Abstract
Purpose
To report a case of intratumoral gene expression profile discordance in a malignant uveal melanoma, associated with intratumoral heterogeneity based upon histopathologic features.
Methods
The clinical history, fundus findings, imaging and histopathologic features, and DecisionDx-UM gene expression profile results (Castle Biosciences, Inc., Phoenix, AZ, USA) of the tumor were reviewed.
Results
A trans-retinal fine-needle aspiration biopsy was performed for a thin, pigmented choroidal tumor in a 33-year-old man. Cells obtained from this biopsy were tested using the DecisionDx-UM gene expression profile test and the tumor was classified as class 1A. Cytology confirmed melanoma. The patient subsequently elected to undergo enucleation. On microscopic examination of the globe, the tumor was composed primarily of spindle B cells, but had a focal area composed of epithelioid cells. This portion of the tumor was subsequently tested and demonstrated a class 1B gene expression profile.
Conclusion
Intratumoral discordance in gene expression profile results has been described in uveal melanomas. Here we demonstrate that this discordance may be associated in some cases with intratumoral heterogeneity based upon histopathologic features.
Keywords: Uveal melanoma, Gene expression profile testing, Fine-needle aspiration biopsy, Tumor heterogeneity
Introduction
Uveal melanoma is associated with high rates of metastatic disease, despite good local control of the intraocular tumor with radiation or enucleation. Gene expression profile (GEP) testing is now a common method used by clinicians to clarify risk for metastatic disease for individuals being treated for uveal melanoma [1]. It is an RNA-based transcriptional analysis of 12 discriminant genes and 3 control genes, which has been shown in a prospective clinical trial to differentiate between tumors with high risk for metastasis (class 2 tumors) and lower risk for metastasis (class 1) [2,3]. Class 1 tumors more closely resemble normal uveal melanocytes in their gene expression patterns, whereas class 2 tumors more closely resemble primitive neural/ectodermal stem cells with lost expression of melanocytic genes [4]. Further subclassification of class 1 tumors into 1A and 1B tumors suggests a better prognosis for class 1A tumors, thus making it clinically relevant to distinguish between the two subgroups [3,5,6].
With increasing use of GEP testing and increasing reliance of clinicians on the GEP results for ongoing management of tumors, metastatic surveillance decisions, and clinical trial enrollment, a comprehensive understanding of the reliability of this testing modality is critical. Intratumoral genetic heterogeneity is a known phenomenon in uveal melanoma, and has now been described with both chromosomal analyses and GEP testing [2,7,8]. One concern with chromosomal analysis of tumors is that this testing has been demonstrated to be subject to sampling error due to the variance of distribution within tumors [7]. The Coupland group has shown that multiplex ligation-dependent probe amplification testing of microdissected formalin-fixed uveal melanoma tissue demonstrated variation in chromosomal status for chromosomes 1, 3, 6, and 8 in 75% of tumors evaluated, suggesting that single-site biopsy testing may not be representative of the chromosomal status of the whole tumor. However, while there was variation present, the overall determination of chromosome 3 status was generally the same between sites and when compared with the entire tumor as a whole [7,9]. Indeed, only exceptional cases have clearly demonstrated genetic differences which correlate to distinct histopathologic areas within the same tumor [10].
In initial studies, GEP testing appeared to demonstrate less heterogeneity across tumors than monosomy 3 testing [2]. However, Augsburger and colleagues [8] recently reported an 11% rate of GEP discordance among tumors tested with two fine-needle aspiration biopsy (FNAB) samples. Here we report a case in which histopathologic evidence of tumor heterogeneity is associated with intratumoral discordance of GEPs, providing the first clinical pathologic correlation of GEP discordance.
Case Report
A 33-year-old patient presented with gradual decrease in vision in the right eye for 5 years. His medical history included neurofibromatosis type 1 and developmental delay. He was a glaucoma suspect based upon optic nerve appearance.
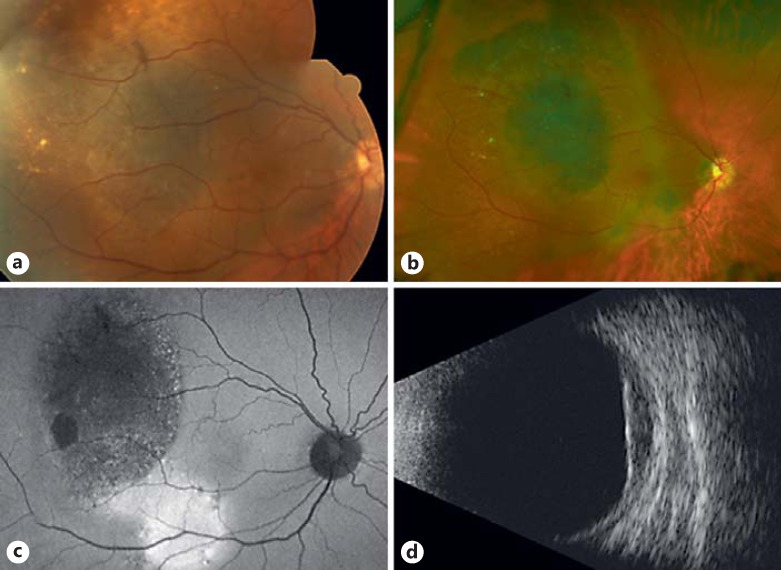
At presentation, best-corrected visual acuity was count fingers at 3 feet for the right eye and 20/60 for the left eye. Intraocular pressures were within normal limits. Slit-lamp examination revealed Lisch nodules in both eyes. Dilated fundoscopic examination revealed a broad-based, mildly elevated, pigmented lesion involving the macula of the right eye, with overlying retinal pigment epithelial metaplasia (Fig. 1a-c). There was an epiretinal membrane present that involved the entire macula and extended onto the disc margin. Drusen were present overlying the lesion, suggestive of chronicity, and there was no lipofuscin or subretinal fluid observed. The dilated funduscopic findings in the left eye were unremarkable. B-scan ultrasound showed a choroidal lesion occupying the entire macula from the disc to the temporal mid-periphery of the globe with low internal reflectivity (Fig. 1d). The tumor thickness was 2.5 mm. The sclera appeared intact by B-scan echography. A review of historic photographs revealed no melanocytic lesion in photos taken 22 years prior, at age 11. There was a melanocytic lesion present in the macula in photos taken at age 21, but the lesion appeared to have enlarged substantially since that time. Based upon these findings, a uveal melanoma was suspected and after a discussion of management options, the patient elected to proceed with diagnostic FNAB of the lesion.
Fig. 1.
Clinical imaging of the choroidal tumor at initial evaluation in the ocular oncology clinic. a Color fundus photograph. b Optos fundus photograph. c Fundus autofluorescence. d B-scan echography (longitudinal).
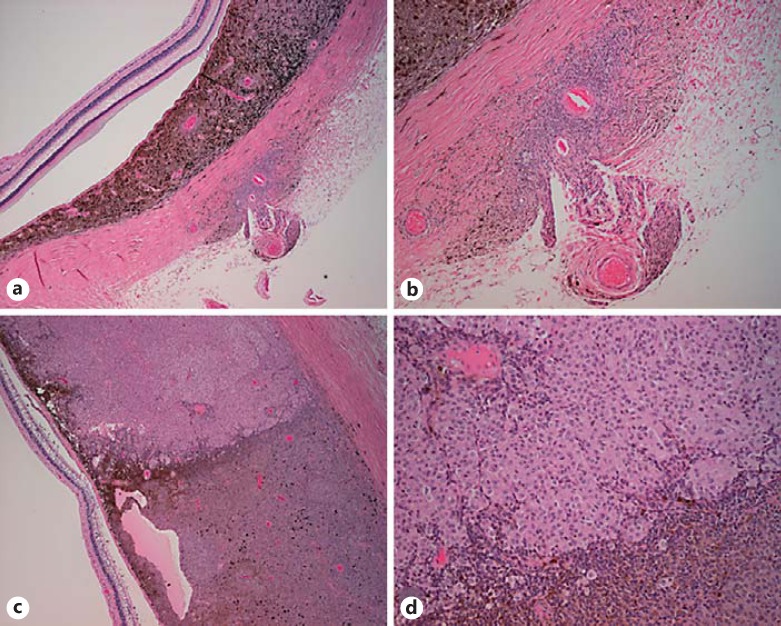
A trans-retinal FNAB was performed for diagnostic and prognostic purposes. Cytology revealed pigment-containing cells with small nuclei, condensed chromatin, and prominent nucleoli. The atypical cells were consistent with melanoma, though other pigment-containing neoplasms could not be completely excluded. The DecisionDx-UM GEP test classified the tumor as class 1A with a discriminant value of 0.96, indicating a low risk for metastatic disease. Systemic imaging did not reveal findings consistent with metastatic uveal melanoma. The options of close observation, proton beam radiotherapy, and enucleation were discussed with the patient. The patient and family preferred enucleation. On gross examination, the globe showed evidence of extrascleral extension in the macula near the optic nerve. On microscopic examination, there were nests of tumor cells in the sclera adjacent to the optic nerve with extrascleral extension along a ciliary artery (Fig. 2a, b). The tumor appeared consistent with a mixed-cell-type malignant melanoma. The majority of the melanoma was composed of spindle B cells with high nuclear-to-cytoplasmic ratio and coarse granular chromatin. However, there was a distinct area occupying 25% of the choroidal mass composed of cells with an epithelioid appearance (Fig. 2c, d). Given the marked intratumoral heterogeneity, there was concern that the initial FNAB might not have sampled the higher-grade portion of the tumor. Therefore, repeat GEP testing was performed on paraffin-embedded tissue from the epithelioid portion of the tumor, which demonstrated a class 1B GEP with a discriminant value of 0.85. Based upon the histopathologic and GEP results, the prognosis with regards to risk for metastatic disease for the patient was revised to be poorer, with risk for clinically apparent metastatic disease at 5 years increasing 10-fold from 2 to 21%. To date, the patient has not developed metastatic disease after 2 years of follow-up.
Fig. 2.
Histopathology. a Low magnification of choroidal tumor (H&E, ×4). b Extrascleral extension along ciliary artery (H&E, ×20). c Tumor heterogeneity (H&E, ×20) with eosinophilic appearance of the epithelioid portion of the tumor in comparison to the spindle B cell portion of the tumor. d High-magnification view of tumor heterogeneity (H&E, ×40).
Discussion
Intratumoral heterogeneity with regards to chromosomal analyses in uveal melanomas is a well-recognized issue which can affect accuracy of prognostication using this technique [7]. Many groups now favor GEP testing over chromosomal analyses for prognostic biopsy in uveal melanoma, and GEP testing using intraoperative FNAB is now commonly used for prognostic classification of primary uveal melanomas [1]. Early results suggested this testing offered a “snapshot” of the tumor as a whole, with little intratumoral heterogeneity, thus offering improved accuracy of prognostication from a single needle sampling. GEP analysis requires substantially less tissue than chromosomal detection methods and the rate of test failure from insufficient tissue is low in comparison [11].
There are limited data on GEP intratumoral heterogeneity and the frequency of this phenomenon is not yet known; however, it appears to be more common than initially suggested. Onken et al. [12] investigated regional heterogeneity and gene expression signatures in eight tumors and found regional discordance within one tumor with a low discriminate value that reduced the result confidence. More recent work has demonstrated a low but consequential rate of intratumoral GEP signature discordance among two-site tumor biopsies [8]. Augsburger's study reported discordance in 11.3% (n = 80) of the uveal melanomas that were biopsied at two distinct sites and even greater discordance in the tumors ≤3.5 mm thick (23.8%) [8]. Tumors with discordant results were observed to behave more closely to class 2 tumors. These studies preceded the class 1A and class 1B designations, so no data evaluating discordance within class 1 categories was collected. Some have advised two-site testing because it has been shown to decrease the odds of underestimating the risk of metastasis [8]. However, multi-site testing of small tumors can be associated with increased risk of surgical complications and may be associated with an increased cost of testing. The Collaborative Ocular Oncology Group recommended in Report No. 1 to sample areas separately when morphologically distinct tumor components are present based upon clinical evaluation [2]. In this case, no morphologically distinct areas were noted on clinical exam; however, histopathologic and GEP heterogeneity was nonetheless present.
The case presented provides the first clinicopathologic correlation in which histopathologic intratumoral heterogeneity of a primary uveal melanoma is associated with heterogeneity of the GEP classification. The higher-grade portion of the tumor on histopathology demonstrated a more worrisome GEP class than the initial biopsy, although the discordance was mild (class 1A vs. class 1B). The clinical implications of intratumoral heterogeneity and GEP discordance remain unclear, but GEP testing to assist in determining the suitability of observation for small or indeterminate tumors should be interpreted with caution, as underestimation of metastatic risk is possible. In addition, when counseling patients with uveal melanoma, it is important to consider other factors known to impact survival, including the anatomic disease staging, rather than considering the results of genetic testing in isolation [13,14,15,16]. It is likely that there is an interplay between these multiple prognostic factors that ultimately will determine an individual's prognosis, and a prognostication algorithm that integrates genetic testing data with other clinical and pathologic criteria will likely prove to be most predictive for survival.
Statement of Ethics
The study protocol was exempted by the Oregon Health and Science University Institutional Review Board. The study was conducted in accordance with the provisions of the Declaration of Helsinki and was performed in compliance with the Health Insurance Portability and Accountability Act.
Disclosure Statement
The authors have no conflicts of interest or financial disclosures to declare.
Author Contributions
Study design: A.K.M., D.J.W., A.H.S. Data analysis: A.K.M., D.J.W., A.H.S. Manuscript preparation: A.K.M., M.J.B, D.J.W., A.H.S. A.H.S. has had full access to all the data and takes responsibility for the integrity and accuracy of the data presented.
Acknowledgements
Supported by a Lloyd Research Endowment Faculty Grant (A.H.S.) and by an unrestricted departmental grant from Research to Prevent Blindness (New York, NY, USA).
References
- 1.Aaberg TM, Cook RW, Oelschlager K, Maetzold D, Rao PK, Mason JO. Current clinical practice: differential management of uveal melanoma in the era of molecular tumor analyses. Clin Ophthalmol. 2014;8:2449–2460. doi: 10.2147/OPTH.S70839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E, Aaberg TM, Altaweel MM, Bardenstein DS, Finger PT, Gallie BL, Harocopos GJ, Hovland PG, McGowan HD, Milman T, Mruthyunjaya P, Simpson ER, Smith ME, Wilson DJ, Wirostko WJ, Harbour JW. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119:1596–1603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landreville S, Agapova OA, Harbour JW. Emerging insights into the molecular pathogenesis of uveal melanoma. Future Oncol. 2008;4:629–636. doi: 10.2217/14796694.4.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onken MD, Worley LA, Dávila RM, Char DH, Harbour JW. Prognostic testing in uveal melanoma by transcriptomic profiling of fine needle biopsy specimens. J Mol Diagn. 2006;8:567–573. doi: 10.2353/jmoldx.2006.060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Worley L, Onken MD, Harbour JW. Integrative genomic analysis of aneuploidy in uveal melanoma. Clin Cancer Res. 2008;14:115–122. doi: 10.1158/1078-0432.CCR-07-1825. [DOI] [PubMed] [Google Scholar]
- 6.Gill HS, Char DH. Uveal melanoma prognostication: from lesion size and cell type to molecular class. Can J Ophthalmol. 2012;47:246–253. doi: 10.1016/j.jcjo.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Dopierala J, Damato BE, Lake SL, Taktak AFG, Coupland SE. Genetic heterogeneity in uveal melanoma assessed by multiplex ligation-dependent probe amplification. Invest Ophthalmol Vis Sci. 2010;51:4898–4905. doi: 10.1167/iovs.09-5004. [DOI] [PubMed] [Google Scholar]
- 8.Augsburger JJ, Corrêa ZM, Augsburger BD. Frequency and implications of discordant gene expression profile class in posterior uveal melanomas sampled by fine needle aspiration biopsy. Am J Ophthalmol. 2015;159:248–256. doi: 10.1016/j.ajo.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coupland SE, et al. Concordant chromosome 3 results in paired choroidal melanoma biopsies and subsequent tumour resection specimens. Br J Ophthalmol. 2015;99:1444–1450. doi: 10.1136/bjophthalmol-2015-307057. [DOI] [PubMed] [Google Scholar]
- 10.Callejo SA, et al. Sudden growth of a choroidal melanoma and multiplex ligation-dependent probe amplification findings suggesting late transformation to monosomy 3 type. Arch Ophthalmol. 2011;129:958–960. doi: 10.1001/archophthalmol.2011.181. [DOI] [PubMed] [Google Scholar]
- 11.Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol. 2014;252:131–135. doi: 10.1007/s00417-013-2515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010;12:461–468. doi: 10.2353/jmoldx.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter SD, et al. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol. 2016;134:734–740. doi: 10.1001/jamaophthalmol.2016.0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correa ZM, Augsburger JJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am J Ophthalmol. 2016;162:20–27. doi: 10.1016/j.ajo.2015.11.019. e1. [DOI] [PubMed] [Google Scholar]
- 15.Damato B, et al. Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res. 2011;30:285–295. doi: 10.1016/j.preteyeres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.The AJCC Ophthalmic Oncology Task Force International Validation of the American Joint Committee on Cancer's 7th Edition Classification of Uveal Melanoma. JAMA Ophthalmol. 2015;133:376–383. doi: 10.1001/jamaophthalmol.2014.5395. [DOI] [PubMed] [Google Scholar]