Abstract
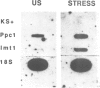
Molecular mechanisms of osmotic stress tolerance were studied in Mesembryanthemum crystallinum (ice plant), a facultative halophyte capable of adjusting to and surviving in highly saline conditions. We screened a subtracted cDNA library enriched for salt stress-induced mRNAs to identify transcripts involved in this plant's adaptation to salinity. One mRNA, Imt1, was found to be up-regulated in leaves and, transiently, in roots. Nuclear run-on assays indicated that this mRNA is transcriptionally regulated. Imt1 encoded a predicted polypeptide of M(r) 40,250 which exhibited sequence similarity to several hydroxymethyl transferases. Expression of the protein in Escherichia coli and subsequent activity assays identified the protein as a novel myoinositol O-methyl transferase which catalyzes the first step in the biosynthesis of the cyclic sugar alcohol pinitol. Pinitol accumulates in salt-stressed M.crystallinum and is abundant in a number of salt- and drought-tolerant plants. The presence of high levels of sugar alcohols correlates with osmotolerance in a diverse range of organisms, including bacteria, fungi and algae, as well as higher plants. The stress-initiated transcriptional induction of IMT1 expression in a facultative halophyte provides strong support for the importance of sugar alcohols in establishing tolerance to osmotic stress in higher plants.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels D., Engelhardt K., Roncarati R., Schneider K., Rotter M., Salamini F. An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein. EMBO J. 1991 May;10(5):1037–1043. doi: 10.1002/j.1460-2075.1991.tb08042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hess F. D., Bressan R. A., Hasegawa P. M. Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol. 1988 Feb;86(2):607–614. doi: 10.1104/pp.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Plant productivity and environment. Science. 1982 Oct 29;218(4571):443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Briehl M. M., Flomerfelt F. A., Wu X. P., Miesfeld R. L. Transcriptional analyses of steroid-regulated gene networks. Mol Endocrinol. 1990 Feb;4(2):287–294. doi: 10.1210/mend-4-2-287. [DOI] [PubMed] [Google Scholar]
- Bugos R. C., Chiang V. L., Campbell W. H. cDNA cloning, sequence analysis and seasonal expression of lignin-bispecific caffeic acid/5-hydroxyferulic acid O-methyltransferase of aspen. Plant Mol Biol. 1991 Dec;17(6):1203–1215. doi: 10.1007/BF00028736. [DOI] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Plant A. L., Moses M. S., Bray E. A. Organ-Specific and Environmentally Regulated Expression of Two Abscisic Acid-Induced Genes of Tomato : Nucleotide Sequence and Analysis of the Corresponding cDNAs. Plant Physiol. 1991 Dec;97(4):1367–1374. doi: 10.1104/pp.97.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Hanson A. D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Cushman J. C., Meyer G., Michalowski C. B., Schmitt J. M., Bohnert H. J. Salt stress leads to differential expression of two isogenes of phosphoenolpyruvate carboxylase during Crassulacean acid metabolism induction in the common ice plant. Plant Cell. 1989 Jul;1(7):715–725. doi: 10.1105/tpc.1.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rocher E. J., Harkins K. R., Galbraith D. W., Bohnert H. J. Developmentally regulated systemic endopolyploid in succulents with small genomes. Science. 1990 Oct 5;250(4977):99–101. doi: 10.1126/science.250.4977.99. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gepstein S., Heikkila J. J., Dumbroff E. B. Use of a scanning densitometer or an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem. 1988 Mar;169(2):227–233. doi: 10.1016/0003-2697(88)90278-3. [DOI] [PubMed] [Google Scholar]
- Gowri G., Bugos R. C., Campbell W. H., Maxwell C. A., Dixon R. A. Stress Responses in Alfalfa (Medicago sativa L.): X. Molecular Cloning and Expression of S-Adenosyl-l-Methionine:Caffeic Acid 3-O-Methyltransferase, a Key Enzyme of Lignin Biosynthesis. Plant Physiol. 1991 Sep;97(1):7–14. doi: 10.1104/pp.97.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J., Sánchez-Martínez D., Stiefel V., Rigau J., Puigdomènech P., Pagès M. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature. 1988 Jul 21;334(6179):262–264. doi: 10.1038/334262a0. [DOI] [PubMed] [Google Scholar]
- Iraki N. M., Bressan R. A., Hasegawa P. M., Carpita N. C. Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol. 1989 Sep;91(1):39–47. doi: 10.1104/pp.91.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida I., Obinata M., Deguchi T. Molecular cloning and nucleotide sequence of cDNA encoding hydroxyindole O-methyltransferase of bovine pineal glands. J Biol Chem. 1987 Feb 25;262(6):2895–2899. [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem J. A., Olson S. W., Schmitt J. M., Bohnert H. J. Salt Stress Increases the Level of Translatable mRNA for Phosphoenolpyruvate Carboxylase in Mesembryanthemum crystallinum. Plant Physiol. 1987 Aug;84(4):1270–1275. doi: 10.1104/pp.84.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem J. A., Vernon D. M., Bohnert H. J. Increased expression of a gene coding for NAD:glyceraldehyde-3-phosphate dehydrogenase during the transition from C3 photosynthesis to crassulacean acid metabolism in Mesembryanthemum crystallinum. J Biol Chem. 1990 Feb 25;265(6):3497–3502. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant A. L., Cohen A., Moses M. S., Bray E. A. Nucleotide sequence and spatial expression pattern of a drought- and abscisic Acid-induced gene of tomato. Plant Physiol. 1991 Nov;97(3):900–906. doi: 10.1104/pp.97.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B. Is there an osmotic regulatory mechanism in algae and higher plants? J Theor Biol. 1977 Sep 7;68(1):17–26. doi: 10.1016/0022-5193(77)90224-7. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Vernon D. M., Ostrem J. A., Schmitt J. M., Bohnert H. J. PEPCase Transcript Levels in Mesembryanthemum crystallinum Decline Rapidly upon Relief from Salt Stress. Plant Physiol. 1988 Apr;86(4):1002–1004. doi: 10.1104/pp.86.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. M. Version 5 of the Mount-Conrad-Myers Sequence Analysis Software Package now available. Comput Appl Biosci. 1988 Mar;4(1):211–211. doi: 10.1093/bioinformatics/4.1.211. [DOI] [PubMed] [Google Scholar]
- Winter K., Gademann R. Daily Changes in CO(2) and Water Vapor Exchange, Chlorophyll Fluorescence, and Leaf Water Relations in the Halophyte Mesembryanthemum crystallinum during the Induction of Crassulacean Acid Metabolism in Response to High NaCl Salinity. Plant Physiol. 1991 Mar;95(3):768–776. doi: 10.1104/pp.95.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]