that semantic memory was influenced remarkably in the AD group compared to the DPD group.
Colliot et al. [28] investigated the specificity and sensitivity of hippocampal volume measurement in the AD, MCI, and control groups. In differentiating AD from controls, both the sensitivity and specificity of hippocampal volume measurements were 84%. In differentiating MCI from controls, the sensitivity and specificity were 75 and 70%, respectively. In the present study, we did not investigate specificity and sensitivity. However, it was assumed that a decrease in the left hippocampal volume might be specific to AD. Smith et al. [29] compared the results of temporal lobe volume and neurocognitive test scores between 20 patients with moderate-to-severe AD and 20 controls. They found a significant correlation between neurocognitive test scores and severity of temporal lobe atrophy. The present study included patients with mild-to-moderate AD. Since the groups were similar in stage of disease, the relationship between disease severity and hippocampal volume could not be assessed.
Volumetric studies in the depression group versus controls demonstrated the presence of notable atrophy in the temporal lobe [30]. Cole et al. [31] measured hippocampal volume in 191 depressive patients and in 282 healthy controls. They found a significant difference in both right and left hippocampal volumes between the depressive cases versus the controls. Based on this finding, they indicated that a decrease in the hippocampal volume may be important in the diagnosis of depression. In the present study, a significant difference was found between the hippocampal volumes of the AD and DPD groups. However, the absence of a control group does not allow us to comment on hippocampal atrophy patterns in DPD patients.
Mounton et al. [32] in 1998 and Baxter et al. [33] in 2006 suggested the presence of a strong correlation between a decrease in MMSE scores and cortical volume loss in AD patients. A significant correlation was found between left hippocampal volume and MMSE scores in the AD group. It has been reported that hippocampal volume is also influenced by the duration and frequency of depression.
Some studies report right-sided hippocampal atrophy while some others report left-sided hippocampal atrophy, whereas some other studies report no statistically significant hippocampal atrophy in depression [33, 34, 35, 36]. Greenberg et al. [37] failed to show a significant relation between subtypes of depression and hippocampal volumes. In the present study, we only used GDS, but did not identify subtypes of depression. Also, we have no data about the duration of depression. For this reason, we have no detailed conclusion regarding the relation between depression and hippocampal volume.
The normalization of regional volumes of the brain by intracranial volume (ICV) would be necessary to show the effect of disease-related atrophy [38]. However, in a study of the Alzheimer's Disease Neuroimaging Initiative, which evaluated MRI data from two large cohorts, the researchers found that the association between hippocampal volumes and cognition was not altered by ICV normalization [39]. Thus, ICV normalization was not performed in our study.
One of the widely used tests developed by Benton et al. [40] is the facial recognition test and another is the line orientation test. The present study used the BFRT. These tests assess the visuoperceptual skills of the parietal, parieto-occipital and occipitotemporal configurations of the right hemisphere. Tranel et al. [41] investigated the anatomical relation of the BFRT and line orientation test in patients with a focal cerebral lesion. They found that impairment in the BFRT is particularly associated with lesions of the right postero-inferior parietal and right ventral occipitotemporal regions. In another study, the BFRT was administered to healthy subjects aged between 20 and 92 years and the perceptual processing speed was compared with the ratio of frontal lobe volume/cerebral ventricle. Consequently, a correlation was found between cerebral atrophy and perceptual processing speed in approximately 35% of patients [42]. In the present study, a significant correlation was found between the BFRT score and both the right and left hippocampal volume in the AD group.
Conclusion
To the best of our knowledge, this is the first published study comparing AD and DPD based on hippocampal volumetry. Our study addresses two important issues: the first one is the necessity of considering left hippocampal volumetry in dementia practice and the second, keeping in mind that BFRT is more sensitive than other neurocognitive tests used in hippocampal research. Further studies including a larger patient population and control group combined with functional imaging techniques are needed.
Disclosure Statement
The authors declare no competing interests related to the research covered in this paper.
References
- 1.Fita IG, Enciu AM, Stănoiu BP. New insights on Alzheimer's disease diagnostic. Rom J Morphol Embryol. 2011;52:975–979. [PubMed] [Google Scholar]
- 2.Cosgray RE, Fawley RW. Could it be Ganser's syndrome? Arch Psychiatr Nurs. 1989;3:241–245. [PubMed] [Google Scholar]
- 3.da Silva Novaretti TM, D'Ávila Freitas MI, Mansur LL, Nitrini R, Radanovic M. Comparison of language impairment in late-onset depression and Alzheimer's disease. Acta Neuropsychiatr. 2011;23:62–68. doi: 10.1111/j.1601-5215.2011.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed RM, Paterson RW, Warren JD, Zetterberg H, O'Brien JT, Fox NC, Halliday GM, Schott JM. Biomarkers in dementia: clinical utility and new directions. J Neurol Neurosurg Psychiatry. 2014;85:1426–1434. doi: 10.1136/jnnp-2014-307662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien J, Barber B. Neuroimaging in dementia and depression. Adv Psychiatr Treat. 2000;6:109–119. [Google Scholar]
- 7.Dolek N, Saylisoy S, Ozbabalik D, Adapinar B. Comparison of hippocampal volume measured using magnetic resonance imaging in Alzheimer's disease, vascular dementia, mild cognitive impairment and pseudodementia. J Int Med Res. 2012;40:717–725. doi: 10.1177/147323001204000236. [DOI] [PubMed] [Google Scholar]
- 8.Boccia M, Acierno M, Piccardi L. Neuroanatomy of Alzheimer's disease and late-life depression: a coordinate-based meta-analysis of MRI studies. J Alzheimers Dis. 2015;46:963–970. doi: 10.3233/JAD-142955. [DOI] [PubMed] [Google Scholar]
- 9.Karakas S, Kafadar H, Eski R. Wechsler Bellek Ölçeği Geliştirilmiş Formunun test-tekrar test güvenirliği. Türk Psikoloji Dergisi. 1996;11:46–52. [Google Scholar]
- 10.Karakas S, Erdogan E, Sak L, Soysal AS, Ulusoy T, Yuceyurt Ulusoy I, Alkan S, Stroop Test TBAG Form Standardisation for Turkish culture, reliability and validity. J Clin Psychiatry. 1999;2:75–88. [Google Scholar]
- 11.Keskinkilic C. Standardization of Benton Face Recognition Test in a Turkish normal adult population. Türk Nörol Derg. 2008;14:179–190. [Google Scholar]
- 12.Keskinoglu P, Ucku R, Yener G, Yaka E, Kurt P, Tunca Z. Reliability and validity of revised Turkish version of Mini Mental State Examination (rMMSE-T) in community-dwelling educated and uneducated elderly. Int J Geriatr Psychiatry. 2009;24:1242–1250. doi: 10.1002/gps.2252. [DOI] [PubMed] [Google Scholar]
- 13.Ertan T, Eker E. Reliability, validity, and factor structure of the geriatric depression scale in Turkish elderly: are there different factor structures for different cultures? Int Psychogeriatr. 2000;12:163–172. doi: 10.1017/s1041610200006293. [DOI] [PubMed] [Google Scholar]
- 14.Soininen H, Partanen K, Pitkänen A, Hallikainen M, Hänninen T, Helisalmi S, Mannermaa A, Ryynänen M, Koivisto K, Riekkinen P., Sr Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E epsilon 4 allele. Neurology. 1995;45:391–392. doi: 10.1212/wnl.45.2.391. [DOI] [PubMed] [Google Scholar]
- 15.Fisman M. Pseudodementia. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:481–484. doi: 10.1016/0278-5846(85)90005-3. [DOI] [PubMed] [Google Scholar]
- 16.Watson C, Anderman F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 17.Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chupin M, Gérardin E, Cuingnet R, Boutet C, Lemieux L, Lehéricy S, Benali H, Garnero L, Colliot O. Alzheimer's Disease Neuroimaging Initiative. Fully automatic hippocampus segmentation and classification in Alzheimer's disease and mild cognitive impairment applied on data from ADNI. Hippocampus. 2009;19:579–587. doi: 10.1002/hipo.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien JT. Is hippocampal atrophy on magnetic resonance imaging a marker for Alzheimer's disease? Int J Geriatr Psychiatr. 1995;10:431–435. [Google Scholar]
- 20.Bottino CM, Castro CC, Gomes RL, Buchpiguel CA, Marchetti RL, Neto MR. Volumetric MRI measurements can differentiate Alzheimer's disease, mild cognitive impairment, and normal aging. Int Psychogeriatr. 2002;14:59–72. doi: 10.1017/s1041610202008281. [DOI] [PubMed] [Google Scholar]
- 21.Eckerström C, Olsson E, Borga M, Ekholm S, Ribbelin S, Rolstad S, Starck G, Edman A, Wallin A, Malmgren H. Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: the Göteborg MCI study. J Neurol Sci. 2008;15(272):48–59. doi: 10.1016/j.jns.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D's – delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15:365. doi: 10.1007/s11920-013-0365-4. [DOI] [PubMed] [Google Scholar]
- 24.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, Botteron KN, Luby JL, Belden AC, Gaffrey MS, Babb CM, Nishino T, Miller MI, Ratnanather JT, Barch DM. Structural-functional correlations between hippocampal volume and cortico-limbic emotional responses in depressed children. Cogn Affect Behav Neurosci. 2013;13:135–151. doi: 10.3758/s13415-012-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almkvist O. Neuropsychological features of early Alzheimer's disease; preclinical and clinical stages. Acta Neurol Scand. 1996;165:63–71. doi: 10.1111/j.1600-0404.1996.tb05874.x. [DOI] [PubMed] [Google Scholar]
- 27.Karakas S, Irkeç C. Alzheimer hastaligi kliniginin nöropsikolojik profili. Turkiye Klinikleri J Neur. 2003;1:13–22. [Google Scholar]
- 28.Colliot O, Chételat G, Chupin M, Desgranges B, Magnin B, Benali H, Dubois B, Garnero L, Eustache F, Lehéricy S. Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology. 2008;248:194–201. doi: 10.1148/radiol.2481070876. [DOI] [PubMed] [Google Scholar]
- 29.Smith CD, Malcein M, Meurer K, Schmitt FA, Markesbery WR, Pettigraw LC. MRI temporal lobe volume measures and neuropsychologic function in Alzheimer's disease. Neuroimaging. 1999;9:2–9. doi: 10.1111/jon1999912. [DOI] [PubMed] [Google Scholar]
- 30.Altshuler LL, Conrad A, Hauser P, Li XM, Guze BH, Denikoff K, Tourtellotte W, Post R. Reduction of temporal lobe volume in bipolar disorder: a preliminary report of magnetic resonance imaging. Arch Gen Psychiatry. 1991;48:482–483. doi: 10.1001/archpsyc.1991.01810290094018. [DOI] [PubMed] [Google Scholar]
- 31.Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. Affect Disord. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 32.Mouton PR, Martın LJ, Calhoun ME, DalForno G, Price DL. Cognitive decline strongly correlates with cortical atrophy in Alzheimer's dementia. Neurobiol Aging. 1998;19:371–377. doi: 10.1016/s0197-4580(98)00080-3. [DOI] [PubMed] [Google Scholar]
- 33.Baxter LC, Sparks DL Johnson SC, Lenoski B, Lopez JE, Connor DJ, Sabbagh MN. Relationship of cognitive measures and gray and white matter in Alzheimer's disease. J Alzheimers Dis. 2006;9:253–260. doi: 10.3233/jad-2006-9304. [DOI] [PubMed] [Google Scholar]
- 34.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 35.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 36.Sheline YI. Neuroimaging studies of mood disorder effects of the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg DL, Payne ME, MacFall JR, Steffens DC, Krishnan RR. Hippocampal volumes and depression subtypes. Psychiatry Res. 2008;163:126–132. doi: 10.1016/j.pscychresns.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scahill RI, Frost C, Jenkins R, Whitwell J L, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 39.Voevodskaya O, Simmons A, Nordenskjöld R, Kullberg J, Ahlström H, Lind L, Wahlund LO, Larsson EM, Westman E. Alzheimer's Disease Neuroimaging Initiative. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front Aging Neurosci. 2014;6:264. doi: 10.3389/fnagi.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benton AL, Sivan AB, Hamsher KS, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. New York: Oxford University Press; 1994. pp. 35–53. [Google Scholar]
- 41.Tranel D, Vianna E, Manzel K, Damasio H, Grabowski T. Neuroanatomical correlates of the Benton Facial Recognition Test and Judgment of Line Orientatıon Test. J Clin Exp Neuropsychol. 2009;31:219–233. doi: 10.1080/13803390802317542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schretlen DJ, Pearlson GD, Anthony JC, Yates KO. Determinants of Benton facial recognition. Neuropsychology. 2001;15:405–410. doi: 10.1037//0894-4105.15.3.405. [DOI] [PubMed] [Google Scholar]