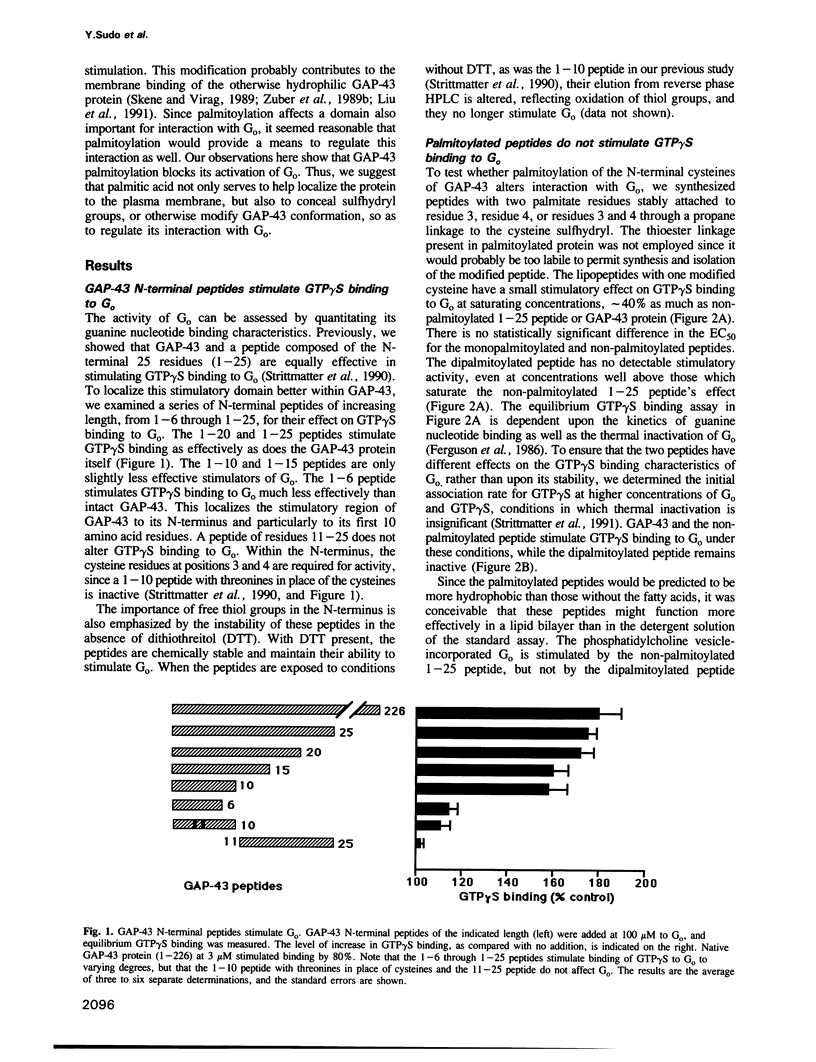
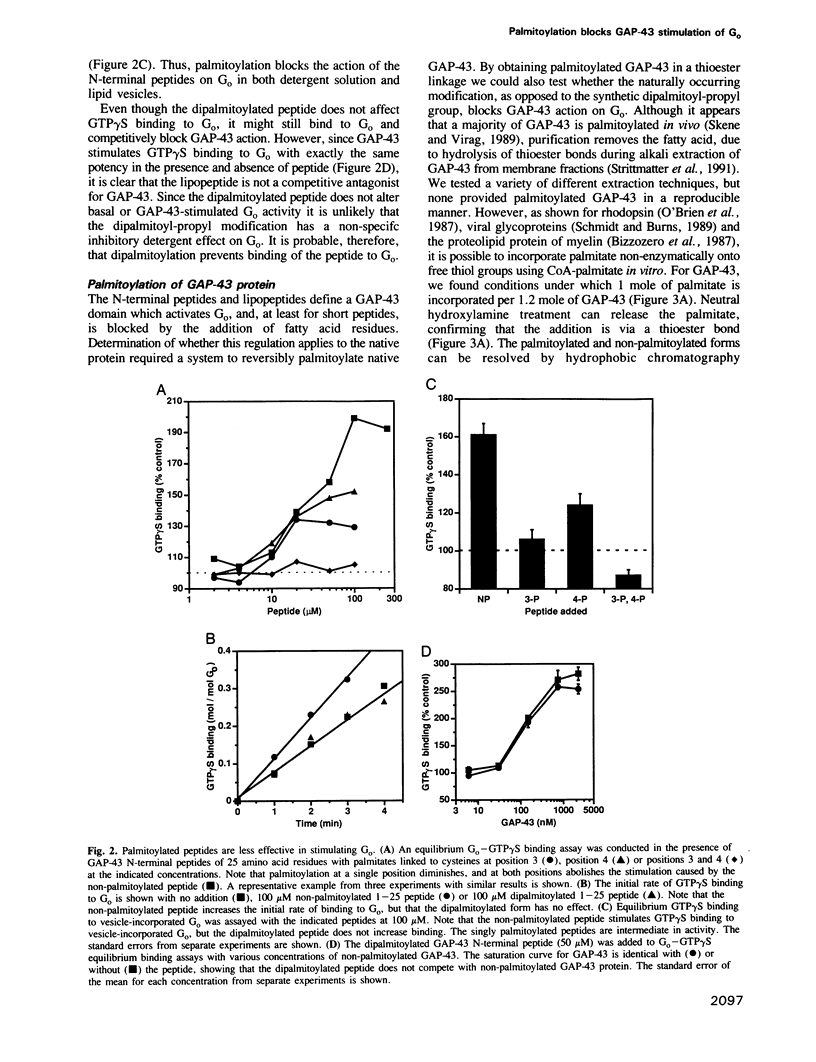
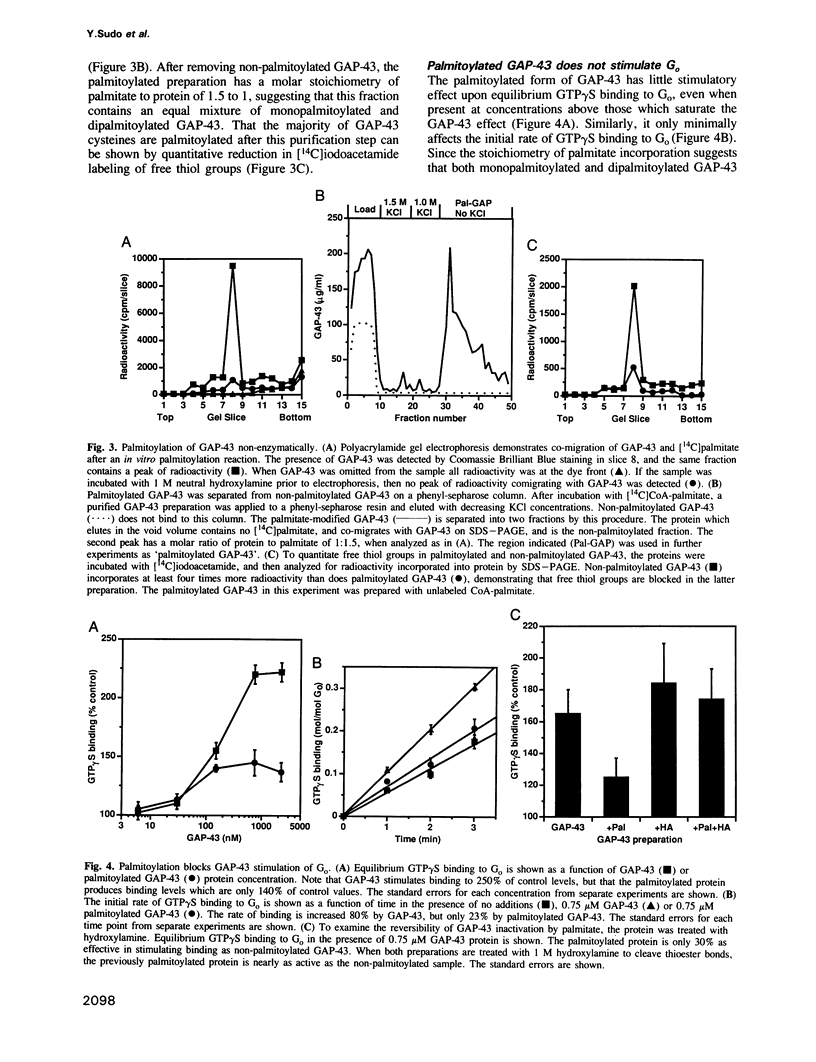
Abstract
The addition of palmitate to cysteine residues enhances the hydrophobicity of proteins, and consequently their membrane association. Here we have investigated whether this type of fatty acylation also regulates protein-protein interactions. GAP-43 is a neuronal protein that increases guanine nucleotide exchange by heterotrimeric G proteins. Two cysteine residues near the N-terminus of GAP-43 are subject to palmitoylation, and are necessary for membrane binding as well as for G(o) activation. N-terminal peptides, which include these cysteines, stimulate G(o). Monopalmitoylation reduces, and dipalmitoylation abolishes the activity of the peptides. The activity of GAP-43 protein purified from brain also is reversibly blocked by palmitoylation. This suggests that palmitoylation controls a cycle of GAP-43 between an acylated, membrane-bound reservoir of inactive GAP-43, and a depalmitoylated, active pool of protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez E., Gironès N., Davis R. J. Inhibition of the receptor-mediated endocytosis of diferric transferrin is associated with the covalent modification of the transferrin receptor with palmitic acid. J Biol Chem. 1990 Sep 25;265(27):16644–16655. [PubMed] [Google Scholar]
- Benowitz L. I., Lewis E. R. Increased transport of 44,000- to 49,000-dalton acidic proteins during regeneration of the goldfish optic nerve: a two-dimensional gel analysis. J Neurosci. 1983 Nov;3(11):2153–2163. doi: 10.1523/JNEUROSCI.03-11-02153.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Schmidt M. F. Cell-free fatty acid acylation of Semliki Forest viral polypeptides with microsomal membranes from eukaryotic cells. J Biol Chem. 1984 Jun 10;259(11):7245–7252. [PubMed] [Google Scholar]
- Berger M., Schmidt M. F. Protein fatty acyltransferase is located in the rough endoplasmic reticulum. FEBS Lett. 1985 Aug 5;187(2):289–294. doi: 10.1016/0014-5793(85)81261-8. [DOI] [PubMed] [Google Scholar]
- Bizzozero O. A., McGarry J. F., Lees M. B. Autoacylation of myelin proteolipid protein with acyl coenzyme A. J Biol Chem. 1987 Oct 5;262(28):13550–13557. [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Chapman E. R., Au D., Alexander K. A., Nicolson T. A., Storm D. R. Characterization of the calmodulin binding domain of neuromodulin. Functional significance of serine 41 and phenylalanine 42. J Biol Chem. 1991 Jan 5;266(1):207–213. [PubMed] [Google Scholar]
- Dekker L. V., De Graan P. N., Oestreicher A. B., Versteeg D. H., Gispen W. H. Inhibition of noradrenaline release by antibodies to B-50 (GAP-43). Nature. 1989 Nov 2;342(6245):74–76. doi: 10.1038/342074a0. [DOI] [PubMed] [Google Scholar]
- Doherty P., Ashton S. V., Moore S. E., Walsh F. S. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991 Oct 4;67(1):21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- Ferguson K. M., Higashijima T., Smigel M. D., Gilman A. G. The influence of bound GDP on the kinetics of guanine nucleotide binding to G proteins. J Biol Chem. 1986 Jun 5;261(16):7393–7399. [PubMed] [Google Scholar]
- Florio V. A., Sternweis P. C. Mechanisms of muscarinic receptor action on Go in reconstituted phospholipid vesicles. J Biol Chem. 1989 Mar 5;264(7):3909–3915. [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I. Characterization of an acyltransferase acting on p21N-ras protein in a cell-free system. Biochim Biophys Acta. 1991 Jun 24;1078(2):147–154. doi: 10.1016/0167-4838(91)99003-b. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Huang E. M. Agonist-enhanced palmitoylation of platelet proteins. Biochim Biophys Acta. 1989 May 10;1011(2-3):134–139. doi: 10.1016/0167-4889(89)90200-0. [DOI] [PubMed] [Google Scholar]
- James G., Olson E. N. Identification of a novel fatty acylated protein that partitions between the plasma membrane and cytosol and is deacylated in response to serum and growth factor stimulation. J Biol Chem. 1989 Dec 15;264(35):20998–21006. [PubMed] [Google Scholar]
- Jing S. Q., Trowbridge I. S. Nonacylated human transferrin receptors are rapidly internalized and mediate iron uptake. J Biol Chem. 1990 Jul 15;265(20):11555–11559. [PubMed] [Google Scholar]
- Jochen A., Hays J., Lianos E., Hager S. Insulin stimulates fatty acid acylation of adipocyte proteins. Biochem Biophys Res Commun. 1991 Jun 14;177(2):797–801. doi: 10.1016/0006-291x(91)91859-b. [DOI] [PubMed] [Google Scholar]
- Karns L. R., Ng S. C., Freeman J. A., Fishman M. C. Cloning of complementary DNA for GAP-43, a neuronal growth-related protein. Science. 1987 May 1;236(4801):597–600. doi: 10.1126/science.2437653. [DOI] [PubMed] [Google Scholar]
- Kater S. B., Mills L. R. Regulation of growth cone behavior by calcium. J Neurosci. 1991 Apr;11(4):891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. C., Chapman E. R., Storm D. R. Targeting of neuromodulin (GAP-43) fusion proteins to growth cones in cultured rat embryonic neurons. Neuron. 1991 Mar;6(3):411–420. doi: 10.1016/0896-6273(91)90249-y. [DOI] [PubMed] [Google Scholar]
- Lovinger D. M., Akers R. F., Nelson R. B., Barnes C. A., McNaughton B. L., Routtenberg A. A selective increase in phosporylation of protein F1, a protein kinase C substrate, directly related to three day growth of long term synaptic enhancement. Brain Res. 1985 Sep 16;343(1):137–143. doi: 10.1016/0006-8993(85)91167-9. [DOI] [PubMed] [Google Scholar]
- Mack D., Berger M., Schmidt M. F., Kruppa J. Cell-free fatty acylation of microsomal integrated and detergent-solubilized glycoprotein of vesicular stomatitis virus. J Biol Chem. 1987 Mar 25;262(9):4297–4302. [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Meiri K. F., Pfenninger K. H., Willard M. B. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986 May;83(10):3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- O'Brien P. J., St Jules R. S., Reddy T. S., Bazan N. G., Zatz M. Acylation of disc membrane rhodopsin may be nonenzymatic. J Biol Chem. 1987 Apr 15;262(11):5210–5215. [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989 May 5;264(13):7564–7569. [PubMed] [Google Scholar]
- Pearce E. J., Magee A. I., Smithers S. R., Simpson A. J. Sm25, a major schistosome tegumental glycoprotein, is dependent on palmitic acid for membrane attachment. EMBO J. 1991 Oct;10(10):2741–2746. doi: 10.1002/j.1460-2075.1991.tb07822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Burns G. R. Hydrophobic modifications of membrane proteins by palmitoylation in vitro. Biochem Soc Trans. 1989 Aug;17(4):625–626. doi: 10.1042/bst0170625. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea T. B., Perrone-Bizzozero N. I., Beermann M. L., Benowitz L. I. Phospholipid-mediated delivery of anti-GAP-43 antibodies into neuroblastoma cells prevents neuritogenesis. J Neurosci. 1991 Jun;11(6):1685–1690. doi: 10.1523/JNEUROSCI.11-06-01685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Jacobson R. D., Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986 Aug 15;233(4765):783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Virág I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J Cell Biol. 1989 Feb;108(2):613–624. doi: 10.1083/jcb.108.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981 Apr;89(1):96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany A., Liau Y. H., Takagi A., Laszewicz W., Slomiany B. L. Characterization of mucus glycoprotein fatty acyltransferase from gastric mucosa. J Biol Chem. 1984 Nov 10;259(21):13304–13308. [PubMed] [Google Scholar]
- Staufenbiel M. Fatty acids covalently bound to erythrocyte proteins undergo a differential turnover in vivo. J Biol Chem. 1988 Sep 25;263(27):13615–13622. [PubMed] [Google Scholar]
- Staufenbiel M., Lazarides E. Ankyrin is fatty acid acylated in erythrocytes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):318–322. doi: 10.1073/pnas.83.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Fishman M. C. The neuronal growth cone as a specialized transduction system. Bioessays. 1991 Mar;13(3):127–134. doi: 10.1002/bies.950130306. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Sudo Y., Linder M. E., Fishman M. C. An intracellular guanine nucleotide release protein for G0. GAP-43 stimulates isolated alpha subunits by a novel mechanism. J Biol Chem. 1991 Nov 25;266(33):22465–22471. [PubMed] [Google Scholar]
- Zuber M. X., Goodman D. W., Karns L. R., Fishman M. C. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989 Jun 9;244(4909):1193–1195. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]
- Zuber M. X., Strittmatter S. M., Fishman M. C. A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43. Nature. 1989 Sep 28;341(6240):345–348. doi: 10.1038/341345a0. [DOI] [PubMed] [Google Scholar]
- Zwiers H., Verhaagen J., van Dongen C. J., de Graan P. N., Gispen W. H. Resolution of rat brain synaptic phosphoprotein B-50 into multiple forms by two-dimensional electrophoresis: evidence for multisite phosphorylation. J Neurochem. 1985 Apr;44(4):1083–1090. doi: 10.1111/j.1471-4159.1985.tb08728.x. [DOI] [PubMed] [Google Scholar]