Abstract
Detachment of the rear of the cell from its substratum is an important aspect of locomotion. The signaling routes involved in this adhesive release are largely unknown. One of the few candidate proteins to play a role is RhoA, because activation of RhoA in many cell types leads to contraction, a mechanism probably involved in detachment. To study the role of RhoA in detachment regulation, we analyzed several subsets of expert migratory leukocytes by video microscopy. In contrast to fast-migrating neutrophils, eosinophils do not detach the rear of the cell unless stimulated with serum. When measuring the amount of active RhoA, with the use of a GST-Rhotekin pulldown assay, we found that serum is an excellent activator of RhoA in granulocytes. Inhibition of RhoA or one of Rho's target proteins, the kinase ROCK, in neutrophils leads to the phenotype seen in eosinophils: the rear of the cell is firmly attached to the substratum, whereas the cell body is highly motile. ROCK-inhibition leads to impaired migration of granulocytes in filters, on glass, and through endothelial monolayers. Also, the ROCK signaling pathway is involved in changes of integrin-mediated adhesion. Eosinophil transduction by a tat-fusion construct containing active RhoA resulted in detachment stimulation in the presence of chemoattractant. From these results we conclude that activation of the RhoA-ROCK pathway is essential for detachment of migratory leukocytes.
INTRODUCTION
When a eukaryotic cell migrates, the leading lamella protrudes, then the cell body moves forward, and finally the rear of the cell (or uropod) releases the binding from the extracellular environment. When the process of rear release is slow compared with the protrusion of the leading lamella, it will determine the migration rate (Lauffenburger and Horwitz, 1996). The mechanisms by which adhesions release are largely unknown. First, cytoskeletal contraction may overcome the negative force that is exerted by the adhesion molecules bound to the extracellular matrix. Contraction of actin filaments can pull on filaments connected to integrins that link the cell to the extracellular matrix (Small et al., 1999). Alternatively, ligand-induced signaling is proposed to play a role in adhesive release. Calcineurin, a calcium-regulated serine-threonine phosphatase, plays a role in recycling of integrins in neutrophils (Lawson and Maxfield, 1995). Also implicated in adhesive release is RhoA: inhibitors of RhoA induce cytoskeletal breakdown and cell rounding (Jalink et al., 1994) and inhibit migration in several cell types (Allen et al., 1998).
RhoA is a member of the Ras superfamily of small GTP-binding proteins that regulate formation of actin stress fibers and focal adhesions. RhoA activation can be blocked by the ADP-ribosyltransferase from Clostridium botulinum, C3-exoenzyme. In this way the importance of RhoA in several cellular processes, such as cell morphology, migration, cytokinesis, DNA-synthesis, and cell growth, was established (reviewed in Hall, 1998). Like Ras, RhoA cycles between a GDP-bound inactive state and a GTP-bound active state. RhoA-bound nucleotides are regulated by several groups of proteins: guanine nucleotide exchange factors, GTPase-activating proteins, and guanine nucleotide dissociation inhibitors. When GTP-bound, RhoA binds to and activates a number of downstream effectors (Aspenström, 1999) such as Rho kinase, lipid kinases, and Rhophilin.
Rho kinase, also known as ROCKII or ROKα is closely related to p160ROCK (ROCKI) or ROKβ (Ishizaki et al., 1996). ROCKII is implicated to mediate actomyosin-based contractility stimulated by RhoA. It phosphorylates myosin phosphatase, resulting in elevated myosin light chain (MLC) phosphorylation (Kimura et al., 1996). MLC phosphorylation is correlated with myosin II filament assembly and actin-activated myosin ATPase activity (Chrzanowska-Wodnicka and Burridge, 1996). Apart from this, Rho kinase may also phosphorylate MLC directly (Amano et al., 1996). With the use of the specific inhibitor Y27632, ROCKs were shown to be important for many of the Rho-mediated cellular processes, including smooth muscle contraction (Uehata et al., 1997), myosin light chain phosphorylation (Klages et al., 1999), tumor cell invasion (Itoh et al., 1999), and motility (Niggli, 1999).
Until recently, RhoA activity was established by the appearance of stress fibers or focal adhesions. In leukocytes however, stress fibers and focal adhesions are not formed. Several groups have now published an activation assay for RhoA, with the use of the RhoA-binding domain of Rhotekin (Reid et al., 1996), and were able to show RhoA activation upon stimulation of 3T3 cells with the integrin ligand fibronectin or with lysophosphatidic acid (Ren et al., 1999; Sander et al., 1999).
We have studied the role of RhoA and its downstream kinase ROCK in the process of detachment of highly motile leukocyte subsets. Serum stimulation of detachment-defective eosinophils leads to RhoA-activation and enhanced migration. Inhibition of RhoA or ROCK in motile neutrophils results in a phenotype similar to eosinophils: the leading lamella is protruding, whereas the rear of the cell stays firmly attached to the substratum. Furthermore, we investigated the effects of ROCK activity on adhesion. The results are discussed with respect to the theoretical background of rear release and its role during cell migration.
MATERIALS AND METHODS
Reagents and Antibodies
Eotaxin was obtained from R&D Systems (Abingdon, United Kingdom); platelet-activating factor (PAF) (1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphoryl-choline) and formyl-methionyl-leucyl-phenylalanine (fMLP) were from Sigma (St. Louis, MO); recombinant human interleukin-5 (IL-5) was a gift from M. McKinnon (GlaxoSmithKline, Stevenage, United Kingdom); Ficoll-paque and Percoll were from Amersham Pharmacia Biotech (Uppsala, Sweden); human serum albumin (HSA) and pasteurized plasma solution were from CLB (Amsterdam, The Netherlands); calcein-AM and Alexa-phalloidin were from Molecular Probes (Eugene, OR); vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) were from R&D Systems; neutralizing antibodies against α4-integrin (HP2/1) were from Immunotech (Marseille, France); antibodies against β2-integrins (IB4) were isolated from culture medium of hybridoma obtained from the American Type Culture Collection (Rockville, MD); anti-RhoA was from Santa Cruz Biotechnologies (Santa Cruz, CA), anti-phospho-Erk1/2, anti-phospho-p38, and anti-phospho-protein kinase B (PKB) were from BioLabs (Surrey, Canada). The ROCK-inhibitor Y27632 was kindly provided by Welfide (Osaka, Japan).
Cell Isolation
Blood was obtained from healthy volunteers via the Red Cross Blood Bank (Utrecht, The Netherlands). Eosinophils were isolated as previously described (Koenderman et al., 1988). In short, buffy coats of citrate-anticoagulated (0.4% wt/vol) blood were subjected to Ficoll centrifugation. Erythrocytes were removed by isotonic shock (NH4Cl), and the mixed granulocyte fraction, after 10-min treatment with fMLP (10−8 M), was subjected to discontinuous Percoll gradient (1.082/1.1 g/ml) centrifugation. Dense eosinophils were washed once with phosphate-buffered saline (PBS) containing 0.3% citrate and 10% pasteurized plasma then taken up in RPMI-HEPES supplemented with 0.5% HSA. They were >95% pure and >98% viable. Cell viability was not affected by treatment with Y27632 (100 μM) for up to 2.5 h.
Video Microscopy and Tracking of Granulocytes
Glass coverslips (0.3 mm) were coated with a solution of 1% HSA. Purified neutrophils or eosinophils, suspended in RPMI-HEPES with 0.5% HSA were attached to the coverslip for 10–15 min at room temperature. The coverslip was then inverted in a droplet of medium containing the desired ligands or antibodies and sealed with a mixture of beeswax, paraffin, and petroleum jelly (1:1:1, wt/wt/wt). Cell migration at 37°C was monitored by time-lapse microscopy and analyzed by custom-made macro (A.L.I.) in image analysis software (Optimas 6.2; Media Cybernetics, Silver Spring, MD). Cells were followed for 8 min (Figures 1 and 3) or 20 min (Figure 7). As expected with the use of primary cells, a heterogeneous population of different age and priming, there is variation in the rate and the amount of movement seen in the figures. However, the results shown are representative for a larger field of cells (usually 50–100 cells) and repeated at least three times for every assay condition.
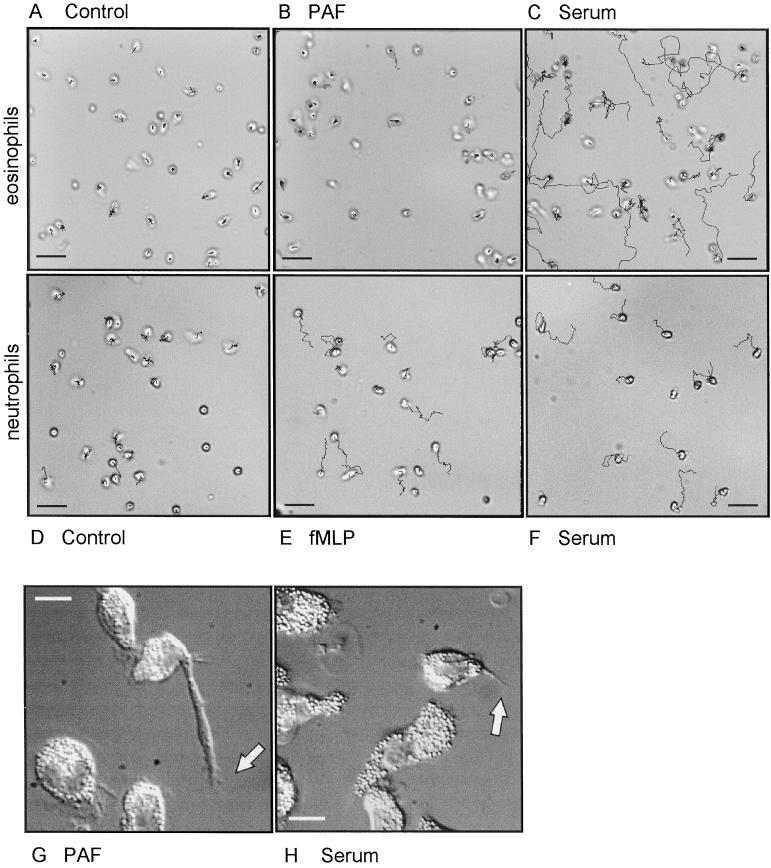
Figure 1.
Cell tracks of randomly moving eosinophils (A, B, C, G, and H) and neutrophils (D–F). The cells were attached to albumin-coated glass coverslips in RPMI-HEPES containing 0.5% HSA. Then they were transferred to the same buffer completed as indicated without stimulus (A and D) with PAF (10−7 M; B and G), fMLP (10−8 M; E), or 10% human pooled serum (C, F, and H). The cells were warmed to 37°C and then monitored for 20 min (A–C), 8 min (D–F), or 30 min (G and H). Bar, 30 μm (A–F) or 10 μm (G and H). Video material available for A–H.
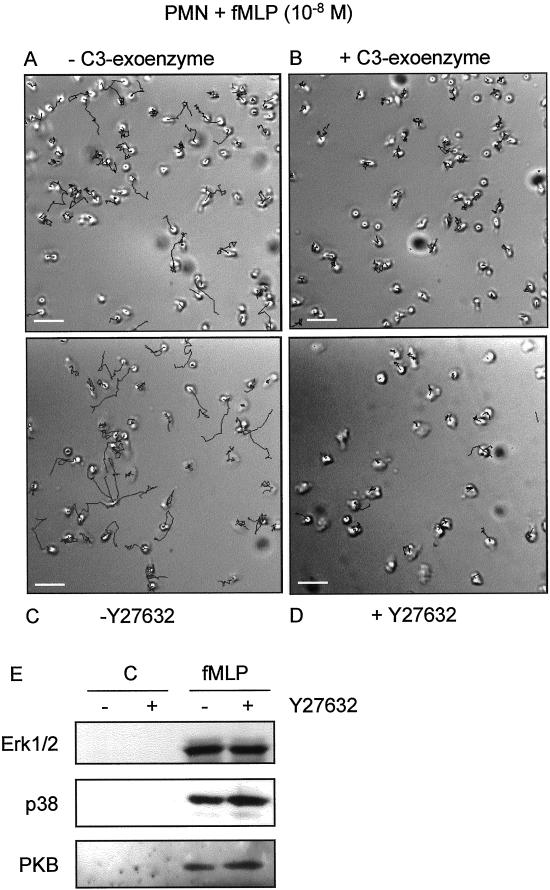
Figure 3.
Migration of neutrophils on albumin-coated glass coverslips. The cells were untreated (A and C) or pretreated with C3-exoenzyme (10 μg/ml; 4 h, B) or Y27632 (10−5 M; 30 min, D) then attached to the coverslips. The coverslips were inverted in assay medium containing fMLP (10−8 M) and embedded. Random movement of the cells was recorded at 20-s intervals. Shown are tracks recorded during an 8-min period. Bar, 30 μm. (E) fMLP-induced activity of Erk1/2, p38, and PKB in neutrophils in the absence or presence of Y27632 (10−5 M; 30 min) with the use of phosphospecific antibodies. Video material available for A–D.
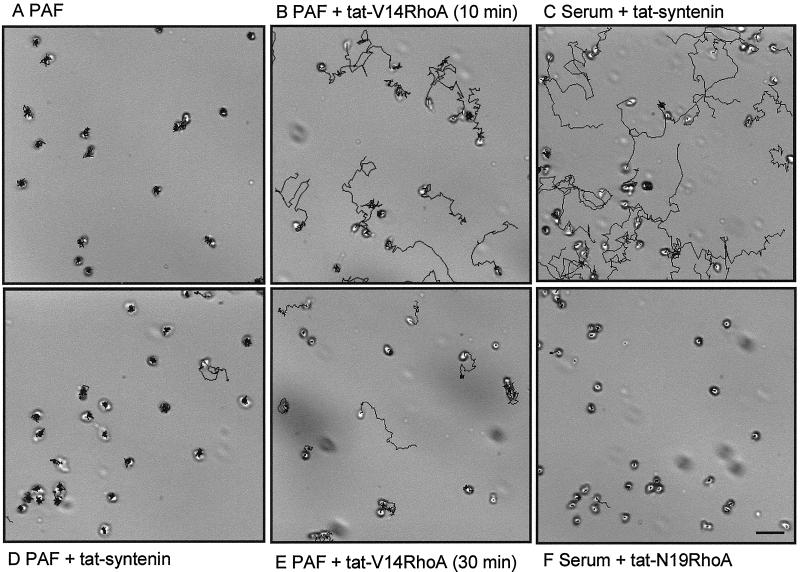
Figure 7.
Video microscopy of migrating eosinophils in the presence of tat-fusion proteins. Cells were attached to albumin-coated glass coverslips, transferred to medium containing 1 μM of the indicated tat-fusion proteins and/or PAF (10−7 M) or human pooled serum (10%), and embedded at t = 0. Images were recorded with 20-s intervals during 20 min at 37°C. Shown are calculated cell tracks of a representative experiment. Bar, 30 μm. Video material is available for A–F.
RhoA Activation Assay
RhoA activity assay was performed as described (Sander et al., 1999). Neutrophils or eosinophils were stimulated in suspension then lysed in 50 mM Tris pH 7.5, 150 mM NaCl, 10% glycerol, 1% NP-40, 0.1% Triton X-100, 5 mM MgCl2, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptine, 10 μg/ml aprotinin). Cleared lysates were incubated with bacterially produced GST-RBD[Rhotekin] (Reid et al., 1996) bound to glutathion-agarose beads for 45 min at 4°C. The beads were washed three times with lysis buffer then bound proteins were eluted in SDS-sample buffer and analyzed by Western blotting with the use of anti-RhoA monoclonal antibody from Santa Cruz Biotechnologies.
Migration Assay with Use of Boyden Chamber
Eosinophil migration was measured in the modified Boyden chamber assay exactly as described (Schweizer et al., 1996). Cellulose nitrate filters (pore width, 8 μm; thickness, 150 μm; Sartorius, Goettingen, Germany) were soaked in 0.5% HSA. The assays were performed in HEPES-buffered RPMI (Life Technologies, Breda, The Netherlands) supplemented with 0.5% HSA for 2.5 h at 37°C in a CO2 incubator. Filters were fixed, stained with hematoxilin (Weigert's method), and embedded in malinol. Analysis of the filters was done by an image analysis system (Quantimet 570 C) and an automated microscope to score the number of cells at 15 intervals of 10 μm in the Z-direction of the filters. The results are expressed as the chemotactic index, indicating the mean migrated distance (μm), excluding cells with migration zero.
Transendothelial Migration
Human umbilical vein endothelial cells were plated in Transwell chambers (Costar, Bucks, UK; pore width, 8 μm; diameter, 6 mm), coated with fibronectin. When confluence was reached, purified eosinophils (105/well) were calcein-AM (Molecular Probes) loaded according to the manufacturer, washed, resuspended in RPMI-HEPES, and placed in the upper wells. The lower wells were filled with RPMI-HEPES containing the chemoattractant. The chambers were placed for 1 h at 37°C in a CO2 incubator. At the end of the experiment, the migrated cells in the lower wells, the inserts, as well as the cells from the upper wells were lysed in 1% Triton-X100-containing buffer. Fluorescence intensities were measured with the use of a FluorImager (Molecular Dynamics, Sunnyvale, CA). Standard curves with the use of fixed amounts of calcein-AM-loaded cells were prepared each time. Values are given as the percentage of migrated cells of total cells loaded.
Adhesion Assay
Static adhesion of eosinophils was performed in 96-well Elisa plates (Nunc, Naperville, CT), coated for 1 h at room temperature with 0.1% HSA or VCAM-1 (5 μg/ml) in PBS. Cells were calcein-AM loaded, incubated with antibody or inhibitors, and added to the wells already containing medium with or without serum. After 5 min, the plates were placed at 37°C for 5 min then they were washed once with PBS containing 0.1% bovine serum albumin and lysed in TX-100 containing buffer. Remaining cells were quantified with the use of a FluorImager.
Tat-Fusion Constructs
Polymerase chain reaction products comprising the coding region of constitutively active V14RhoA, dominant negative N19RhoA, or dominant negative N17Rac-1 (Hall, 1998) were cloned into the pTAT-HA vector (Nagahara et al., 1998), sequenced, and transformed into the BL21(DE3) strain. Bacteria were obtained from a overnight culture, resuspended and sonicated in Z-buffer (8 M urea, 100 mM NaCl, and 20 mM HEPES, pH 8.0), and supernatants containing 10 mM imidazol were loaded onto a Ni-NTA column (Qiagen, Chatsworth, CA). Tat-fusion proteins were eluted with 1 M imidazol in Z buffer, diluted five times with 20 mM HEPES buffer pH 8.0 and applied to a Source 30Q column (Amersham Pharmacia Biotech). After washing, bound proteins were eluted with 1 M NaCl, desalted on a PD-10 columns into PBS/1 mM CaCl2, flash frozen in 10% glycerol, and stored at −80°C.
With the use of tat-V14RhoA constructs in eosinophil migration assays, such as shown in Figure 7, we found in a minority of the experiments no effect of the tat-constructs, which we cannot explain. The effects of tat-V14 Rho decreased after incubations of >45 min.
RESULTS
Imaging of Cellular Detachment
To study the migration of expert migratory leukocytes, we performed video microscopy on human eosinophils. Tracking of cells attached to albumin-coated glass slides allows the study of speed, direction, and morphology of each cell. Figure 1 shows the tracks of eosinophils and neutrophils migrating in the absence or the presence of stimulus. Eosinophils in the control situation, or when stimulated with the chemoattractant PAF, are highly motile. In these circumstances eosinophils move the cell body but are not able to detach the uropod and therefore hardly migrate (Figure 1G; also see video). Whereas the addition of chemoattractants has no effect on eosinophil detachment, serum-stimulation of the cells leads to rear release. More cells were able to detach their uropod, resulting in a higher mean migration speed, as can be concluded from the longer tracks. Neutrophils, however, are optimally stimulated with the chemoattractant fMLP and serum does not further enhance neutrophil migration (Figure 1, D–F).
Involvement of RhoA in Detachment of Uropods
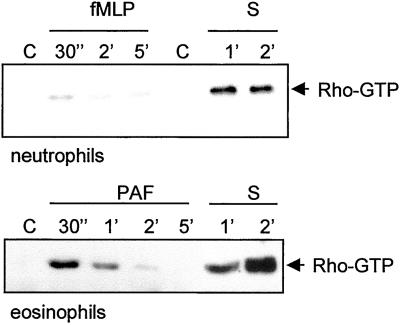
Because serum is able to stimulate detachment, we hypothesized that serum may activate the RhoA-ROCK pathway. We used a recently developed RhoA activation assay (Sander et al., 1999) to test this hypothesis. In this assay a GST-Rhotekin pulldown is performed on lysates of activated leukocytes then the amount of active RhoA present in the cells is determined by Western blotting. Figure 2 shows that serum is an excellent activator of RhoA, both in neutrophils and eosinophils. Compared with serum, the activity of RhoA after stimulation by the chemoattractant fMLP and PAF is rather weak. However, these latter experiments show that a relatively small and transient activation of RhoA per se is not sufficient for rear release (see below). These results indicate that increased detachment observed after serum stimulation correlates with strong RhoA activation. This RhoA activation is not dependent on a cycle of attachment and detachment because the experiments were performed with cells in suspension.
Figure 2.
RhoA activation assay in neutrophils and eosinophils. The cells were stimulated with fMLP (10−7 M), PAF (10−7 M), or serum for the time-periods indicated, lysed, and subjected to GST-Rhotekin pulldown as described in MATERIALS AND METHODS. Shown are Western blots of active RhoA (arrow).
To determine whether RhoA is involved in stimulation of detachment, we used a specific inhibitor of RhoA, C3-exoenzyme. We studied the migration of neutrophils because these cells have no rear release problems when monitored on a glass coverslip in the presence of chemoattractant. Figure 3A shows tracks of neutrophils that were control treated or preincubated with C3-exoenzyme (Figure 3B). Those cells show a morphology similar to eosinophils: the uropod is attached, but the cell body is still highly motile, as can be seen in the videos. These results indicate that RhoA is involved in cellular detachment of the uropod.
ROCKs Are Involved in Rear Release Regulation
ROCKI and II are downstream targets of RhoA involved in MLC phosphorylation and thereby in force generation and contraction of cells. To investigate whether ROCKs are involved in detachment, we used the ROCK-inhibitor Y27632 (Uehata et al., 1997) in several migration assays. Figure 3C and D shows tracks of neutrophils that are stimulated with fMLP either in the absence or in the presence of Y27632. After ROCK-inhibition by Y27632, the cells show a decreased net cell translocation and thus a decreased mean migration speed, which can be concluded from the track length. However, these cells are still highly motile, as can be seen in the videos provided with Figure 3. The uropod of the cells is again attached to the substratum, whereas the cell body moves around it. It is obvious that the cells treated with Y27632 cannot detach. The specificity of the ROCK inhibitor Y27632 is tested in vitro by Uehata et al. (1997). Several important kinases were tested: PKC, PKA, MLCK, and PAK. Furthermore, Y27632 did not have any effect on RhoA-dependent transcription and Rac-dependent membrane ruffling. In neutrophils, we tested fMLP-stimulated activation of PKB, Erk1/2, and p38 (Figure 3E). We found no inhibitory effect of Y27632 on these key signaling routes.
Importance of ROCKs during Transendothelial Migration
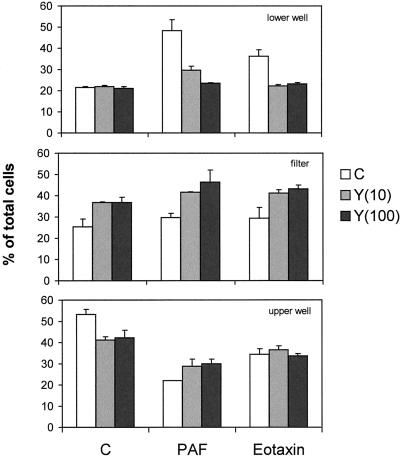
Migration of eosinophils across a monolayer of endothelial cells is complicated by the fact that the endothelial cells not only form the substrate over which the eosinophils have to move and the barrier they have to cross but also the endothelial cells provide signals to the eosinophils, for instance, by secreted chemokines. In the past, attention has focussed on the role of RhoA and Rho kinase in the contraction and permeability of endothelial cell layers (Essler et al., 1998). We investigated the involvement of ROCK in eosinophils when transmigrating an endothelial cell layer in the absence of serum. Figure 4 shows a transwell assay performed on eosinophils pretreated with Y27632. The transmigration (top) induced by PAF or eotaxin is completely blocked by Y27632 at 100 μM, whereas the control values are hardly affected. Analysis of the filters (middle) shows that after inhibition of ROCK by Y27632, a considerable number of cells remain present in the filter, independent of the stimulus. This could indicate an increase of adhesion of eosinophils to the endothelial cells or a decreased detachment. To rule out the first possibility, we performed adhesion assays of eosinophils to endothelial monolayers. We never found an increased adhesion after Y27632 treatment (unpublished results). Therefore, we conclude that the high number of Y27632-treated cells present in the filter is probably the result of a defect in detachment.
Figure 4.
Transendothelial migration of eosinophils in the presence of Y27632. Eosinophils migrating over confluent monolayers of human umbilical vein endothelial cells (details in MATERIALS AND METHODS) were stimulated with PAF (10−7 M) or eotaxin (10−7 M). Pretreatment of the eosinophils with Y27632 was for 30 min as indicated (in μM). After 1 h of migration, the cells were taken out of the lower wells, filters, and upper wells and separately quantified with the use of calcein. The results are expressed as percentage of total cells (mean ± SEM) of a representative experiment performed in duplicate.
ROCK Involvement in Chemotaxis and Chemokinesis
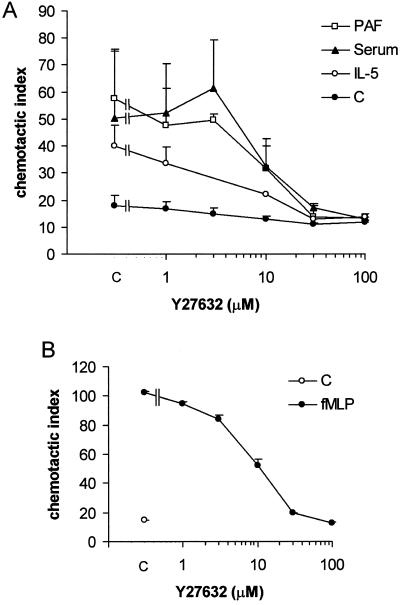
In the Boyden chamber assay, neutrophils or eosinophils migrate through a three-dimensional filter of nitrocellulose. Migration of cells in filters occurs under less stringent circumstances. The main difference between these experimental setups is the presence of a two- versus a three-dimensional substrate. Eosinophils migrating through filters show efficient migration after stimulation with PAF or IL-5, which is not the case when migrating over albumin-coated glass coverslips. Preincubation of eosinophils with the ROCK-inhibitor Y27632 leads to a dose-dependent inhibition of chemotaxis toward PAF (Figure 5A). When serum is present in both the upper and the lower wells of the chemotaxis chamber, chemokinesis is observed. The ROCK-inhibitor is effective in inhibition of chemokinesis stimulated by IL-5 or serum as well as chemotaxis stimulated by PAF. The observed IC50 for the different stimuli is 3–10 μM, in range with the published value in smooth muscle cells (Uehata et al., 1997). Similarly, chemotaxis of neutrophils induced by fMLP is inhibited by Y27632 (Figure 5B). Thus, activation of ROCK is necessary for the migration of granulocytes.
Figure 5.
Granulocyte chemotaxis in the presence of ROCK-inhibitor Y27632. (A) Eosinophils migrating in the Boyden chamber were stimulated with PAF (10−7 M) present in the lower wells, IL-5 (10−10 M) in the upper well, or with serum (10%) in both wells. (B) Boyden chamber chemotaxis assay of neutrophils stimulated with fMLP (10−8 M). Y27632 pretreatment was in all cases for 30 min. The results are expressed as chemotactic index (mean ± SEM of 2–6 experiments performed in duplicate).
ROCKs Influence Attachment
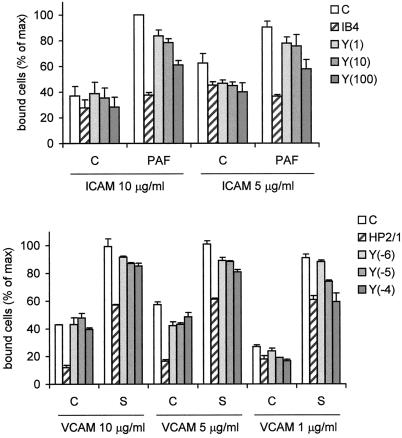
Activation of ROCK leads to phosphorylation of MLC, an event that is correlated with force generation and contraction of cells. It can be envisioned how a higher contractility of a cell leads to better detachment from its substrate. Alternatively, RhoA activation is implicated in adhesion of neutrophils (Laudanna et al., 1996). Therefore, RhoA's downstream kinase ROCK could elicit a signal toward adhesion molecules, thereby regulating the affinity for ligands present on the substrate. To address this hypothesis, we measured the effect of ROCK-inhibitor on the activity of two important adhesion molecules present on eosinophils (Weber et al., 1996): α4β1-integrin (binding to VCAM) and β2-integrins (binding to ICAM-1). Figure 6 shows that Y27632 has an inhibitory effect on the activity of α4- as well as β2-containing integrins. The effects of Y27632 are rather small, as seen by the partial inhibition of cells binding to ICAM and the fact that inhibition of binding to VCAM is only observed at limiting ligand concentration. This means that ROCK activation does influence attachment as well as detachment.
Figure 6.
Adhesion of eosinophils to ICAM-1 or VCAM. Eosinophils were calcein-loaded, incubated for 30 min with Y27632 at the indicated concentrations (between brackets, in μM), or with neutralizing antibodies against α4- (HP2/1) or β2- (IB4) integrins. Then the cells were placed in 96-well plates coated with ICAM-1 (5–10 μg/ml) or VCAM (0.1–5 μg/ml) containing control buffer or serum-containing buffer and incubated for 5 min at 37°C. The cells were washed and lysed and fluorescence intensity was measured. The results are expressed as percentage of maximal binding (mean ± SEM of 2–5 experiments performed in duplicate).
Is RhoA Activity Sufficient to Stimulate Detachment?
To investigate whether RhoA activation is the primary mechanism of detachment, we transduced eosinophils with activated RhoA (V14RhoA). Because classic transfection procedures are unsuccessful on these primary cells, we made use of the cell permeability of tat-fusion proteins (Nagahara et al., 1998). When tat-V14RhoA is applied to the cells in the presence of the chemoattractant PAF, tat-V14RhoA is able to replace serum and stimulate detachment (Figure 7B). The experiments are performed in the continuous presence of tat-fusion protein to avoid diffusion out of the cell. The stimulation of detachment increases in time, probably due to a higher concentration of tat-V14RhoA in the cell, but decreases again after ∼45 min (Figure 7E). As a control, a fusion-protein containing syntenin is used, which had no effect on detachment (Figure 7, C and D). To substantiate the hypothesis we did the reverse experiment. Tat-N19RhoA was applied to the cells in the presence of serum. This resulted in an inhibition of detachment (Figure 7F), whereas in the control situation, eosinophils incubated with tat-syntenin in the presence of serum show normal detachment. From these results we conclude that activation of the Rho-ROCK pathway alone is necessary but not sufficient to stimulate detachment. Other, as yet unidentified signals, elicited by the stimulus PAF contribute to the stimulation of detachment.
DISCUSSION
We have shown that the Rho-ROCK pathway is essential in the regulation of the process of uropod detachment or rear release, a field in migration research that has not received much attention until now. One of the possible explanations is the difficulty in monitoring rear release. The only method to appreciate cellular rear release is through time-lapse (video) microscopy of cells migrating on a two-dimensional substratum. Highly motile cells that are detachment-defective can easily be recognized by direct viewing of the cells. Such a cell has a uropod that is firmly attached to the substratum. Under high-magnification viewing it becomes clear that the cell body is moving actively around the cell.
We found that high motility and stimulation of rear release can be separated. In eosinophils, a situation of high motility and no detachment is found in the absence of serum. This situation can be mimicked in neutrophils by inhibition of RhoA or ROCK. This leads us to the conclusion that motility and rear release have a different molecular basis.
The mechanisms underlying rear release are largely unknown. Based on our findings, ROCK-mediated cytoskeletal contraction turns out to be very important. The measurement of force generation in cells is expected to correlate with rear release stimulation.
A second mechanism that may explain rear release involves integrin modulation. In this model, ligand-stimulated signaling is responsible for affinity or avidity changes of integrins (Lauffenburger and Horwitz, 1996; Hughes and Pfaff, 1998). It is clear that the strength of adhesions determines the migration speed of cells (Palecek et al., 1997): at decreased adhesive strength migration speed is enhanced. This occurs in a situation without addition of external stimuli, which implies no changes in cytoskeletal contraction. Whether RhoA signaling plays a role in integrin modulation is still a debate. RhoA was found to be important for β2-integrin–dependent lymphocyte aggregation (Tominaga et al., 1993) and β1- and β2-integrin–dependent leukocyte adhesion (Laudanna et al., 1996) but not for inside-out signaling toward platelet αIIbβ3 (Leng et al., 1998).
From previous work we know that interference with the ligand-binding capacity of α4-containing integrins can improve rolling of eosinophils (Ulfman et al., 1999) and rear release and migration of the cells (our unpublished results). We found a contribution of Rho's immediate downstream target ROCK in signaling toward α4β1- or β2-integrin–dependent attachment. This means that at the same time ROCK stimulates detachment and attachment. Detachment of the rear of the cell leads to migration, not necessarily to detachment of the entire cell. This can be seen in Figure 6: serum stimulation, which very efficiently stimulates detachment of the uropod, leads to increased adhesion, i.e., attachment. Adhesion per se does not correlate with migration properties of cells. Whether integrins are turned off during rear release has to be further explored.
In migrating cells, Rac and Cdc42 are responsible for lamellipodial protrusions, whereas RhoA regulates uropod detachment by stimulation of actomyosin filament contraction. Coordination of the activity of these three GTPases is necessary for optimal migration. In adherent cells that contain focal adhesions, Rac is able to counteract Rho-stimulated formation of stress fibers and focal adhesions directly or through activation of PAK (Sanders et al., 1999). In leukocytes however, focal adhesions are not present. Here, the formation of contractile forces is enough to ensure adhesive release and Rac or Cdc42 may be activated in concert with RhoA (Geijsen et al., 1999). Clearly, the adhesive state of cells influences RhoA activation by soluble factors (Ren et al., 1999). In conclusion, our results point to an essential role for the Rho-ROCK pathway in migration of leukocytes, namely in the relatively unknown process of rear release.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. J.A.M. van der Linden for help with image analysis. Welfide Corporation (Osaka, Japan) kindly provided us with the ROCK-inhibitor Y27632. The fusion protein construct of GST-RBD[Rhotekin] was a gift of Dr. J.G.Collard (Amsterdam, The Netherlands). The pTAT-HA-fusion vector was kindly provided by Dr. S.F. Dowdy. This work was supported by Glaxo-Wellcome B.V., The Netherlands.
Footnotes
Online version of this article contains video material for some figures. Online version is available at www.molbiolcell.org.
REFERENCES
- Allen WE, Zicha D, Ridley AJ, Jones GE. A role for cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Aspenström P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Geijsen N, van Delft S, Raaijmakers JAM, Lammers JJ, Collard JG, Koenderman L, Coffer PJ. Regulation of p21rac activation in human neutrophils. Blood. 1999;94:1121–1130. [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hughes, P.E., and Pfaff, M. (1998). Integrin affinity modulation. Trends Cell Biol. 359–364. [DOI] [PubMed]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watenabe N, Saitor Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kD Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med. 1999;5:221–225. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakatuku M, Yamamori B, Feng V, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderman L, Kok PT, Hamelink ML, Verhoeven AJ, Bruijnzeel PL. An improved method for the isolation of eosinophilic granulocytes from peripheral blood of normal individuals. J Leukoc Biol. 1988;44:79–86. doi: 10.1002/jlb.44.2.79. [DOI] [PubMed] [Google Scholar]
- Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Leng L, Kashiwagi H, Ren XD, Shattil SJ. RhoA and the function of platelet integrin αIIbβ3. Blood. 1998;91:4206–4215. [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani AM, Snyder EC, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Niggli V. Rho-kinase in human neutrophils: a role in signaling for myosin light chain phoshporylation and cell migration. FEBS Lett. 1999;445:69–72. doi: 10.1016/s0014-5793(99)00098-8. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1021. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Schweizer RC, van Kessel-Welmers BA, Warringa RA, Maikoe T, Raaijmakers JA, Lammers JW, Koenderman L. Mechanisms involved in eosinophil migration. Platelet-activating factor-induced chemotaxis and interleukin-5-induced chemokinesis are mediated by different signals. J Leukoc Biol. 1996;59:347–356. doi: 10.1002/jlb.59.3.347. [DOI] [PubMed] [Google Scholar]
- Small JV, Rottner K, Kaverina I. Functional design in the actin cytoskeleton. Curr Opin Cell Biol. 1999;11:54–60. doi: 10.1016/s0955-0674(99)80007-6. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh M, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Imir J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Ulfman LH, Kuijper PH, van der Linden JA, Lammers JW, Zwaginga JJ, Koenderman L. Characterization of eosinophil adhesion to TNF-alpha-activated endothelium under flow conditions: alpha 4 integrins mediate initial attachment, and E-selectin mediates rolling. J Immunol. 1999;163:343–350. [PubMed] [Google Scholar]
- Weber C, Katayama J, Springer TA. Differential regulation of beta 1 and beta 2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.