Abstract
One of the challenges in evaluating arguments for extending the conceptual framework of evolutionary biology involves the identification of a tractable model system that allows for an assessment of the core assumptions of the extended evolutionary synthesis (EES). The domestication of plants and animals by humans provides one such case study opportunity. Here, I consider domestication as a model system for exploring major tenets of the EES. First I discuss the novel insights that niche construction theory (NCT, one of the pillars of the EES) provides into the domestication processes, particularly as they relate to five key areas: coevolution, evolvability, ecological inheritance, cooperation and the pace of evolutionary change. This discussion is next used to frame testable predictions about initial domestication of plants and animals that contrast with those grounded in standard evolutionary theory, demonstrating how these predictions might be tested in multiple regions where initial domestication took place. I then turn to a broader consideration of how domestication provides a model case study consideration of the different ways in which the core assumptions of the EES strengthen and expand our understanding of evolution, including reciprocal causation, developmental processes as drivers of evolutionary change, inclusive inheritance, and the tempo and rate of evolutionary change.
Keywords: domestication, niche construction theory, coevolution, evolvability, ecological inheritance, cooperation
1. Introduction
First proposed in the mid-twentieth century, the modern synthesis (MS) has proven to be a powerful conceptual framework for studying evolution that extends far beyond biology into the social sciences and the humanities. By bringing Mendalian genetics together with neo-Darwinism, the MS provided the mechanisms on which Darwinian selection operated, and that, through the agency of population genetics, could be operationalized into a coherent understanding of the process of evolution [1–3]. In the MS, natural selection is recognized as the pre-eminent and ultimate causal force in evolution that sorts variation arising through random mutation, and passes on adaptive variations at a higher rate than less adaptive ones, resulting in an evolutionary process that proceeds at a gradual pace made up of small micro-evolutionary changes in the composition of individual genes and alleles within genes (table 1).
Table 1.
Core assumptions of the MS and the EES—after Laland et al. [1].
| modern synthesis | extended evolutionary synthesis | |
|---|---|---|
| causality | natural selection is the pre-eminent cause of evolution, responsible for sorting variation on the basis of adaptive fitness; operates as an asymmetrical, unidirectional process in which organisms are shaped by selection to match features in the environment | causation is a reciprocal process in which organisms shape and are shaped by selective developmental environments; phenotypic changes and acquired characteristics may lead rather than invariably follow genetic change |
| directionality | variation arises through random genetic mutations with no directionality | variation arises through a combination of genetic and constructive processes; biases in phenotypic variation provide directionality |
| targets of selection | targets of selection are genes and alleles of genes; evolution consists of changes in gene frequencies | targets of selection may be alleles, genes, organisms or groups of organisms; evolution consists of change on any of these levels |
| inheritance | genetic inheritance is the only inheritance system; only genetically encoded traits can be inherited | multiple systems shape transgenerational inheritance, both internal to the organism (genetic, epigenetic, maternal) and external (ecological, social learning, cultural); acquired traits may be inherited |
| tempo and pace of change | evolution proceeds at a gradual pace made up of micro-evolutionary processes shaped primarily by selection, but also drift, mutation and gene flow | evolution proceeds at an uneven pace with periods of stasis punctuated by periods of rapid macro-evolutionary change |
Early calls for emendation of the core principles of the MS noted the importance of directional processes that sort and pass on variation in ways that may not always conform to adaptive fitness criteria, and envisioned evolution as proceeding at an uneven rate with long periods of stasis punctuated by events of major macro-evolutionary change [4–6]. Subsequent insights from developmental biology, ecology and the social sciences have built on these initial calls for revision, highlighting areas in which core MS principles may require additional re-evaluation and extension. These include insights about the evolutionary impacts of ecosystem engineering in influencing the selective environments of both engineers and others sharing constructed niches—and their descendants [7]; about the role of multiple constructive developmental processes in leading rather than following genetically driven change [8]; and the recognition of additional inheritance systems—internal and external to an organism—that shape evolutionary trajectories [9,10]. These insights have recently been integrated into a general call for a revision of the classical MS by an extended evolutionary synthesis (EES) [11,12]. Proponents of the EES maintain that, in addition to natural selection, other powerful forces shape evolution through interactive bouts of development and selection operating on variation that, along with genetic mutation, arises via the agency of constructive processes. They further contend that these variations are passed from one generation to the next by multiple interacting inheritance systems, resulting in directional, non-random change at the level of genes, whole organisms or even groups of organisms, and that evolution is more likely to proceed at variable, often quite rapid, rates (table 1).
One of the major challenges in making the case for the EES is to demonstrate that the core principles of the EES can generate empirically testable predictions about evolutionary process that are not considered under the MS [13,14]. While many studies have attempted to empirically evaluate elements of the EES framework, advocates of emendation recognize that more is needed.
Although many systems can reveal individual pieces of a larger puzzle, the gold standard is … for a single system … to understand the complete set of links between environmental variation, developmental responses, and changes to genetic and epigenetic architecture over the course of evolution. Schlichting [15, p. 666]
Here, I argue that the initial domestication of plants and animals by humans provides a compelling example of such a gold standard system. Domestication is a process with clear and dramatic evolutionary impact. It created new organisms that cannot live outside of a domesticatory relationship with humans; contributed to significant declines in overall biodiversity through the extirpation or even extinction of free-living species; transformed landscapes and atmospheric conditions; and reshaped the trajectory of human cultural evolution [16]. Beyond its unquestionable status as a major evolutionary event, what makes domestication an exceptionally valuable model system is that it encompasses all the central issues involved in ongoing debates over the need for an EES—from the developmental processes EES advocates require greater attention, to the multiple forms of inheritance held to shape evolutionary trajectories. Moreover, new archaeological, archaeobiological and genetic data are producing robust records of initial domestication in multiple world areas, all set within an increasingly well-resolved temporal framework that allows for an assessment of how these multiple constructive processes and inheritance systems affect the pace, tempo and direction of evolutionary change.
The case for domestication as a model system for exploring major tenets of the EES is made here first by discussing the novel insights that niche construction theory (NCT, one of the pillars of the EES) provides into the domestication processes as they relate to five key areas: coevolution, evolvability, ecological inheritance, cooperation and the pace of evolutionary change. This discussion is next used to frame testable predictions about the initial domestication of plants and animals that contrast with those grounded in standard evolutionary theory, and to demonstrate how these predictions might be tested in the many different world areas that witnessed the independent domestication of a wide range of different species of plants and animals. I conclude with a broader consideration of the value of domestication as a model system for evaluating core assumptions of the overall EES as it relates to issues of reciprocal causation, the role of constructive developmental processes, inclusive inheritance, the targets of selection and rates of change.
2. Explanatory frameworks for initial domestication
Archaeologists have a long history of looking to evolutionary biology as a source of explanatory frameworks for initial domestication. To date, most of these frameworks have been grounded in neo-Darwinian principles. One approach, promoted by researchers adhering to the selectionist school of archaeological theory, embraces a MS trait-level view of evolution (with individual human behaviours and their artefact proxies taking the place of genes and alleles) and envisions the process of cultural evolution as made up of small micro-evolutionary trait adjustments [17–21]. Another is based in optimal foraging theory, which is itself a theory borrowed from micro-economics that self-identifies with Neo-Darwinism on the assumption that foragers who optimize for returns enjoy selective advantage over those who do not [22–24]. Both selectionist and OFT explanations characterize domestication as a slow incremental coevolutionary process between humans and target plant and animal species initiated in the context of resource depression, caused either by environmental change or by human impacts on resource availability, which forced foragers to turn to lower ranked, less preferred resources (such as the progenitors of domesticates) garnered through increasingly more intensive delayed-return strategies. Natural selection serves as the primary driving force, guiding both the coevolutionary relationships between humans and target species, as well as the individual decisions foragers make about resource choice and exploitation strategy (for a more extended discussion of neo-Darwinian explanatory frameworks for initial domestication, see references [25] and [26]).
More recently, archaeologists have begun to look to NCT, for an alternative explanatory framework for initial domestication [26–33]. NCT focuses on the evolutionary impacts of ecosystem engineering activities that modify selection pressures acting on present and future generations living within altered niches [7]. Broadly defined, NCT encompasses the niche-altering activities of all organisms, with humans characterized as ‘the ultimate niche constructors' [7, p. 28]. The inclusion, and indeed emphasis, in NCT on ‘cultural niche construction’ has drawn social scientists to NCT who see it as a way of bridging the divide that has traditionally separated the study of cultural evolution from that of biological evolution [34–39]. This has been particularly true of archaeologists interested in the origins of plant and animal domestication [26–33]. An obvious strength of NCT is the extent to which it provides special insight into domestication in five key areas: coevolution, evolvability, ecological inheritance, cooperation and rates of change [32, p. 327).
2.1. Coevolution
A central tenet of NCT is that niche-constructing activities cause eco-evolutionary feedback in which ‘ecological interactions drive evolutionary change in organismal traits that, in turn, alter the form of ecological interactions’ ([40], p. 1629; [41]). Eco-evolutionary feedbacks that arise in the context of niche construction are likely to foster coevolutionary interactions between organisms living in constructed niches [42–44]. These relationships are considered diffuse when the impacts of the niche-constructing activities of one species on another are mediated by some change in abiotic conditions or intermediary biota. Diffuse coevolutionary interactions may have wide-ranging evolutionary and ecological effects that ripple outward from the niche-constructing organism and affect other organisms in ways that may or may not have a reciprocal impact on the original niche-constructor. Coevolutionary relationships are particularly potent, however, when they are pairwise and involve interacting organisms that are both actively engaged in and affected by niche-constructing activities that drive reciprocal evolutionary responses in each [44].
NCT insights into the development of coevolutionary relationships in the context of niche construction have clear and obvious implications for understanding domestication, best defined as a pairwise, multi-generational coevolutionary mutualism between a domesticator and a domesticate that arises in the context of niche construction [31,32]. By assuming some degree of influence over the care, protection and/or reproduction of the target domesticate, the domesticator engages in niche-constructing activities aimed at increasing the supply or predictability of a resource provided by the domesticate. The domesticate, in turn, engages in reciprocal niche-constructing activities that enable it to benefit from its association with humans in a way that enhances its fitness over con-species that remain outside this relationship. And this is true of all the major pathways that humans and target species have followed into domestication [45]. The harvest, or prey, pathway, for example, is initiated by humans through ecosystem engineering activities that manipulate the conditions of growth of an organism or its environment with the goal of increasing its relative abundance or predictability. Moving plants into protected areas, channelling water, landscape burning, building traps or corrals, selective culling can all be considered niche-constructing activities initiated by humans that can result in the development of coevolutionary relationships between human niche constructors and target plant or animal populations that may lead to domestication [28]. This is the pathway that brought many perennial plant and tree species, as well as most livestock animals, into domestication. The commensal pathway, on the other hand, is initiated by plants and animals that, through relocative niche construction, move into anthropogenic environments to take advantage of new opportunities available there. Many ‘weedy’ annual plants followed this pathway into domestication as they colonized soils disturbed by anthropogenic activities [46], as did animals such as wolves and wild cats that ventured into human settlements to feed off human refuse or to prey on other animals drawn to these environments [45]. Finally, the directed pathway to domestication in which humans deliberately set out to domesticate a species to extract some resource of interest also involves a significant degree of niche construction as humans tightly control the target species' movements, nutrition and breeding (to the point of direct genetic manipulation). This is the pathway followed by all recent domesticates, with the explosion of aquatic species brought into domestication in the last 50–100 years [47]—whose rearing requires major transformation of aquatic environments—being a particularly compelling example of the role of niche construction in directed domestication.
In each case, however, the coevolutionary relationships that develop in these contexts will only result in domestication if the niche-constructing activities initiated by one partner (the domesticator or the domesticate) are met by those of the other in ways that enhance the benefits both derive from the relationship, increasing the mutual investment of each in maintaining it [31]. Domestication, then, requires a sustained multi-generational coevolutionary relationship between two niche-constructing species with each undergoing changes (either genetically driven or plastic) that enhance the benefits each derives from the relationship and/or that make further investment in the relationship more attractive to its partner. When humans sow seed in prepared substrates, for example, receptive plants may respond by altering the timing of germination and subsequent ripening—a form of developmental niche construction [48]—in ways that increase the likelihood of their harvest by humans and subsequent inclusion in the next season's seed stock [49]. Animals practicing relocative niche construction by moving into anthropogenic environments experience selection for reduced reactivity to humans that enhances their reproductive success in that setting and encourages reciprocal niche-constructing activities on the part of humans directed towards the animals' care, protection and breeding [45,50].
2.2. Evolvability
Whether organisms engage in coevolutionary relationships in the context of niche construction depends in large measure on their evolutionary potential or evolvability—their ability to respond to the new selective pressures in constructed niches in ways that result in heritable evolutionary change in descendant populations [40,41]. Insights from NCT about the potential of organisms to respond to altered niches in evolutionary significant ways have special resonance for understanding the process of domestication that merit a more extended consideration.
2.2.1. Evolutionary potential
In domestication, the capacity of different potential partners to respond to new selective pressures created by niche construction plays a major role in determining which will embark on pathways leading to domestication and the directions those pathways will take. In plants, annual species with fewer specific habitat requirements and which are able to colonize disturbed habitats are thought to be good candidates for domestication [46]. Behavioural traits such as reduced wariness, hierarchical herd structure, and generalized feeding and habitat preferences make certain animal species more likely potential domesticates than species that lack these traits [45]. Variables that make certain human groups more likely to engage in sustained coevolutionary relationships leading to domestication include their willingness/ability to modify technology and alter existing subsistence strategies in the light of new opportunities or challenges, their assessment of the current and potential demands and returns of available resources, as well as their physiological capacity to use resources [31,32]. In each case, on both the plant/animal and human side of the equation, a certain degree of plasticity or flexibility in the ability to take advantage of new opportunities and respond to new challenges is an important pre-requisite for embarking on pathways to domestication.
2.2.2. Phenotypic plasticity
To a major degree, this flexibility may be attributable to a capacity for engaging in constructive developmental processes that shape evolutionary trajectories in non-random, directional ways [12,51]. Of these processes, the ability of an organism to match its phenotype to different environments through the plastic expression of novel morphological, physiological or behavioural traits [52] may be one of the most important factors behind an organisms’ potential for engaging in a domesticatory relationship [53]. To date, most of the research on the role of phenotypic plasticity in domestication has concentrated on the impact of climate change in the expression of cryptic variation in progenitors of crop plants. Experimental studies have, for example, demonstrated that teosinte grown under reduced CO2 atmospheric conditions, similar to those of the Late Pleistocene/Early Holocene, exhibit a number of maize-like traits not seen in teosinte grown under modern CO2 conditions [54]. These traits would have made these plants more productive and easier to harvest, it is argued, leading to coevolutionary relationships between foragers and certain teosinte morphotypes that, eventually, resulted in the fixation of these traits in domesticated maize. An additional trigger of plastic responses to Early Holocene climate change may be the impact of more marked, but predictable, seasonality in climate during this time [55], environmental conditions which Donohue posits may be responsible for triggering ‘phenological cuing’ in organisms—a form of developmental niche construction in which phenotypic responses to conditions experienced in one stage of an organism's life cycle determine those displayed during subsequent life stages [48].
Another promising area for future research is the role of niche construction in setting parameters for the accumulation and expression of phenotypic plasticity in emergent domesticates. Exposure to novel environments can result in the release of otherwise cryptic variation that facilitates rapid adaptive evolution in receptive species [56–58]. This process of developmental selection involves sampling a range of phenotypes, with feedback from the novel environment helping to reinforce the high performing phenotypes [59]. The introduction of plants and animals to novel environments created by anthropogenic disturbance or manipulation is then likely to have resulted in the expression of otherwise cryptic phenotypic variation in species with the ability to alter phenotypes in response to different environmental conditions, allowing plants and animals entering anthropogenic environment to rapidly adjust to the new selective pressures encountered there. Contemporary studies, in fact, find that plastically induced trait changes occur in much higher rates in organisms entering anthropogenic environments than in natural contexts [60], with human harvesting of organisms being a particularly potent factor in inducing dramatic phenotypic changes [61–63].
Once within these environments, plants and animals living under human management and care would have been shielded from many of the selective pressures faced by con-species living outside this relationship, creating the kind of homeostatic mechanisms thought to encourage the accumulation of additional genetic variation and novel combinations of variants in populations that may be released at some later point [15,59]. The effect of human agency in buffering managed plants and animals may have played an especially important role in reducing the costs of developmental selection, which include expenditures of energy and material needed to express different phenotypes and the risks incurred by the expression of potentially deleterious traits during the trial-and-error exploratory phase of adjustment to a new environment [52]. Human intervention and buffering, in fact, frequently favoured traits in target species of interest to humans that would otherwise be selected against outside the managed environment (i.e. indehiscent seed dispersal traits that increase the yield of human harvested plants, but that face strong negative selective pressures in the wild [49]).
The release of cryptic variation within anthropogenic environments may well, then, be responsible for the high degree of variability in trait expression documented in crop plants during the initial stages of domestication, as has been suggested for the non-directional expression of variable seed sizes and coat thickness in managed Chenopodium in Eastern North America [64]. Similarly, the high degree of variability observed in the expression of the non-shattering rachis in cereals and in seed size among both cereals and pulses during the initial stages of their cultivation in the Near East, commonly attributed to restocking or introgression of managed plants with free-living ones [65], may, instead, be attributable to the release of phenotypic plasticity in plants living within anthropogenic managed environments.
2.2.3. Pleiotropy
Plastic responses to novel environments may also elicit the expression of pleitropically linked traits across the life cycle of an organism in ways that can both foster and constrain its potential for evolutionary change [48,56,59,66]—a process that may be responsible for the constellation of traits that often co-occur in domesticates. In plants [46,67], these include morphological and phenological traits that affect: (i) germination (changes in dormancy thresholds, changes in seed size and tests thickness), (ii) dispersal (synchronization in ripening, development on non-shattering seed heads and pods), (iii) plant architecture (reduction in the number of branches, plant stature and apical dominance), and (iv) productivity (increases in density of plants and in the number and size of plant organs of economic interest to humans). In animals [45], these include: (i) reduction in brain size (especially in regions of the brain that control aggression and reactivity to outside stimuli), (ii) changes in developmental rates and sequences (usually a delay in rates of development or paedomorphisis), (iii) a corresponding neotonization in morphology (often manifested in changes in cranial morphology), (iv) retention of juvenile behaviours, (v) early sexual maturity, (vi) lop ears (thought to be the result of a failure of cartilage fully develop due to early sexual maturity), and (vii) piebald coats (possibly an artefact of the shared pathways travelled by melatonin and serotonin, an example of the way in which similar signalling and resource mobilization pathways can induce pleiotropic responses in organisms [48]).
Some researchers maintain that these constellations of domestication traits are best viewed as pleiotropic cascades that arise from the orchestration of gene expression during development caused by mutations in a limited number of regulatory genes [68]. Earlier studies suggested that this was indeed the mechanism for the manifestation of these traits in domesticates. A comparative mapping of qualitative trait loci in pearl millet, for example, suggested that the same loci controlling spikelet structure in this species were also responsible for seed shattering in maize, rice and sorghum [69]. Another early study demonstrated the pleiotropic linkage between loci responsible for the expression of traits affecting growth habit and phenology, seed dispersal and dormancy, and fruit and seed size in the common bean [70].
Subsequent research employing whole-genome sequencing and genome-wide association studies, however, has shown that the origins of these traits are more complicated than originally thought. The non-shattering trait found in domestic cereals, for example, is now known to be controlled by different genes in different species, with, in some cases, different linked loci implicated in the formation of the tough rachis in different independent domestication events involving the same species [49]. Studies of gene expression in different pairs of domesticated and wild animals find little or no overlap in genes or gene complexes controlling wariness and aggression, and no evidence for a genomic association between behaviour and coat colour in domesticated rats [50,71,72]. Another recent study suggests that linked traits in domestic animals arise not through the action of individual genes or gene complexes, but through the multi-genic downstream consequences of selective factors operating on neural crest cells during embryonic development [73]—an example, perhaps, of the role that organismal levels above genes play in an organisms' ability to shape its own developmental trajectory [12]. Another study maintains that neuro-endocrine mechanisms, specifically the role of single hormones in targeting multiple tissues, underlie the expression of pleiotropic behavioural traits seen in domestic animals [74].
2.2.4. Hybridization
Another way in which novel traits can be acquired is by horizontal transfers of DNA through hybridization and genome doubling that can result in major epigenetic change in a single generation [75,76]. Introgressive hybridization and polyploidy is a common feature of many domestic crop species (i.e. cotton, bread wheat, tobacco, sugar cane, potatoes and other related tubers), with sometimes complicated histories of auto- and allo-polyploidy in the same species occurring both prior to and concurrent with human manipulation [77]. Hybrid origins without gene doubling has been suggested for at least one common animal domesticate, with the yellow skin characteristic of the domestic chicken shown to be the result of the hybridization of the red junglefowl with the closely related grey junglefowl [78]. And we know that at least the later history of South American camelids has been shaped by a complicated process of hybridization between domesticated llamas and alpacas and their progenitors, guanacos and vicuñas [79]. The capacity for successful hybridization resulting in dramatic genomic reorganization, or ‘cataclysmic evolution’ [75,76], may, in fact, have been another trait that predisposed certain species to rapidly adapt to anthropogenic environments and eventual domestication, as well as one that assisted in the dispersal of domesticates [50,77].
2.2.5. Niche construction and constructive development
While the mechanics of the acquisition and expression of these domestication traits is an active and productive area of study, it may be equally interesting to focus on the role of niche construction in creating the selective environments in which they arose in the first place. Viewing these traits as constellations of environmentally mediated genetic associations (EMGAs in NCT parlance [43]) that arise in the context of altered environments raises a number of promising new avenues of inquiry. One direction for future research in this vein would be examining the role of co-constructed niches in generating selective pressures that triggered the appearance of the same constellation of traits in different species. Such research might investigate the commonalities in human/plant interaction that resulted in the same constellation of traits in different cereal crops such as wheat, barley, rice and millet domesticated (in some cases multiple times) in the Near East, East Asia and Africa [49]. Another promising goal for future research would be identifying the conditions that trigger plastic responses and pleiotropic cascades in certain species within certain engineered environments, but not others living in the same or similar environments. Continuing with the example of cereals, here one might ask why wild oats that were intensively collected in the Early Holocene Levant failed to move on to domestic status at this time, while wheat and barley that were exploited along with wild oats responded to human manipulation by developing traits that led to their domestication [80]. A better understanding of how these co-constructed niches change selective pressures on plants and animals undergoing domestication may give new insight into the variability in the timing and sequence of the appearance of different domestication traits in different species. A goal might here be discovering why seed size increase preceded the development of indehiscence in Near Eastern cereals (emmer and einkorn wheat and barley) by hundreds of years or more, while in Near Eastern pulses (chickpeas, fava beans and lentils), indehiscence seems to have developed long before any clear trends towards seed size increase, even though these crops were brought into domestication at the same time by the same early cultivator groups [49,81]. And finally, new research might explore how domesticatory relationships arising in the context of co-constructed niches served to buffer certain species from the deleterious effects of hybridization and encouraged the perpetuation of traits that outside this relationship would have conferred no selective advantage or even been selected against, but that within it proved highly advantageous to both human and the domesticate. A profitable line of inquiry here would be examining the role of humans in fostering crosses between a species of goat grass and domesticated emmer wheat—itself derived from a polyploid progenitor created by a cross between other species of goat grass and wheat—to form bread wheat, a hexaploid free-threshing and highly productive wheat species grown today around the world but not found in the wild [82].
2.2.6. Genetic accommodation and assimilation
In order for traits arising in the context of niche construction to have evolutionary impact, there must be some mechanism by which they are stabilized within a population and become heritable traits with evolutionary impact—a process EES advocates call genetic accommodation [56–58,83,84]. In domesticates, genetic accommodation of certain traits may come about through the adoption of new harvesting, culling or processing practices, as has been proposed for the fixation of the non-shattering rachis in wheat and barley once sickles were used as the primary harvesting tool ([85] but see [86]). Deliberate human selection for certain desirable traits may also have been responsible for their fixation, as may have been the case in the selection for the woolly undercoat, visible only during moulting season in wild sheep, that became the dominant fibre in the coats of domestic sheep [87]. But perhaps the most significant factor in this process was the movement of managed plants and animals into new areas where factors like genetic drift and founders effect responses to new selective pressures encountered in these new environments, and, especially, reproductive isolation from free-living sympatrics served to fix traits that arose in the context of initial management.
Domesticates likely also underwent a process of genetic assimilation [15,52,57,58,83,88], a subset of genetic accommodation, that results in the reduction or loss of plasticity, and the costly machinery underlying plasticity, so that the plastic traits that made the plant or animal an attractive domestic partner to humans no longer required environmental cuing for expression. Despite the loss of plasticity and the major genetic bottlenecks caused by factors such as genetic drift, founders effect and reproductive isolation [49], domesticates likely retained at least some capacity for the plastic expression of traits that made it possible to successfully disperse far beyond the natural habitats of their progenitors and the homeland of initial domestication. The dispersal of Near Eastern cereal crops into Europe, for example, could only have been accomplished if these domesticates retained the ability to undergo changes in genetic controls on seasonality, especially in flowering phenology and accommodations for length of growing season, that allowed them to adjust to the very different seasonal regimes and climatic conditions they encountered in Europe [89,90]. Similarly, plasticity in the expression of vernalization genes among different land races of wheat and barley may have been key in determining the pace and direction of their eastward dispersal across and around the Tibetan Plateau into Asia [89,91], as well as the dispersal of rice out of the Yangtze basin into northern China [89], and of maize from central Mexico throughout North America [92–94].
2.3. Ecological inheritance
A basic tenet of NCT is that the niche-altering activities leave an ecological inheritance that persists beyond the lifetime of the original niche-constructing organism and bequeaths a legacy of modified selection pressures to its own and other organisms' descendants [95,96]. This concept has obvious relevance to the niche-constructing activities by humans and plants/animals involved in emergent domestication. Human landscape management aimed at promoting species of economic interest and their alterations of the population structure of biotic communities, as well as the relocative and developmental niche-constructing activities of plants and animals living within anthropogenic environments, all serve to create reinforcing eco-evolutionary feedbacks that deepen the commitment of all partners to maintaining the co-constructed ecological niche that they bequeath to their descendants [32].
The impact of this ecological legacy in shaping the evolutionary trajectories of subsequent generations is so great, NCT advocates argue, that it qualifies as a second general inheritance system on a par with genetic inheritance [7,96]. Further, they maintain that there are multiple interacting channels, in addition to genetic and ecological inheritance, over which information is transmitted to subsequent generations [97,98]. Channels internal to an organism include not only genetic inheritance, but also developmental processes like epigenetic inheritance and maternal effects. External channels that operate outside the organism include the inheritance of modified selective environments and, importantly, acquired and learned behaviours. While template copying systems like genetic inheritance ensure a high degree of fidelity in information transfer over internal channels, the targets of these high-fidelity transfers are limited to parental organisms and their direct descendants. Information transferred through external channels, on the other hand, is more prone to transcription and other errors, but its targets are far wider [99]. One way to increase the fidelity of information transfer over external channels, at least in animals, is through improvements in the transmission of acquired behaviours that enhance an animal's ability to learn from one another and allow novel traits to spread widely through a population [42]. In humans, the capacity for language and symbolic communication vastly enhances information transfer, making it possible to modify and fine-tune behaviours and so ‘ratchet’ up their complexity and efficiency [100].
Small-scale human societies, such as those engaged in initial domestication, use this capacity to amass stores of ecological knowledge (tradition ecological knowledge or TEK) that become the primary vehicles for transmitting this ecological inheritance to their descendants [29,101]. Extensively documented in small-scale societies around the world [102–105], TEK consists of continually updated cognitive maps of recourse distributions, information about the life cycles of economically important resources, their seasons of availability, behaviour and the ways in which environments or biotic communities can be manipulated to enhance the supply and predictability of target resources [29]. Played out in stories, myths, ritual performance and lessons passed on from elders to younger members of social groups, TEK provides a coherent framework for how the world works and the human place within it [29,101]. Protecting, preserving and continually updating this store of knowledge is essential for the survival of societies engaged in the kinds of ecosystem engineering that both forges and deepens the coevolutionary dependencies between humans and emergent domesticates. Including these cultural behaviours among the external channels that shape evolutionary trajectories, then, provides fresh insight into the how the generation and transmission of TEK plays a catalytic role in the domestication process.
2.4. Cooperation
By modulating resource availability, niche-constructing activities create the potential for a tragedy of the commons in which some individuals reap the benefits of resource-enhancing activities without contributing to them [106]. The perpetuation of constructed niches that form the basis of ecological inheritance, and the means by which niche construction becomes an evolutionary process, requires conditions that counter disincentives for cooperation and reward individuals that contribute to collective activities. Clearly, there are pressures for cooperative behaviours among human groups involved in the niche-altering activities leading to domestication, at the very least to combat the advantage that cheaters would enjoy from the collective activities that they themselves shirk [107].
One way in which cooperative behaviours can be encouraged, NCT advocates argue, is by broadening the focus of the rewards of collective actions from those enjoyed by an individual over contemporary competitors, to those that the individual bequeaths to relatives living in the future. In this way, activities of the individual that benefit relatives living in the future can be seen as directly benefiting the individual [108]. This form of ‘transgenerational altruism’ [109] is particularly effective in philopatric organisms whose long-term investments in altered niches make it more likely that they and their descendants will continue to reap the benefits of these collective activities. Incentives for cooperative behaviours, by contrast, are diminished under conditions of high mobility and dispersal where there is a smaller pay-off from niche-altering behaviours and a decreased probability of relatedness among aggregating individuals [108]. Another factor thought to encourage the cooperative behaviours is the ability to monopolize constructed niches, thereby ensuring that niche-constructing populations continue to benefit from collective activities, while minimizing the extent to which interlopers are able to take advantage of them [106]. It is likely no accident, then, that initial domestication of plants and animals usually took place in the context of semi- to fully sedentary groups that had long-term investments in the exploitation of abundant, diverse resources that could be predictably found within well-defined catchment areas [26,29,111]. These are contexts that could have been both monopolized and defended in ways that encouraged the kinds of cooperative behaviours needed to perpetuate niche-constructing activities and set the coevolutionary relationships responsible for domestication into motion.
In addition to providing a more reliable supply of critical resources, however, resource management promotes notions of ownership of the resources and the manipulated environments in which they are grown, while also creating a real potential for uneven access to the rewards of collective activities [111]. These forces are evident in Near Eastern societies where, with agricultural emergence, we see a change from communal storage of vital resources in locations accessible to the whole community, to the development of household storage facilities within individual households outside the view of others, as well as the beginning of increasing differentiation in access to basic resources [112,113]. The centrifugal tensions that arise with increased reliance on managed resources extracted from constructed niches make behaviours that combat them even more important to the survival of the group. These tensions, then, likely created additional pressures for behaviours that promote community cohesion and keep it from splintering into competing subgroups.
Here is where NCT insights about the human capacity for language, symbolic communication and information transfer through social learning can be seen to a play a clear role in promoting the cooperative behaviours essential to the success of small-scale societies [114]. Rather than engaging in a continual process of calculation of self-interest and punishment of non-cooperators, humans, it is argued, have developed a means of maintaining cooperative behaviour through systems of shared norms, customs and values, that are played out and reinforced in an array of everyday and ritual activities, and that set unambiguous, widely acknowledge rules for cooperative behaviour that make failure to abide by these rules expensive. These behaviours are on full display in societies on the threshold of plant and animal domestication which experience an uptick in social and ritual activities directed primarily at enhancing social cohesion and collective activities. In the Near East, these activities are reflected in the construction of communal houses and megalithic structures, elaboration of symbolic lexicons, large-scale feasting activities and ritualized practice of day-to-day activities [115], all of which can be seen as vehicles for promoting the cooperative behaviours needed to perpetuate the collective activities and social cohesions that sustain these groups [116].
2.5. Rates of change
Niche-constructing activities are also held to have a profound impact on rates of evolutionary change, both speeding up and slowing down responses to selection [43,95]. Domestication provides a prime opportunity for exploring these impacts. Plastic responses to novel environments have a far greater potential for rapid evolutionary change than those induced through mutation. This is because mutation-induced novelties affect the survival of only one individual and must await a slow process of selection and transmission to spread more widely. By contrast, environmentally induced novelties, such as those that arise in the context of niche construction, affect numerous individuals, and can persist, even in the absence of strong positive selection, simply through the presence of the inducing factor [57,58]. Moreover, just as exposure to anthropogenic environments has been demonstrated to result in a dramatic increase in plastically driven trait changes [60], anthropogenically induced trait changes have also been shown to occur at a rate roughly twice as fast as changes stemming from non-human drivers. The rate of trait changes stemming from human harvest of wild populations is particularly rapid, estimated at about three times faster than other anthropogenically induced trait changes [61]. Thus, the introduction of plants and animals to novel managed environments, whether they sought out these environments themselves or were relocated there by humans, is likely to have induced the expression of otherwise cryptic variation that would have resulted in rapid population-wide evolutionary change in receptive species. Periods of evolutionary stasis might be expected to follow the initial introduction to anthropogenic environments as plants and animals living within these environments experience conditions of relaxed selection during which genetic variation accumulates in managed populations [56].
Humans, with their ability to spontaneously invent or alter goal-directed behaviours and to widely pass on effective behaviours, are particularly potent factors in setting the rate of evolutionary change [99]. These goal-directed behaviours can be expected to have had a profound impact on the rates of evolution in managed plants and animals, both as they respond to human management in ways that make them better equipped to benefit from their relationship with humans and as humans deliberately encourage traits of interest (even otherwise deleterious ones) and discourage those that make the partnership with the plant or animal less attractive. This is especially the case when humans begin to tinker with genomic reorganization by either encouraging the persistence of naturally occurring hybrids that have traits of interest, or becoming directly engaged in cross-breeding individuals or species for directed goals.
3. Contrasting neo-Darwinian and niche construction theory informed explanatory frameworks
We can distill the fundamental differences between NCT informed perspectives and neo-Darwinian ones (briefly outlined in §2 and explored at greater length in [25] and [26]) into two contrasting explanatory frameworks for initial domestication that generate distinct predictions as to how the process unfolded (table 2) [32]. A neo-Darwinian framework (table 2: column 3) begins with some event that causes an imbalance between human population levels and environmental carrying capacity [117,118]. Niche-constructing activities are only adopted as a way of compensating for the lower returns of less intensive resource extraction strategies and are considered a sign of a decrease in foraging efficiency [119]. Social and ritual activities, if mentioned at all, are portrayed as responses to tensions arising from resource depression and are directed towards increasing the competitive (and reproductive) advantage of some foragers over others [120,121]. And the coevolutionary relationships between humans and target domesticates at the heart or the process of domestication unfolds at a gradual, incremental pace as the result of the accumulation of hundreds of decisions of individual foragers shaped by natural selection [21,24].
Table 2.
Test implications and datasets of explanatory frameworks for initial domestication.
| category | variables | selectionist/OFT predictions | NCT predictions | archaeological data |
|---|---|---|---|---|
| context of initial domestication | climate/environmental change | change resulting in reduction in relative biomass | either stable or improving, resulting in increase in relative biomass | Palaeo-environmental proxies |
| population levels | marked increase or stable; reaching or surpassing carrying capacity | little or no change; population levels remains well below carrying capacity | number and size of sites and intensity of occupation | |
| mobility | decrease in mobility due to population packing | decrease in mobility with settlements targeting resource-rich areas within circumscribed catchment, without population packing | settlement pattern data and correlation with regional resource mapping | |
| resource depression | decrease in high ranking resources; intensification of procurement and processing activities | broadening of resource base, with either no reduction in available biomass or intensification in processing | archaeological plant and animal remains | |
| role of niche construction in initial domestication | modification of environments and biotic communities | only adopted as a response to decline in availability in high ranking prey | continuous and intensifying alteration of landscape or modification of biotic communities in the absence of evidence of resource pressure | evidence of human manipulation of environments (burning, changes in weed assemblages, appearance of species outside habitats) |
| role of social behaviours | social interaction, ritual behaviours, feasting | intensification in these activities aimed at promoting competitive advantage of successful foragers over others | intensification aimed at transmitting environmental knowledge, enhancing community cohesion and levelling intra-community differences in access to resources | evidence of feasting, changes in community structure, burial practices |
| pace of change | dates of appearance of domesticates and agricultural emergence | gradual and incremental | periods of stasis and periods of rapid change in domesticates and humans | AMS dating of material culture and archaeobiological materials, aDNA |
An NCT informed framework (table 2: column 4), by contrast, is set within environments in which abundant resources can be reliably found within circumscribed areas and population levels are below carrying capacity [27,32,110]. Niche-constructing activities should be evident from the outset and will likely intensify through time as they keep carrying capacity in balance with population [30]. Social and ritual activities play a key, catalytic role in NCT informed frameworks, providing vehicles for transmitting environmental knowledge from one generation to the next, enhancing community cohesion and combatting forces that encourage intra-group differences in access to basic resources [115]. Throughout, the process proceeds at an uneven pace, with periods of stasis punctuated by periods of rapid change, driven by both the rapid expression and accumulation of variation resulting from developmental processes and turbo-charged by the human capacity for inventing and transmitting goal-directed behaviours [33].
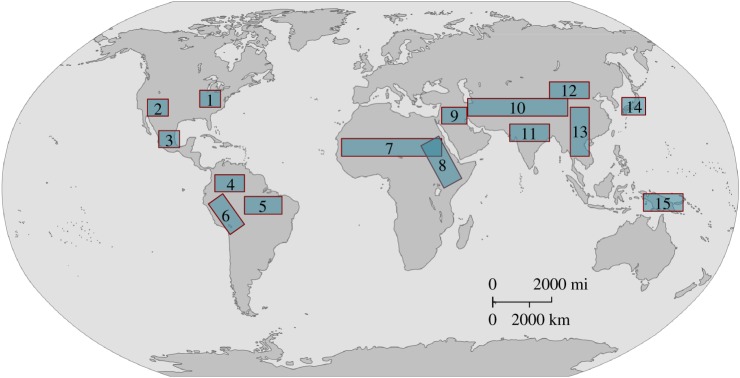
Not only can one draw clear, testable, predictions for these two contrasting frameworks, there is also a growing body of empirical data available to perform these tests (table 2: column 5), including enhanced climate proxies [122], ways of detecting human ecosystem engineering [123], improved methods (morphometric and genetic) for tracing the process of domestication [124], large archaeobiological assemblages and more precise direct dating techniques [125]. Domestication is, moreover, a global phenomenon that arose independently in multiple areas around the world (figure 1). Records for initial domestication in the Near East and eastern North America are particularly robust [110], those for China and the Andes are becoming increasing well resolved [27,126–128], and there are ongoing efforts at documenting domestication in other world areas, including Mesoamerica, the Amazon, West and East Africa, and South East and Island Southeast Asia [129].
Figure 1.
Currently recognized areas of domestication of plants and animals with some examples of plants and animals domesticated in each centre. 1, Eastern North America: chenopodia, squash, sunflower, knotweed and maygrass; 2, Southwest US: turkeys; 3, Mesoamerica: maize, squash, beans and turkeys; 4, northern Peru/Ecuador: squash and lima beans; 5, Amazonia: manioc, yams, peanuts and Muscovy duck; 6, Andes: oca, potato, quinoa, amaranth, llama, alpaca and guinea pigs; 7, sub-Saharan Africa: pearl millet, sorghum and African rice; 8, Horn of Africa/Nile Valley: asses, tef; 9, Near East: wheat, barley, lentils, peas, sheep, goats, taurine cattle and pigs; 10, Central Asia: horses, golden hamster; 11, South Asia: browntop millet, water buffalo and zebu cattle; 12, North China: foxtail and broomcorn millet; 13, South China/Southeast Asia: rice and chickens; 14, Japan: barnyard millet, mung bean, burdock; and 15, New Guinea: bananas, yams and taro. Note: list of domesticates not exhaustive.
While systematic head-to-head evaluations of the different neo-Darwinian and NCT predictions about initial domestication are only just beginning to be performed [30,110], a review of empirical data from regions with the more complete records tend to support NCT generated predictions (see the electronic supplementary material for a longer discussion). Mounting evidence from several world areas suggests that initial domestication took place in the context of small semi- to fully sedentary settlements strategically located at the junction of multiple resource-rich eco-zones in the absence of demonstrable resource pressure [27,30–32,110,126,130]. Niche-constructing activities in each of these areas are evident well before any evidence of initial domestication [30,123,131]. Moreover, the uptick of social and ritual activities that accompanies increasing reliance on managed resources seems to be directed towards promoting community cohesion and combatting centrifugal tendencies towards competition [116,132]. And while the process of initial domestication and subsequent agricultural emergence unfolded over a millennium or more in most regions [31], the pace of change seems to be one in which long periods of relative stasis are punctuated by periods of more rapid change [25,33,125].
4. Domestication as a model system for the extended evolutionary synthesis
Beyond providing a tractable system for exploring the value of NCT as an explanatory framework for initial domestication, a case can also be made that domestication serves as an ideal model system for examining broader issues at the heart of the argument for extending the MS to accommodate recent developments in evolutionary biology, ecology and the social sciences. Not only is NCT one of the cornerstones of the EES, it is also the case, as the above review of the relevance of NCT to initial domestication makes clear, that essentially all the key concepts that fall under the EES umbrella are involved in the process. Recent advances in the study of initial domestication of plants and animals offer particularly promising potential avenues for empirical evaluation of core EES concepts of reciprocal causation, constructive development, hierarchy in targets of selection, inclusive inheritance and the tempo of evolutionary process highlighted in table 1. Some of these avenues of future research are outlined here.
4.1. Causality
Adherents of neo-Darwinian informed explanatory frameworks tend to characterize domestication as a one-way adaptive response to new selective pressures [133] in which niche construction, and other developmental processes involved in domestication are portrayed either as adaptive responses to changing selective pressures [134] or as sources of environmental variability no different from stochastically derived variations in environmental background conditions that elicit adaptive responses [135]. This view stands in strong contrast to an EES perspective that envisions evolutionary events such as domestication as driven by ‘interacting bouts’ of constructive development and selection that may actually lead and shape genetically encoded adaptive change rather than follow it [96]. The increasingly fine-grained record of initial domestication in multiple world areas provides an opportunity for assessing which of these contrasting views of the nature of causation and the primacy of natural selection in evolution is best supported by being able to more precisely track both selective and developmental processes involved in plant and animal domestication.
4.2. Directionality
All the constructive processes that proponents of the EES maintain shape evolutionary trajectories in non-random, directional ways are in play in plant and animal domestication, with niche construction, developmental phenotypic plasticity and genetic accommodation playing especially prominent roles [32]. Whole-genome sequencing has been accomplished for many of today's domestic crops and livestock, as well as for many of their progenitors [136–139], providing a remarkably detailed understanding of the genetic basis of key domestication traits. Advances in the analysis of DNA extracted from archaeobiological remains and the ability to precisely date these remains, moreover, provide excellent opportunities to monitor the interplay among these developmental processes and manifestations of heritable genetic changes in emergent domesticates. Recent studies of ancient DNA extracted from ancient maize, for example, have made it possible to track the appearance of specific domestication genes in this important crop plant as it moved from the heartland of initial domestication in southwestern Mexico up through central Mexico and into the southwestern USA [94,140,141]. Another recent study of ancient DNA extracted from horse skeletal remains finds evidence of enrichment for genes involved in androgen and steroid hormone receptor binding indicative of the selective pressures for behavioural and cognitive changes central to animal domestication [142]. This same study also found evidence for enrichment of genes affecting tissues and cell types derived from the neural crest that lends support for the hypothesis that the neural crest development is involved in the variety of associated traits commonly found in domestic animals [73].
New molecular techniques are coming online that make it possible to perform population-wide screenings for epigenetic markers [51]. Application of such techniques to directly dated archaeobiological remains might allow for an empirical assessment of the hypothesis advanced here that developmental plasticity plays a leading, perhaps dominant, role in shaping the genotype of domesticates.
4.3. Targets of selection
Domestication also provides a case study for exploring the targets of selection, especially the impact of selection operating above the level of individual alleles in driving evolutionary change. The possibility that the constellation of traits found in domestic animals arise through selection operating on neural crest cells [73] is one such opportunity for assessing the role of ‘exploratory behaviour’ among systems above the level of genes in facilitating evolutionary change [143]. The importance of the transmission of ecological inheritance and the reinforcement of cooperative behaviours in domestication, especially as they relate to human behaviour, widens the focus of selection beyond gene frequencies of individuals to one that targets the distribution of traits across transgenerational populations, thus opening the way for an examination of the role of group selection in evolution [9,10,12,144].
4.4. Inheritance
As outlined above, domestication offers an opportunity to assess the impact of all the additional channels of information transfer that EES proponents maintain are equally as important in evolution as genetic inheritance, including epigenetic inheritance, ecological inheritance, social learning and culture. Consideration of the domestication process promises new insights into the role of these inclusive or ‘soft’ forms of inheritance in shaping evolutionary change [9,10,145].
The importance of culture as a form of soft inheritance is particularly noteworthy. Evolutionary biologists operating within the framework of the MS have traditionally had a very difficult time accounting for the role of culture in evolutionary change [144,146]. Human culture is often relegated to the status of a proximate causal process that might help ‘fine-tune’ the variation on which selection (the ultimate cause of evolutionary change) acts, but that has little influence on directional change in evolution [147,148]. This view is embraced by archaeologists working within MS informed frameworks who dismiss the role of human agency or intentionality in evolutionary change—biological or cultural [19,21,23]. This discomfort might also be a reason that many social scientists and scholars working within the humanities tend to down-play or even dismiss the role of human/environmental interaction, and the relevance of evolutionary biology, focusing instead on more purely social, ritual and symbolic behaviours in explanatory frameworks of domestication and culture change in general [7,32]. A view of culture as a form of soft inheritance builds a bridge between the biological and social sciences, and the humanities, that neither negates or downplays the role of culture in evolution, nor glorifies it as something exceptional that operates outside of nature. Recognizing culture and other human cognitive abilities as extensions of the use of acquired knowledge and social learning by non-human animals to enhance the fidelity of information transmitted along external inheritance channels, brings these behaviours squarely within the parameters of evolutionary processes, while also acknowledging the unique and powerful roles they play in shaping the evolutionary trajectory of humans and biota affected by human actions.
The niche construction literature has made significant contributions to documenting the role of domestication in shaping gene–culture interactions and their effect on human biological evolution, with the study of the development of lactose tolerance and resistance to sickle cell anaemia in humans practicing dairying and yam cultivation primary examples of this important work [149]. In addition to having had some impact on the human genome, however, domestication has also played a major role in influencing the course of cultural change, thus providing an opportunity for exploring the importance of human goal-directed behaviour and cultural transmission in human cultural evolution. Consideration of domestication as a model system for evaluating core concepts of the EES also allows for an examination of the role of human agency and intentionality in shaping the evolutionary trajectories of partner species in domesticatory relationships, as well as in the evolution of other non-participant organisms affected by these behaviours. Moreover, a view that sees target species as assuming an active part in directing the course of domesticatory relationships provides an opportunity for examining the reciprocal impact they have in shaping the trajectory of human cultural and biological evolution.
4.5. Tempo and pace of change
Finally, the ability to set the process of domestication in an increasingly well-constrained temporal framework is perhaps one of the most appealing aspects of domestication as a model system for exploring core assumptions of the extended synthesis. Advocates of the EES sometimes lament the lack of empirical examples of the role of genetic accommodation in fixing traits arising from developmental processes, maintaining that the signs of this process may disappear so quickly from a natural population as to make it appear as if these traits arose through standard selection operating on genetic variants following the MS paradigm [51]. Possible systems suggested for documenting these fast-moving processes include natural environments undergoing dramatic rapid modification through climate change or anthropogenically induced habitat destruction [84], invasive species moving into and adapting to novel environments [51,52] and comparisons of the capacities for the expression of phenotypic plasticity in extant ancestral populations with those of derived ones [56].
Domestication resembles these potential model systems in that it involves the introduction and subsequent adaptation of species to novel anthropogenically modified environments that provide optimal settings for assessing the role of constructive processes in the genetic fixation of adaptive traits. In many cases, there are living representatives of both domesticates and their progenitors that might allow for an assessment of the extent to which ancestral plasticity served as a source for fixed genetic traits in their domestic descendants, and thus to assess whether these traits arose as a response to selective pressures, or through the plastic expression of existing previously cryptic traits that were subsequently fixed through the process of genetic accommodation [56]. Perhaps even more exciting is the potential of ancient DNA for determining the timing of the sequence of plastic expression and genetic accommodation behind the fixation of domestication traits in ‘real time’ as it unfolded during the process of initial domestication. Domestication is then a model system that combines multiple interacting constructive processes and multiple internal and external inheritance systems resulting in significant evolutionary change in multiple species, set within an increasingly well-resolved temporal sequence. As such, it would seem that domestication meets all the requirements laid out by EES advocates of a "gold standard" system for assessing the case for revision of standard evolutionary theory [59].
5. Conclusion
Darwin used domestication as a model system for exploring mechanisms of variation in plants and animals under human control to support his thesis of evolution through natural selection [150]. Experimental cross-breeding of domesticates (both plants and animals) also provided model systems that allowed Greger Mendel to discover the rules of heredity responsible for the transmission of variation across generations that provided the fuel for Darwinian selection. Domesticates and the process of domestication were then foundational systems for the modern evolutionary synthesis that brought Darwinian theory of evolution together with Mendelian genetics through the application of population genetics [1–3]. It seems only appropriate that those seeking to build on this important foundation turn once again to domestication as a model system for assessing how recent insights into the role of multiple shaping processes and forms of inheritance should be incorporated into and extended understanding of evolution.
Supplementary Material
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Huxley J. 1942. Evolution: the modern synthesis. New York, NY: Harper and Brothers. [Google Scholar]
- 2.Mayr E. 1942. Systematics and the origin of the species from the viewpoint of a zoologist. New York, NY: Columbia University Press. [Google Scholar]
- 3.Simpson GG. 1977. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 4.Gould S J, Lewontin RC. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationalist programme. Proc. R. Soc. Lond. B 205, 581–598. ( 10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- 5.Eldredge N, Gould SJ. 1972. Punctuated equilibria: an alternative to phyletic gradualism. In Models in paleobiology (ed. Schopf TJ.), pp. 82–115. San Francisco, CA: Freeman, Cooper. [Google Scholar]
- 6.Gould SJ. 2002. The structure of evolutionary theory. Cambridge, MA: Belknap. [Google Scholar]
- 7.Odling-Smee FJ, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution. Monographs in population biology 37. Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Jablonka E, Lamb MJ. 2010. Trangenerational epigenetic inheritance. In Evolution: the extended synthesis (eds Pigliucci M, Müller GD), pp. 137–174. Cambridge, MA: MIT Press. [Google Scholar]
- 10.Jablonka E, Lamb MJ. 2008. Soft inheritance: challenging the modern synthesis. Genet. Mol. Biol. 31, 389–395. ( 10.1590/S1415-47572008000300001) [DOI] [Google Scholar]
- 11.Pigliucci M, Müller G (eds). 2010. Evolution: the extended synthesis. Cambridge, MA: The MIT Press. [Google Scholar]
- 12.Laland KM, Uller T, Feldman MW, Sterenly K, Müller G, Moczek A, Jablonka E, Odling-Smee J. 2015. The extended evolutionary synthesis: its structure, assumptions and predictions. Proc. R. Soc. B 282, 20151019 ( 10.1098/rspb.2015.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott-Phillips TC, Laland KN, Shuker DM, Dickins TE, West SA. 2014. The niche-construction perspective. A critical appraisal. Evolution 68, 1231–1243. ( 10.1111/evo.12332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futuyma D. 2017. Evolutionary biology today and the call for an extended synthesis. Interface Focus 7, 2016045 ( 10.1098/rsfs.2016.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlichting CD, Wund MA. 2014. Phenotypic plasticity and epigenetic marking: an assessment of evidence for genetic accommodation. Evolution 68, 656–672. ( 10.1111/evo.12348) [DOI] [PubMed] [Google Scholar]
- 16.Smith BD, Zeder MA. 2013. The onset of the Anthropocene. Anthropocene 4, 8–13. ( 10.1016/j.ancene.2013.05.001) [DOI] [Google Scholar]
- 17.Dunnell R. 1980. Evolutionary theory and archaeology. Adv. Archaeol. Method Theory 3, 35–99. ( 10.1016/B978-0-12-003103-0.50007-1) [DOI] [Google Scholar]
- 18.O'Brien MJ, Lyman RL. 2000. Applying evolutionary archaeology. New York, NY: Kluwer Academic/Plenum. [Google Scholar]
- 19.Lyman RL, O'Brien MJ. 1998. The goals of evolutionary archaeology: history and explanation. Curr. Anthropol. 39, 615–652. ( 10.1086/204786) [DOI] [Google Scholar]
- 20.Rindos D. 1985. Darwinian selection, symbolic variation, and the evolution of culture . Curr. Anthropol. 26, 65–77. ( 10.1086/203227) [DOI] [Google Scholar]
- 21.Rindos D. 1984. The origins of agriculture: an evolutionary perspective. Orlando, FL: Academic Press. [Google Scholar]
- 22.Hawkes K, O'Connell JF. 1992. On optimal foraging models and subsistence transitions. Curr. Anthropol. 33, 63–65. ( 10.1086/204035) [DOI] [Google Scholar]
- 23.Gremillion KJ, Barton L, Piperno D. 2014. Particularism and the retreat from theory in the archaeology of agricultural origins. Proc. Natl Acad. Sci. USA 111, 6171–6177. ( 10.1073/pnas.1308938110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winterhalder B, Kennett DJ. 2006. Behavioral ecology and the transition from hunting and gathering to agriculture. In Behavioral ecology and the transition to agriculture (eds Kennett DJ, Winterhalder B), pp. 1–21. Berkeley, CA: University of California Press. [Google Scholar]
- 25.Zeder MA. 2009. Evolutionary biology and the emergence of agriculture: the value of co-opted models of evolution in the study of culture change. In Macroevolution in human prehistory (eds Prentiss A, Kuijt I, Chatters J), pp. 157–210. New York, NY: Springer. [Google Scholar]
- 26.Zeder MA. 2012. The broad spectrum revolution at 40: resource diversity, intensification, and an alternative to optimal foraging explanations. J. Anthropol. Archaeol. 31, 241–264. ( 10.1016/j.jaa.2012.03.003) [DOI] [Google Scholar]
- 27.Crawford G. 2011. Advances in understanding early agriculture in Japan. Curr. Anthropol. 52, S331–S345. ( 10.1086/658369) [DOI] [Google Scholar]
- 28.Smith BD. 2011. General patterns of niche construction and the management of wild plant and animal resources by small-scale pre-industrial societies. Phil. Trans. R. Soc. B 366, 836–848. ( 10.1098/rstb.2010.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith BD. 2012. A cultural niche construction theory of initial domestication. Biol. Theory 6, 260–271. ( 10.1007/s13752-012-0028-4) [DOI] [Google Scholar]
- 30.Smith BD. 2016. Neo-Darwinism, niche construction theory, and the initial domestication of plants and animals. Evol. Ecol. 30, 307–324. ( 10.1007/s10682-015-9797-0) [DOI] [Google Scholar]
- 31.Zeder MA. 2015. Core concepts in domestication research. Proc. Natl Acad. Sci. USA 112, 3191–3198. ( 10.1073/pnas.1501711112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeder MA. 2016. Domestication as a model system for niche construction theory. Evol. Ecol. 30, 325–348. ( 10.1007/s10682-015-9801-8) [DOI] [Google Scholar]
- 33.Kuijt I, Prentiss AM. 2009. Niche construction, macroevolution, and the Late Epipaleolithic of the Near East. In Macroevolution in human prehistory (eds Prentiss AM, Kuijt I, Chatters J), pp. 253–271. New York, NY: Springer. [Google Scholar]
- 34.Laland KN, Odling-Smee FJ, Feldman MW, Kendall JR. 2009. Conceptual barriers to progress within evolutionary biology. Found. Sci. 4, 195–216. ( 10.1007/s10699-008-9153-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laland K, Matthews B, Feldman MW. 2016. An introduction to niche construction theory. Evol. Ecol. 30, 191–202. ( 10.1007/s10682-016-9821-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antón SC, Potts R, Aiello LC. 2014. Evolution of early Homo: an integrated biological perspective. Science 345, 1236828 ( 10.1126/science.1236828) [DOI] [PubMed] [Google Scholar]
- 37.Fuentes A, Wyczalkowski M, MacKinnon KC. 2010. Niche construction through cooperation: a nonlinear dynamics contribution to modeling facets of the evolutionary history in the genus Homo. Curr. Anthropol. 51, 435–444. ( 10.1086/651221) [DOI] [Google Scholar]
- 38.Kendal J, Tehrani JJ, Odling-Smee FJ. 2011. Human niche construction in interdisciplinary focus. Phil. Trans. R. Soc. B 366, 785–792. ( 10.1098/rstb.2010.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flynn E, et al. 2013. Developmental niche construction. Dev. Sci. 16, 296–313. ( 10.1111/desc.12030) [DOI] [PubMed] [Google Scholar]
- 40.Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640. ( 10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendry AP. 2017. Eco-evolutionary dynamics. Oxfordshire, UK: Princeton University Press. [Google Scholar]
- 42.Laland KN, Boogert NJ. 2008. Niche construction, co-evolution, and biodiversity. Ecol. Econ. 69, 731–736. ( 10.1016/j.ecolecon.2008.11.014) [DOI] [Google Scholar]
- 43.Odling-Smee FJ, Erwin D, Palkovacs E, Feldman M, Laland KN. 2013. Niche construction theory: a practical guide for ecologists. Q. Rev. Biol. 88, 3–28. ( 10.1086/669266) [DOI] [PubMed] [Google Scholar]
- 44.Matthews B, de Meester L, Jones CG, Ibelings BW, Bouma TJ, Nuutinen V, de Koppel J, Odling-Smee J. 2014. Under niche construction: an operational bridge between ecology, evolution, and ecosystem science. Ecol. Monogr. 84, 245–263. ( 10.1890/13-0953.1) [DOI] [Google Scholar]
- 45.Zeder MA. 2012. The domestication of animals. J. Anthropol. Res. 68, 161–190. ( 10.3998/jar.0521004.0068.201) [DOI] [Google Scholar]
- 46.Smith BD. 2006. Documenting plant domestication in the archaeological record. In Documenting domestication: new genetic and archaeological paradigms (eds Zeder MA, Emshwiller E, Smith BD, Bradley D), pp. 15–24. Berkeley, CA: University of California Press. [Google Scholar]
- 47.Duarte CM, Marbá N, Holmer H. 2007. Rapid domestication of marine species. Science 316, 382–383. ( 10.1126/science.1138042) [DOI] [PubMed] [Google Scholar]
- 48.Donohue K. 2014. Why ontogeny matters during adaption: developmental niche construction and pleiotropy across the life cycle in Arabidopsis thaliana. Evolution 68, 32–47. ( 10.1111/evo.12284) [DOI] [PubMed] [Google Scholar]
- 49.Fuller DQ, Allaby R. 2009. Seed dispersal and crop domestication: shattering, germination and seasonality in evolution under cultivation. Annu. Plant Rev. 38, 238–295. [Google Scholar]
- 50.Larson G, Fuller DQ. 2014. The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 45, 115–136. ( 10.1146/annurev-ecolsys-110512-135813) [DOI] [Google Scholar]
- 51.Pigliucci M. 2010. Phenotypic plasticity. In Evolution: the extended synthesis (eds Pigliucci M, Müller GD), pp. 354–378. Cambridge, MA: MIT Press. [Google Scholar]
- 52.Snell-Rood EC. 2012. Selective processes in development: implications for the costs and benefits of phenotypic plasticity. Integr. Comp. Biol. 52, 31–42. ( 10.1093/icb/ics067) [DOI] [PubMed] [Google Scholar]
- 53.Gremillion KJ, Piperno DR. 2009. Human behavioral ecology, phenotypic (developmental) plasticity, and agricultural origins: insights from the emerging evolutionary synthesis. Curr. Anthropol. 50, 615–619. ( 10.1086/605360) [DOI] [PubMed] [Google Scholar]
- 54.Piperno DR, Holst I, Winter K, McMillan O. 2015. Teosinte before domestication: experimental study of growth and phenotypic variability in Late Pleistocene and early Holocene environments. Quat. Int. 363, 65–77. ( 10.1016/j.quaint.2013.12.049) [DOI] [Google Scholar]
- 55.Asouti E. In press. Human palaeoecology in southwest Asia during the early pre-pottery Neolithic (9700–8500 cal BC): the plant story. In The construction of Neolithic corporate identities (eds Benz M, Gebel HG, Watkins T), Proc. 9th ICAANE Workshop, SENSPSE 18 Berlin, Germany: ex oriente. [Google Scholar]
- 56.Moczek AP, Sultan S, Foster S, Ledón-Rettig C, Dworkin I, Nijhout HF, Abouheif E, Pfennig D. 2011. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B 278, 2705–2713. ( 10.1098/rspb.2011.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West-Eberhard MJ. 2005. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549. ( 10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West-Eberhard MJ. 2005. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. B Mol. Dev. Evol. B 304, 610–618. ( 10.1002/jez.b.21071) [DOI] [PubMed] [Google Scholar]
- 59.Snell-Rood EC, Van Dyken JD, Cruickshank TE, Wade MJ, Moczek AP. 2010. Toward a population genetic framework of developmental evolution: costs, limits, and consequences of phenotypic plasticity. Bioessays 32, 71–81. ( 10.1002/bies.200900132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hendry AP, Farrugia TJ, Kinnison MT. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29. ( 10.1111/j.1365-294X.2007.03428.x) [DOI] [PubMed] [Google Scholar]
- 61.Palkovacs EP, Kinnison MT, Correa C, Dalton CM, Hendry AP. 2011. Fates beyond traits ecological consequences of human induced trait change. Evol. Appl. 5, 183–191. ( 10.1111/j.1752-4571.2011.00212.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darimont CT, Carlson SM, Kinnison MT, Paquete PC, Reimchena TE, Wilmers CC. 2009. Human predators outpace other agents of trait change in the wild. Proc. Natl Acad. Sci. USA 106, 952–954. ( 10.1073/pnas.0809235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coltman DW, O'Donoghue P, Jorgenson JY, Hogg JT, Strobeck C, Festa-Blanchet M. 2003. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658. ( 10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- 64.Gremillion KJ. 1993. The evolution of seed morphology in domesticated Chenopodium: an archaeological case study. J. Ethnobiol. 13, 149–169. [Google Scholar]
- 65.Wilcox G, Fornite S, Herveux L. 2008. Early Holocene cultivation before domestication in northern Syria. Veg. Hist. Archaeobot. 17, 313–325. ( 10.1007/s00334-007-0121-y) [DOI] [Google Scholar]
- 66.Donohue K. 2014. The epigenetics of adaptation: focusing on epigenetic stability as an evolving trait. Evolution 68, 617–619. ( 10.1111/evo.12347) [DOI] [PubMed] [Google Scholar]
- 67.Allaby. 2014. Domestication syndrome in plants. In Encyclopedia of global archaeology (ed. Smith C.), pp. 2182–2184. New York, NY: Springer. [Google Scholar]
- 68.Jensen P. 2006. Domestication—from behavior to genes and back again. Appl. Anim. Behav. Sci. 97, 3–15. ( 10.1016/j.applanim.2005.11.015) [DOI] [Google Scholar]
- 69.Poncet V, Martel E, Allouis S, Devos K, Lamy F, Sarr A, Robert T. 2002. Comparative analysis of QTLs affecting domestication traits between two domesticated×wild pearl millet (Pennisetum glaucum L., Poaceae) crosses. Theor. Appl. Genet. 104, 965–975. ( 10.1007/s00122-002-0889-1) [DOI] [PubMed] [Google Scholar]
- 70.Koinange EMK, Singh SP, Gepts P. 1996. Genetic control of the domestication syndrome in common bean. Crop. Sci. 36, 1037–1045. ( 10.2135/cropsci1996.0011183X003600040037x) [DOI] [Google Scholar]
- 71.Albert FW, et al. 2012. A comparison of brain expression levels in domesticated and wild animals. PLoS Genet. 8, 1–16. ( 10.1371/journal.pgen.1002962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carneiro M, et al. 2014. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science 345, 1074–1079. ( 10.1126/science.1253714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilkens AS, Wrangham RW, Tecumseh Fitch W. 2014. The ‘Domestication Syndrome’ in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795–808. ( 10.1534/genetics.114.165423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conrad JL, Weinersmith KL, Brodin T, Saltz JB, Sih A. 2011. Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J. Fish Biol. 78, 395–435. ( 10.1111/j.1095-8649.2010.02874.x) [DOI] [PubMed] [Google Scholar]
- 75.Stebbins JGL. 1951. Cataclysmic evolution. Sci. Am. 184, 54–59. ( 10.1038/scientificamerican0451-54) [DOI] [Google Scholar]
- 76.Shapiro JA. 2017. Biological action in Read–Write genome evolution. Interface Focus 7, 20160115 ( 10.1098/rsfs.2016.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Emschwiller E. 2006. Origins of polyploid crops: the example of the octoploid tuber crop Oxalis tuberosa. In Documenting domestication: new genetic and archaeological paradigms (eds Zeder MA, Bradley DG, Emschwiller E, Smith BD), pp. 153–168. Berkeley, CA: University of California Press. [Google Scholar]
- 78.Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Strömstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L. 2008. Identification of yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 4, e1000010 ( 10.1371/journal.pgen.1000010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wheeler JC, Chickhi L, Bruford MW. 2006. Genetic analysis of the origins of South American camelids. In Documenting domestication: new genetic and archaeological paradigms (eds Zeder MA, Bradley DG, Emshwiller E, Smith BD), pp. 329–341. Berkeley, CA: University of California Press. [Google Scholar]
- 80.Weiss E, Kislev M, Hartmann A. 2006. Autonomous cultivation before domestication. Science 312, 1608–1610. ( 10.1126/science.1127235) [DOI] [PubMed] [Google Scholar]
- 81.Tanno K-I, Wilcox G. 2006. How fast was wild wheat domesticated? Science 311, 1886 ( 10.1126/science.1124635) [DOI] [PubMed] [Google Scholar]
- 82.Faris JD. 2014. Wheat domestication: key to agricultural revolutions past and present. In Genomics of plant genetic resources (eds Tuberosa R, Graner A, Frison E), pp. 439–464. The Netherlands: Springer. [Google Scholar]
- 83.Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. 2010. Phenotypic plasticity's impacts on diversification and speciation. Trends Ecol. Evol. 25, 459–467. ( 10.1016/j.tree.2010.05.006) [DOI] [PubMed] [Google Scholar]
- 84.Moczek AP. 2012. The nature of nurture and the future of evodevo: toward a comprehensive theory of developmental evolution. Integr. Comp. Biol. 52, 108–119. ( 10.1093/icb/ics048) [DOI] [PubMed] [Google Scholar]
- 85.Hillman GC, Davies MS. 1992. Domestication rates in wild wheats and barley under primitive cultivation: preliminary results and archaeological implications of field measurements of selection coefficient. In Préhistoire de l'agriculture: nouvelle approches expérimentales et ethnographiques (ed. Anderson PC.), pp. 113–158. Paris, France: Centre National de la Recherche Scientifique. [Google Scholar]
- 86.Maeda O, Lucas L, Silva F, Tanno K-I, Fuller DQ. 2016. Narrowing the harvest: increasing sickle investment and the rise of domesticated cereal agriculture in the Fertile Crescent. Quat. Sci. Rev. 145, 226–237. ( 10.1016/j.quascirev.2016.05.032) [DOI] [Google Scholar]
- 87.Zeuner FE. 1955. A history of domesticated animals. London, UK: Hutchinson. [Google Scholar]
- 88.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 89.Fuller D, Lucas L. In press. Adapting crops, landscapes, and food choices: patterns in the dispersal of domesticated plants across Eurasia. In From colonization to globalization: species movement in human history (eds Boivin N, Crassard R, Petraglia M) Cambridge, UK: Cambridge University Press. [Google Scholar]
- 90.Jones H, et al. 2008. Population-based resequencing reveals that the flowering time adaptation of cultivated barley originated east of the Fertile Crescent. Mol. Biol. Evol. 25, 2211–2219. ( 10.1093/molbev/msn167) [DOI] [PubMed] [Google Scholar]
- 91.d'Alpoim Guedes J, Lu H, Li Y, Spengler RN, Wu X, Aldenderfer MS. 2013. Moving agriculture onto the Tibetan Plateau: the archaeobotanical evidence. Archaeol. Anthropol. Sci. 6, 255–269. ( 10.1007/s12520-013-0153-4) [DOI] [Google Scholar]
- 92.Smith BD. In press. Tracing the initial diffusion of maize in North America. In From colonization to globalization: species movement in human history (eds Boivin N, Crassard R, Petraglia M). Cambridge, UK: Cambridge University. [Google Scholar]
- 93.da Fonseca RR, et al. 2015. The origin and evolution of maize in the southwestern United States. Nat. Plants 1, 1–5. ( 10.1038/nplants.2014.3) [DOI] [PubMed] [Google Scholar]
- 94.Jaenicke-Després V, Buckler ES, Smith BD, Gilbert MTP, Cooper A, Doebley J. 2003. Early allelic selection in maize as revealed by ancient DNA. Science 302, 1206–1208. ( 10.1126/science.1089056) [DOI] [PubMed] [Google Scholar]
- 95.Erwin DH. 2008. Macroevolution of ecosystem engineering, niche construction and diversity. Trends Ecol. Evol. 23, 304–310. ( 10.1016/j.tree.2008.01.013) [DOI] [PubMed] [Google Scholar]
- 96.Laland KN, Odling-Smee FJ, Gilbert SF. 2008. Evo-devo and niche construction: building bridges . J. Exp. Zool. Part B Mol. Dev. Evol. 310B, 549–566. ( 10.1002/jez.b.21232) [DOI] [PubMed] [Google Scholar]
- 97.Odling-Smee FJ. 2010. Niche inheritance. In Evolution: the extended synthesis (eds Pigliucci M, Müller GB), pp. 175–207. Cambridge, MA: MIT Press. [Google Scholar]
- 98.Odling-Smee FJ, Laland KN. 2012. Ecological inheritance and cultural inheritance: what are they and how do they differ? Biol. Theory 6, 220–230. ( 10.1007/s13752-012-0030-x) [DOI] [Google Scholar]
- 99.Laland KN, Sterelny K. 2006. Seven reasons (not) to neglect niche construction. Evolution 60, 1751–1762. ( 10.1111/j.0014-3820.2006.tb00520.x) [DOI] [PubMed] [Google Scholar]
- 100.Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. 2013. Identification of the social and cognitive processes underlying human cumulative culture. Science 335, 1114–1118. ( 10.1126/science.1213969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berkes F. 2008. Sacred ecology, 2nd edn New York, NY: Routledge. [Google Scholar]
- 102.Anderson MK. 2005. Tending the wild. Berkeley, CA: University of California Press. [Google Scholar]
- 103.Deur D, Turner N (eds). 2005. Keeping it living: traditions of plant use and cultivation on the northwest coast of North America. Seattle, WA: University of Washington Press. [Google Scholar]
- 104.Hunn E. 1993. What is traditional ecological knowledge? In Traditional ecological knowledge: wisdom for sustainable development (eds Williams NM, Baines G), pp. 13–15. Canberra, Australia: Centre for Resource and Environmental Studies, Australian National University. [Google Scholar]
- 105.Bliege-Bird R, Bird DW, Codding BF, Parker CH, Jones JH. 2008. The ‘fire stick farming’ hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc. Natl Acad. Sci. USA 105, 14 796–14 801. ( 10.1073/pnas.0804757105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krakauer DC, Page KM, Erwin D. 2009. Diversity, dilemmas, and monopolies of niche construction. Am. Nat. 173, 26–40. ( 10.1086/593707) [DOI] [PubMed] [Google Scholar]
- 107.Bettinger RL, Barton L, Morgan C. 2010. The origins of food production in north China: a different kind of agricultural revolution. Evol. Anthropol. 19, 9–21. ( 10.1002/evan.20236) [DOI] [Google Scholar]
- 108.Lehmann L. 2008. The adaptive dynamics of niche-constructing traits in spatially subdivided populations: evolving posthumous extended phenotypes. Evolution 62, 549–566. ( 10.1111/j.1558-5646.2007.00291.x) [DOI] [PubMed] [Google Scholar]
- 109.Lehmann L. 2007. The evolution of trans-generational altruism: kin selection meets niche construction. J. Evol. Biol. 20, 181–189. ( 10.1111/j.1420-9101.2006.01202.x) [DOI] [PubMed] [Google Scholar]
- 110.Zeder MA, Smith BD. 2009. A conversation on agriculture: talking past each other in a crowded room. Curr. Anthropol. 50, 681–691. ( 10.1086/605553) [DOI] [Google Scholar]
- 111.Bowles S, Choi J-K. 2013. Co-evolution of farming and private property during the early Holocene. Proc. Natl Acad. Sci. USA 110, 8830–8835. ( 10.1073/pnas.1212149110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuijt I. 2000. People and space in early agricultural villages: exploring daily lives, community size, and architecture in the late Pre-Pottery Neolithic. J. Anthropol. Archaeol. 19, 75–102. ( 10.1006/jaar.1999.0352) [DOI] [Google Scholar]
- 113.Kuijt I, Finlayson B. 2009. Evidence for food storage and pre-domestication granaries 11 000 years ago in the Jordan Valley. Proc. Natl Acad. Sci. USA 106, 10 966–10 970. ( 10.1073/pnas.0812764106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sterelny K. 2007. Social intelligence, human intelligence and niche construction. Phil. Trans. R. Soc. B 362, 719–730. ( 10.1098/rstb.2006.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeder MA. 2012. Religion and the revolution: the legacy of Jacques Cauvin. Paléorient 37, 39–60. ( 10.3406/paleo.2011.5437) [DOI] [Google Scholar]
- 116.Kuijt I. (ed.). 2000. Life in Neolithic farming communities: social organization, identity, and differentiation. New York, NY: Plenum. [Google Scholar]
- 117.Piperno DR. 2006. The origins of plant cultivation and domestication in the Neotropics: a behavioral ecological approach. In Behavioral ecology and the transition to agriculture (eds Kennett DJ, Winterhalder B), pp. 137–166. Berkeley, CA: University of California Press. [Google Scholar]
- 118.Stutz AJ, Munro ND, Bar-Oz G. 2009. On increasing the resolution of the broad spectrum revolution in the southern Levantine Epipaleolithic (19–12 ka). J. Hum. Evol. 56, 294–306. ( 10.1016/j.jhevol.2008.10.004) [DOI] [PubMed] [Google Scholar]
- 119.Munro ND, Atici L. 2009. Human subsistence change in the Late Pleistocene Mediterranean Basin: the status of research on faunal intensification, diversification, and specialization. Before farming: the archaeology of old world hunter–gatherers 2009/1, article 1. [Google Scholar]
- 120.Aldenderfer M. 2006. Costly signaling, the sexual division of labor, and animal domestication in the Andean Highlands. In Behavioral ecology and the transition to agriculture (eds Kennett DJ, Winterhalder B), pp. 167–196. Berkeley, CA: University of California Press. [Google Scholar]
- 121.Starkovich BM., Stiner MC. 2009. Hallan Çemi Tepesi: high ranked game exploitation alongside intensive seed processing at the Epipaleolithic Neolithic transition in southeastern Turkey. Anthropozoologica 44, 41–61. ( 10.5252/az2009n1a2) [DOI] [Google Scholar]
- 122.Roberts N, Eastwood WJ, Kuzucuoglu C, Fiorentino G, Carcuta V. 2011. Climatic, vegetation and cultural change in the eastern Mediterranean during the mid-Holocene environmental transition. Holocene 21, 147–162. ( 10.1177/0959683610386819) [DOI] [Google Scholar]
- 123.Asouti E, Kabukcu C, White CE. 2015. Early Holocene woodland vegetation and human impacts in the arid zone of the southern Levant. Holocene 25, 1565–1580. ( 10.1177/0959683615580199) [DOI] [Google Scholar]
- 124.Zeder MA, Emshwiller E, Smith BD, Bradley DG. 2006. Documenting domestication: the intersection of genetics and archaeology. TIG 22, 139–155. ( 10.1016/j.tig.2006.01.007) [DOI] [PubMed] [Google Scholar]
- 125.Byrd B. 2005. Reassessing the emergence of village life in the Near East. J. Archaeol. Res. 13, 231–290. ( 10.1007/s10814-005-3107-2) [DOI] [Google Scholar]
- 126.Ren X, Lemoine X, Mo D, Kiffer TR, Guo Y, Qin Z, Liu X. 2016. Foothills and intermountain basins: does China's fertile arch have ‘Hilly Flanks’? Quat. Int. 334–335, 165–178. ( 10.1016/j.quaint.2016.04.001) [DOI] [Google Scholar]
- 127.Mengoni-Goñalons G, Yacobaccio H. 2006. The domestication of South American camelids: a view from the South-Central Andes. In Documenting domestication: new genetic and archaeological paradigms (eds Zeder MA, Bradley DG, Emshwiller E, Smith BD), pp. 228–244. Berkeley, CA: University of California Press. [Google Scholar]
- 128.Pearsall D. 2008. Plant domestication and the shift to agriculture in the Andes. In Handbook of South American archaeology (eds Silverman H, Isbell WH), pp. 105–120. New York, NY: Springer. [Google Scholar]
- 129.Smith BD. 1998. The emergence of agriculture. New York, NY: Scientific American Library. [Google Scholar]
- 130.Smith BD. 2009. Resource resilience, human niche construction, and the long-term sustainability of pre-Columbian subsistence economies in the Mississippi River Valley Corridor. J. Ethnobiol. 29, 167–183. ( 10.2993/0278-0771-29.2.167) [DOI] [Google Scholar]
- 131.Aceituno FJ, Loaiza N, Delgado-Burbano M, Barrientos G. 2013. The initial settlement of Northwest South America during the Pleistocene–Holocene transition: synthesis and perspectives. Quat. Int. 30, 23–33. ( 10.1016/j.quaint.2012.05.017) [DOI] [Google Scholar]
- 132.Zeder MA, Spitzer MD. 2016. New insights into broad spectrum communities of the Early Holocene Near East: the birds of Hallan Çemi. Quat. Sci. Rev. 151, 140–159. ( 10.1016/j.quascirev.2016.08.024) [DOI] [Google Scholar]
- 133.Williams GC. 1992. Gaia, nature worship, and biocentric fallacies. Q. Rev. Biol. 67, 479–486. ( 10.1086/417796) [DOI] [Google Scholar]
- 134.Coding B, Bird D. 2015. Behavioral ecology and the future of archaeological science. J. Archaeol. Sci. 56, 9–20. ( 10.1016/j.jas.2015.02.027) [DOI] [Google Scholar]
- 135.Broughton JM, Cannon MD, Barelink EJ. 2010. Evolutionary ecology, resource depression, and niche construction theory: applications to central California hunter–gatherers and Mimbres-Mogollon agriculturalists. J. Archaeol. Method Theory 17, 317–421. ( 10.1007/s10816-010-9095-7) [DOI] [Google Scholar]
- 136.Benchley R, et al. 2012. Analysis of the bread wheat genome using whole genome shotgun sequencing. Nature 491, 705–710. ( 10.1038/nature11650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Groenen MAM. 2016. A decade of pig genome sequencing: a window on pig domestication and evolution. Genet. Sel. Evol. 48, 23 ( 10.1186/s12711-016-0204-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rubin C-J, et al. 2010. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464, 587–591. ( 10.1038/nature08832) [DOI] [PubMed] [Google Scholar]
- 139.Weber AL, Briggs WH, Rucker J, Baltazar BM, de Jesús Sánchez-Gonzalez J, Feng P, Buckler ES, Doebley J. 2008. The genetic architecture of complex traits in teosinte (Zea mays ssp. parviglumis): new evidence from association mapping. Genetics 180, 1221–1232. ( 10.1534/genetics.108.090134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramos-Madrigal J, Smith BD, Moreno-Mayar JV, Gopalakrishnan S, Ross-Ibarra J, Gilbert MTP, Wales N. 2016. Genome sequence of a 5310-year-old maize cob provides insights into the early stages of maize domestication. Curr. Biol. 26, 3195–3201. ( 10.1016/j.cub.2016.09.036) [DOI] [PubMed] [Google Scholar]
- 141.Vallebueno-Estrada M, Rodríguez-Arévalo I, Rougon-Cardoso A, Martínez González J, García Cook A, Montiel R, Vielle-Calzada J-P. 2016. The earliest maize from San Marcos Tehuaćan is a partial domesticate with genomic evidence of inbreeding. Proc. Natl Acad. Sci. USA 113, 14 151–14 156. ( 10.1073/pnas.1609701113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Librado P, et al. 2017. Ancient genomic changes associated with the domestication of the horse. Science 356, 442–445. ( 10.1126/science.aam5298) [DOI] [PubMed] [Google Scholar]
- 143.Gerhart JC, Kirschner MW. 2007. The theory of facilitated variation. Proc. Natl Acad. Sci. USA 104, 8582–8589. ( 10.1073/pnas.0701035104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilson DS. 2010. Multilevel selection and major transitions. In Evolution: the extended synthesis (eds Pigliucci M, Müller GD), pp. 81–93. Cambridge, MA: MIT Press. [Google Scholar]
- 145.Dickins T, Rahman Q. 2012. The extended evolutionary synthesis and the role of soft inheritance in evolution. Proc. R. Soc. B 278, 1721–1727. ( 10.1098/rspb.2012.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Laland KN. 2017. Darwin's unfinished symphony: how culture made the human mind. Princeton, NJ: Princeton University Press. [Google Scholar]
- 147.Laland KN, Odling-Smee J, Hoppitt W, Uller T. 2013. More on how and why: cause and effect in biology revisited. Biol. Philos 28, 719–745.. ( 10.1007/s10539-012-9335-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Laland KN, Uller T, Feldman MW, Sterelny K, Müller GB, Moczek A, Jablonka E, Odling-Smee FJ. 2014. Does evolutionary theory need a rethink? Yes, urgently. Nature 514, 161–164. ( 10.1038/514161a) [DOI] [PubMed] [Google Scholar]
- 149.O'Brien MJ, Laland K. 2012. Genes, culture, and agriculture: an example of human niche construction. Curr. Anthropol. 53, 434–470. ( 10.1086/666585) [DOI] [Google Scholar]
- 150.Darwin C. 1868. The variation of animals and plants under domestication. London, UK: John Murphey Murray. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.