Abstract
The concept of a ‘human nature’ or ‘human natures’ retains a central role in theorizing about the human experience. In Homo sapiens it is clear that we have a suite of capacities generated via our evolutionary past, and present, and a flexible capacity to create and sustain particular kinds of cultures and to be shaped by them. Regardless of whether we label these capacities ‘human natures’ or not, humans occupy a distinctive niche and an evolutionary approach to examining it is critical. At present we are faced with a few different narratives as to exactly what such an evolutionary approach entails. There is a need for a robust and dynamic theoretical toolkit in order to develop a richer, and more nuanced, understanding of the cognitively sophisticated genus Homo and the diverse sorts of niches humans constructed and occupied across the Pleistocene, Holocene, and into the Anthropocene. Here I review current evolutionary approaches to ‘human nature’, arguing that we benefit from re-framing our investigations via the concept of the human niche and in the context of the extended evolutionary synthesis (EES). While not a replacement of standard evolutionary approaches, this is an expansion and enhancement of our toolkit. I offer brief examples from human evolution in support of these assertions.
Keywords: extended evolutionary synthesis, niche, Homo sapiens, human nature(s), stone tools, warfare
1. A human nature?
What, if anything, distinguishes humans from other animals? How are the psychological and biological traits that contemporary humans possess related to those of their long-dead ancestors? Answers to these questions have often been framed under the rubric of a search for a ‘human nature’. However, experts from across a diverse range of disciplines disagree on what ‘human nature’ is, what it could be, or even if there is one [1–3]. Many evolutionary biologists, such as Laland & Brown [4], view attempts at describing ‘human nature’ as overly simplistic and as antithetical to contemporary understandings of organismal biology. At the same time, other evolutionary scientists, such as van Schaik & Cartmill [5], see a benefit in describing a ‘unique human nature’ that is distinct from a ‘primate nature’ or ‘ape nature’. Many anthropologists engaged with evolutionary perspectives, Ingold & Sussman [3] for example, maintain that researching human nature is a quest based on questionable philosophical and methodological assumptions as human cultures and behaviours are too diverse to be explained by invoking an innate, universal ‘nature’. Yet for many scholars, from across a range of disciplines, and much of the lay public ‘human nature’ is a cornerstone in the philosophical quest and the scientific study of humanity (see [2,3] for extensive reviews of these perspectives).
One emerging compromise position is a path in which we do not identify human nature as a set of innate cognitive or genetic mechanisms but rather as a suite of potentials generated from our evolutionary history, and present, and our flexible capacity to create and sustain human cultures and to be shaped by them: in short, describing and assessing the actions of evolutionary processes and patterns in the human niche [6]. Such a niche, as with all organisms, consists of the spatial, ecological and social sphere including social partners, structural ecologies, other species and the larger population. However, in examining the human niche we also include the contexts created by the perceptual/behavioural patterns developed and reinforced via hyper-complex manipulation of extra-somatic materials/ecologies, a particularly information-rich (and malleable) communication system, and the full range of complex pressures/affordances introduced by the patterns and modalities of human cultures. In such an approach we are seeking to understand the distinctive patterns and potentialities in human capacities, rather than a single outline that delineates, in near-complete detail, the human experience [6]. This is the perspective explored in this article.
2. Evolution and human nature(s)
Across the Pleistocene (the last approximately 2.5 million years) the genus Homo (the human lineage) underwent increases in brain size and complexity, developed an extreme extended childhood period, and created increasingly complex social structures [5]. During this time members of the genus Homo constructed a niche involving expanded innovation and increased use of extra-somatic materials in a dynamic feedback between action, perception and neural structures, which in turn altered the potentials for information acquisition and problem solving [6]. The past five centuries have seen enormous investment in empirical and philosophical research on this history of our genus, this human niche, and on our specific species with a particular focus on the origins of the mind/brain/consciousness, and of human culture (reviewed in [2,3]). While there is no single ‘best’ discourse on human nature across disciplines, many social and most biological scientists would agree that an evolutionary approach to understanding humans' distinctive histories is valuable. However, at present we are faced with a few different narratives as to exactly what such an approach entails.
On the one hand there is what we can call the ‘standard evolutionary approach’ rooted in the neo-Darwinian paradigm developed over the last 160+ years and refined extensively in the last 40. The standard evolutionary approach defines biological evolution as changes in the frequency of DNA sequences in a population across generations, with a focus on four specific processes: natural selection, genetic drift, mutation and gene flow. Natural selection is the process that sorts biological variants such that over time those variants that enhance fitness become highly prevalent in subsequent generations (adaptation) [7]. In this approach, in regards to human evolution, the actions of natural selection and the resultant functional impacts are the key to the origins and selective mechanisms/histories of evolutionarily relevant human traits.
On the other hand there is what we might term an ‘integrated evolutionary approach’ that extends the standard evolutionary theoretical and methodological toolkit via the extended evolutionary synthesis (EES) [8]. The EES focuses on expanding beyond the selection focused standard approach to include the processes of niche construction, ecological inheritance and multiple modes of inheritance in evolutionary inquiry. The EES retains the fundaments of evolutionary theory, but emphasizes constructive processes in development and evolution, and reciprocal portrayals of causation. In the EES, ‘developmental processes, operating through developmental bias, multiple modes of inheritance and niche construction, share responsibility for the direction and rate of evolution, the origin of character variation and organism–environment complementarity’ [8]. Such an approach expands explanatory options for the investigation of human evolution by incorporating and/or considering how human memory and social learning could influence evolutionary trajectories.
Laland & Brown [4], arguing from within the EES context, suggest that the term ‘human nature’ is not useful and is best replaced with descriptions of human behaviour and/or human cognition that are seen as the products of ‘socially mediated internal and external constructive processes operating over both developmental and evolutionary timescales’. Alternatively, the palaeoanthropologist Cartmill [5], arguing from the standard evolutionary approach, calls for the need to place ‘human nature in an evolutionary, explicitly phylogenetic and comparative perspective’. If this is the case, then discovering something that we might term human nature(s) in an evolutionary perspective means understanding the continuities and discontinuities between humans and other organisms. Such patterns are humans' shared evolutionary histories, or ‘natures’, with others, and our distinctive histories since lineage-defining splits. While acknowledging the importance of substantive continuities with closely related taxa, it is via focusing on the discontinuities that our efforts to elucidate the distinctive components that characterize humans (as opposed to other mammals and primates) are enhanced. This approach illustrates that humans share, in an evolutionary sense, mammalian natures, primate natures, ape natures and, as van Schaik [5] notes, a ‘uniquely human nature’. Van Schaik [5] defines this uniquely human nature as the set of traits that evolved exclusively on the hominin lineage after we became hunters and cooperative breeders and that are likely to be the result of ‘grafting the prosocial motivation of the cooperative breeder on the great-ape level cognitive abilities’. Van Schaik (and many others, see [5] and references therein) also argues that there is a post-Neolithic (Holocene) supplement to human nature that can be termed ‘the novel expression of human nature…that arose due to the rapid and unprecedented post-Neolithic changes in subsistence and social organization, driven mainly by accelerated cultural evolution and gene-culture coevolution’ [5].
Here I propose a clarification of how we can think about evolutionary approaches to human behaviour, culture and cognition, whether we call them ‘natures’ or not. In this review I illustrate the rationale for, and benefits of, an integrated evolutionary approach, including the EES, and a more comprehensive and anthropologically informed conceptualization of human culture. I argue not that humans have a ‘nature’ and other organisms do not, but rather that an integrated evolutionary understanding of humans requires recognition of several distinctive characteristics, resulting from specific processes and patterns in the human lineage since the last common ancestor with the other apes. I offer this not as an argument that humans are unique, or that different evolutionary processes function on humans relative to other organisms, but rather that the particularities of human evolutionary histories, and human contemporary biology/ecology (the human niche), offer a specific set of complexities and that the broader evolutionary toolkit of the EES presents a robust context in which to assess them.
3. The standard evolutionary approach and the problem of human culture
In the standard evolutionary approach, examining behaviour in an evolutionary perspective involves a clear distinction between proximate and ultimate causes (a position developed by Mayr [9,10] and expanded on by multiple scholars (e.g. [11])). Proximate causes are mechanisms and developments that emerge from intrinsic motivations and responses to extrinsic stimuli and can be divided into two forms: immediate causal mechanisms and developmental pathways. Ultimate causes are those of greater relevance in evolutionary ‘human natures’ questions as they reflect the effects of natural selection and are thus statements about the fitness, functional impact, origins and selective mechanisms/histories of the traits in question. The standard evolutionary approach seeks to identify key biological and behavioural processes in humans and explain them using models that focus on a proximate/ultimate distinction with natural selection as the primary process shaping relevant traits (but see [12,13]).
However, effectively understanding distinctive processes in the evolution of human behaviour and morphology proves quite difficult to comprehensively explain using this approach for two key reasons: human culture [14,15] and particularly complex human life-history patterns [16,17].
At this point it is important to note that if ‘culture’ is defined as behaviour transmitted via social facilitation and learning from others, which endures for long enough to generate customs and traditions, then many species have culture [18–20]. In many species culture and cultural evolution are significant phenomena in that they emerge from processes of biological evolution but can develop such that they supplement genetic transmission with social transmission and can play central roles in shaping the behaviour, ecology, and even biology, of populations [18,19,21]. However, when considering human culture here, I am speaking specifically about patterns and processes that characterize human behaviour and society, and this includes many processes that are measurably different in scale and impact than in most other species. For humans, cultural elements include massive extra-somatic material creation, manipulation and use (tools, weapons, clothes, buildings, towns, etc.) and extensive ratcheting (expansion and augmentation of cultural processes based on accumulation and innovation) on scales and with a level of structural and material complexity greater than in other organisms. Additionally, the actions involved in developing and using human culture are rooted in the linguistically mediated beliefs, institutions, histories and practices of human groups. While all species' cultural patterns can potentially influence their evolutionary processes, in humans culture is a ubiquitous primary component, and potential driver, of such processes [5,6,14,15,18,22–24].
Human life-history patterns involve a substantially extended childhood and developmental period, with birth occurring extremely early in the development of neurobiological and motor systems, which is likely to be related to the enhanced complexity, and long duration, of cultural acquisition in humans [16,17,25]. Other species with more extensive cultural/social–behavioural complexity also exhibit extended juvenility life-history patterns (e.g. apes, cetaceans, elephants) but rarely to the extent seen in humans. The human life-history pattern creates a more central and extensive role for social learning and neurobiological plasticity than in most other species [17,18,20].
Human cultures are perceptual, material and behavioural and shared across space and time. They are symbolic, linguistic, dynamic and experienced both communally and individually by their members. But human cultures are more than materials, perceptions, beliefs and behaviours—they are also rules, organizations, etc.… with concrete structures and specified consequences. Human cultural systems are interlaced with patterns of social constraint and facilitation that move beyond social hierarchies and dominance relationships, and represent complex multifarious structures and processes that have specific political and economic histories, inherited ecologies (material, perceptual and symbolic) and institutions [24]. It is the scale and complexity in, not the presence of, cultural patterns and processes that represents a distinctly human set of behavioural and ecological contexts relative to other animals. Given the characteristics of human culture and its central role in human evolution it can be quite difficult to effectively analyse via the standard evolutionary approach, with its primary focus on natural selection as the core driver in explanatory models for the appearance of complex, potentially adaptive, patterns. The challenge to understand the human, in an evolutionary sense, is the challenge to develop a model that integrates the influences of history, biology, culture, language and institutions in the human experience and offers a toolkit that enables connecting these processes with evolutionary outcomes.
The two main ways the issue of human cultural complexity has been dealt with within the standard evolutionary approach are the evolutionary psychology approach and various versions of the cultural evolution approach [22]. At its core evolutionary psychology argues that behind the huge variety of human cultures there lies an adapted universal psychology that guides and constrains the range of expressions of human thinking and behaviour. In this view the various aspects of human universal psychology (our nature) are reflective of adaptations to the challenges that humans confronted in their Pleistocene environment (with some ongoing evolutionary response to contemporary ecologies) [26]. There are extensive critiques of this position over the past two decades and the majority of evolutionary scholars do not ascribe to it currently so I will not engage with it directly here (see [27,28] for an overview of the contemporary evolutionary psychology discourse).
Cultural evolutionary approaches remain in broad usage in efforts to tackle the conundrums poised by human culture. Lewens [22] refers to the main contemporary cultural evolution approaches as selectionist and kinetic. In both categories cultural processes are seen as being affected and shaped by evolutionary forces (natural selection, genetic drift, gene flow and mutation). However, selectionists assume that cultural variants/components are engaged in a competitive struggle for existence, in the same way that biological traits are in a Darwinian natural selection model. The kinetic approach is a broader neo-Darwinian approach wherein selection, drift and population-level processes are all involved as opposed to exclusive trait-based competition as the central driver of change.
The three dominant kinetic approaches that address the human natures issue are dual-inheritance theory [15], cultural group selection [29], and the general human cultural evolution model [5,30]. These approaches seek to understand how biologically based processes (anatomical or physiological traits tied to patterns of DNA sequence variation) interact with cultural dynamics to produce the contents and direction of cumulative cultural evolution in humans [30,31]. A common assumption in these models is that culture and biology evolve under relatively similar neo-Darwinian evolutionary forces, with some recent inclusion of the possibility that other evolutionary processes (e.g. aspects of the EES) are also at play [22,29].
In the majority of cultural evolutionary approaches human culture is characterized as a system composed of heritable, potentially competing, variants and patterns: culture is often modelled as a composite of traits and beliefs. Such approaches tend to see cultural evolution as fundamentally neo-Darwinian in its basic structure with culture variants/processes (expressed at individual or at group levels) being the targets of selection (and subject to drift and flow) [14,23,25]. Researchers infer from such models that the evolutionary relevant aspects of ‘culture’, those that might inform us most about human nature(s), reflect or are derived from some form of culturally adaptive (and on occasion biologically maladaptive) patterns.
Such standard evolutionary explanations, even when deployed in modelling cultural in addition to biological evolution, are increasingly seen as necessary but not sufficient for explaining observed patterns, due to the complexity of the interfaces, and entanglements, between cultural and biological processes in humans [22,32]. Consider the following:
A foetus is formed via the interactions between the genes and developmental processes, laying the baseline for body and behaviour…it is exposed to environmental factors such as diet and stress that shape its development and can set off epigenetic change. After birth an infant may be strapped to a cradleboard, cuddled by the father, or nursed by a number of caretakers with an impact on the physiology of both caretaker and infant. From early on, children …begin to embody the skills to negotiate challenging physical and social terrain. Even basic perceptions such as smell and colour are mutually shaped physiology and cultural experience. Growth and maturity are often ushered in by complex rites of passage, with social selection pressures shaping reproductive chances and outcomes and what those processes mean to the individual and the society. Humans develop in community. Adults carry out economic enterprises in niches built over generations of history. They acquire ideological outlooks that guide their motivations, goals, and loyalties. Humans learn the rules of cultural institutions while individual agents push the limits, bringing about game changes that alter niches and make history. ([24], and see [23,25,33–38] for detailed examples of these patterns/processes)
Any attempt to include the range of active processes involved in the production, maintenance and patterns that characterize the variables in the human niche must be able to accommodate, accurately, the diversity of human developmental, physiological, behavioural, perceptual and cultural action. This is not to say that standard evolutionary approaches are incorrect, rather that they are incomplete.
Under standard evolutionary approaches to human evolution and behaviour there is a focus on particular traits, behavioural and morphological (e.g. mating patterns, warfare, tool use, female menopause, male upper body size, etc.), and an attempt to connect them to specific selection histories or to develop explanations for their presence as reflective of specific adaptive benefits [15,16,26,29,39–41]. In these cases it is often the ‘ultimate’ question that is seen as the evolutionarily relevant one and the ‘proximate’ processes are the ethnographic and behavioural details that facilitate the workings of the broader system. Even when cultural traits are given ‘culturally ultimate’ explanatory status, the model in which they act is still analogous to a fitness-based standard evolutionary approach in a selection-dominated landscape [5,15,26]. Assessing the ultimate causal factors often takes the shape of assessing alternative fitness trade-off models (cultural or biological). Generally organismal ‘fitness’ is assumed to be lifetime reproductive output but with long-lived organisms, like humans, proxy measures are used, such as the likely effect of specific behaviours on potential lifetime reproductive success or measures of potential inclusive fitness, or simply energetic costs and benefits of the behaviours in question (with the assumption that negative balances potentially reduce fitness unless compensatory fitness benefits come from the incursion of the lost energy/effort). A concept of ‘trait fitness’ is also commonly deployed wherein models are constructed to estimate the potential relative fitness of a given trait (be it morphological, behavioural or cultural). The models produce proxy measures that can be used in calculating the ultimate value of the trait. In a nutshell, standard evolutionary approaches see evolutionary pressures as potential impacts on reproductive output, challenges to individuals' energy budgets (and associated health risks), and the variation in future potential fitness via individuals' actions in relation to other individuals and local environmental contexts (see overviews for these models and assumptions in [5,15,22,26,27,40,42]). One can substitute ‘cultural trait/process’ for ‘individual’ in the above description for many (but not all [22]) cultural evolution scenarios. While this approach has provided significant contributions for the construction of models and theory (especially in human behavioural ecology [5,16,40]) it remains incomplete, especially when applied to the human niche [6,32].
This is where Andersson et al. [32], myself [6] and many others [42–45] offer to expand the scope of the evolutionary question. Cultural and behavioural components of the human niche need not be modelled only as proximate or ontogenetic modifiers, or assumed to be the outcomes of specific histories of selection. Rather they might also be driving key aspects of the system. If so, processes other than natural or cultural selection may also be shaping evolutionarily relevant patterns. In the EES behavioural and symbolic inheritances are included as potentially relevant to evolutionary processes. The processes and patterns of social institutions can be modelled as local ecologies influencing behavioural options at multiple levels, and the structures of human landscapes, which have both material and perceptual pressures and topographies for the humans inhabiting them, are active agents in the mutual mutability between humans and their niches [6,32,42,43]. Examining the human experience under the rubric of EES can offer options for expanding beyond ‘selection-focused’ standard evolutionary approaches and enable connection to a systems approach in analyses of evolutionary histories and processes (e.g. [6,8,44–49]).
For the remainder of this paper I summarize the evolutionary background of the genus Homo and the concept of the human niche, and offer two examples from human evolutionary history to demonstrate the benefits of adding the EES to our efforts.
4. Human nature(s): an evolutionary context
Foley [43] argues that there have been at least three major transitions in human evolution relevant to a conceptualization of human natures:
(1) the transition to bipedal hominin (approx. 6–4 Ma);
(2) the transition to stone tool-making forager and high-quality diets; the emergence of the genus Homo (approx. 3–2 Ma); and
(3) the emergence of life history and behavioural/social changes underpinned by increasingly complex cultural and cognitive innovations and processes (approx. 0.5 Ma to the present).
These shifts reflect specific key transitions in the history of the human lineage broadly, but involve multiple genera and species. Where do we place the term ‘human’ in such a framework and how does that relate to the possibility of thinking about human natures?
What evolutionary anthropologists mean by ‘human’ is usually one of three groupings of hominins in the genus Homo: (i) all members of the genus Homo, (ii) members of the genus Homo from approximately 1.75 Ma that are likely to be on the line to contemporary Homo sapiens, or (iii) only anatomically contemporary Homo sapiens (who appear approximately 200 000 years ago). The genus Homo shows up in the fossil record between approximately 2.8 and 2.3 Ma and there is contention as to how early members of the genus, from approximately 2.8 to 1.75 Ma, should be classified [50,51]. There is a growing debate as to what we can term ‘distinctive’ or lineage-defining traits for the early members of the Homo lineage and whether or not it makes much sense to make substantive distinctions between the evolutionarily relevant capacities and behavioural patterns of the individuals grouped under the category of early Homo from other temporally contiguous fossils assigned to the genus Australopithecus [52].
However, from the first appearance of fossils placed into the category of Homo erectus/ergaster at approximately 1.75 Ma there is widespread agreement on the inclusion of nearly all subsequent Homo-like fossil specimens into the genus Homo, but less agreement on how many species and subspecies are represented and which ones are on the lineage that ultimately gave rise to contemporary humans, the only extant hominin lineage [53]. There is also little doubt that multiple populations not directly on the lineage to contemporary humans did contribute genetic material to [53], and may have influenced, behavioural and ecological processes in what Foley [43] terms ‘the human adaptive zone’, or what I prefer to call ‘the human niche’ [6,54].
Regardless of the specific taxonomic affiliations, it is clear that there were multiple Homo populations over the middle to late Pleistocene (approximately the last 2 million years) who contributed biologically and culturally to the lineage of modern humans [50–53]. It is in this stretch of archaeological and fossil evidence that much of the baseline for what we would term distinctively human emerged. By 20 000 years ago all populations of the genus Homo on the planet are of the same subspecies (H. s. sapiens) and the pace and diversity of cultural and material change ratchets up across the Holocene (last 10 000 years or so), exploding to an even greater pace in what many term the Anthropocene (last approximately 3–5 centuries) [54,55].
Here I follow Malone et al. [56] and expand on Foley's [43] three transition model in offering an overview of the key evolutionarily relevant accumulations of patterns and processes that characterize particularly distinctive aspects of human evolution resulting in the development of a ‘human niche’ [57,58]. It is these distinctively human characteristics that develop, in combination with one another, over the course of the Pleistocene history of the genus Homo that are of interest in assessing human (not primate or hominin) natures. The following is a core list of the key developments in the genus Homo during the Pleistocene:
— a substantial expansion in brain size and neurological plasticity [50];
— expanded cultural innovation via manipulation of extra-somatic materials as indicated by the creation of increasingly complex stone, and other material, tools via methodologies and technologies not seen in other mammals, primates or hominins [43,50,53];
— accumulation of cultural complexity (including material technology) via an autocatalytic process involving feedback between creativity and transmission (ratcheting) at a level of structural and material complexity beyond that observed in other tool-using organisms [57];
— shared intentionality, hyper-cooperation and complex theory of mind [25,43,58];
— a coevolutionary interdependence between the ecological, cognitive and neural systems, and skill transmission via an apprentice model [59,60];
— the development of a ‘language-ready brain’ and the eventual emergence of language [61,62]; and
— the Holocene and post-Holocene (Anthropocene) explosions in demography, behavioural and cultural diversity, material complexity, manipulation of other organisms (domestication) and the creation of diverse modes of extra-somatic information transfer/technology [54,55,57].
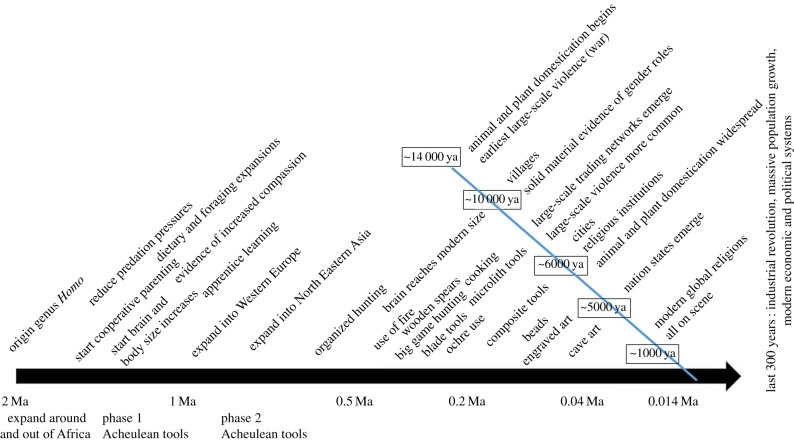
Figure 1 summarizes this pattern with labelled detail associated with specific time referents.
Figure 1.
Key events in Pleistocene and Holocene human evolution. (Online version in colour.)
While components of many of these patterns can be seen in other organisms, their combination, interconnections and the ratcheting-up in complexity of their ecological impacts across the Pleistocene represents the development of a particular human niche. This niche is the context in which we can glean distinctively human patterns that might be considered components of human natures.
In contemporary ecological theory a niche is the structural, temporal and social context in which a species exists. It includes space, structure, climate, nutrients and other physical and social factors as they are experienced, and restructured, by organisms and via the presence of competitors, collaborators and other agents in a shared environment [63]. The human niche is then the spatial and social sphere that includes the structural ecologies (including other species), social partners and the larger local groups/population. But for humans, since at least the mid- to later-Pleistocene, the niches they occupy, structure and interact with also include novel perceptual contexts developed via their increasingly complex manipulation of extra-somatic materials and the patterns and modalities of communication between human individuals and communities. Homo structural and social relationships become perceived and expressed via behavioural, symbolic and material aspects in the development of human culture, creating the human niche (for elaboration on this theme and for specific details of the processes involved see [54,57–60,62,64–71], and for comparisons with other species see [18–20]). The human niche, then, is the context for the lived experience of humans and their communities, where they share ‘kinship’ and social and ecological histories, and where they create and participate in shared knowledge, social and structural security, and development across the lifespan, and thus the human niche is the context in which evolutionary processes act [6]. But how do we identify and model evolutionary processes in this ecologically, behaviourally and culturally hyper-complex niche?
5. Assessing human evolution (human natures): incorporating the extended evolutionary synthesis
Given the specific history and patterns in the evolution of the genus Homo, and the particular complexities introduced by human culture, it is likely that the toolkit offered by standard evolutionary approaches may not be sufficient. Multiple authors suggest that we need an expanded toolkit to develop a richer, and more nuanced, understanding of the genus Homo and the diverse sorts of niches created and occupied by them across the Pleistocene [4,32,50,72,73]. I suggest that the EES can offer at least some of these additional tools and contexts for gaining insight into the processes and functioning of the human niche. Here I provide two examples where EES offers us an enhanced approach.
5.1. Case 1: stone tools
Gamble et al. [74] argue that we need an interdisciplinary framework to understand the specific process of amplification and coevolution of social and technological behaviour in the evolution of our genus [72,73]. Changes in fossil and material evidence of foraging and resource exploitation offer clues to the patterns and processes in human niches and assist in modelling the processes within communities of early Homo [54,74,75]. Approaches relying on connecting specific behavioural or morphological traits associated with stone tool production/use with reproductive success or potential fitness outcomes, and other standard evolutionary models, have not produced sufficiently nuanced and explanatory outcomes [54,60,72,74]. However, there is substantive evidence that niche construction and feedback loops involving neurobiology, ecology and behaviour are central to the evolution of tool use and creation in primates, birds and other organisms (see [76] and [77] for specific examples, and [19] for an overview). In the genus Homo such a system became a central component of evolutionary trajectories, one that gives rise to certain technological and cognitive discontinuities with other species [77,78], thus the application of an EES approach is warranted [54,60,73]
While the earliest stone tool culture (the Lomekwian [79]) appears prior to fossil evidence of the genus Homo (and may be associated with earlier hominin forms, e.g. Australopithecus afarensis, A. garhi or Kenyanthropus platyops) the role of tool creation/manipulation and its impact ratchets up significantly, relative to other animals and other hominins, in Homo across the Pleistocene. Stone tool creation and use had a substantive influence on human morphology, neuroanatomy, ecology and developmental processes [77,80]. Recent work by Stout and colleagues [80], by Hiscock [81] and others shows that there is a rich body of social, neurobiological and ecological information that can be extracted from the evidence offered by early stone toolkits (Oldowan and phase 1 Acheulean approximately 1.5–2.3 Ma). An EES approach to these processes, with emphasis on niche construction, multiple modes of inheritance including behavioural and ecological inheritance, offers a sharper lens and better context for analyses of these data when added to standard evolutionary approaches.
To make Oldowan tools requires a set of manipulations made possible by a specific hand morphology (precision grip and hand–eye coordination), a cognitive capacity for predicting the outcomes of hitting the stone cores in certain ways and some process of sharing information. The most common Oldowan tools are sharp stone flakes created by striking a stone core (often called a ‘cobble’) with another stone called a ‘hammer stone’. In order to successfully construct these tools one needs to be able to do a number of things in sequence. Finding appropriate raw materials is not necessarily easy. Not all stones are equally effective for making flakes as density, grain and crystal structure vary across types of rock. Making an effective tool requires searching for, locating and repeatedly going back to the same sources, or at least being able to assess the same rock types, and sizes, in order to get the best raw materials. All information that must be behaviourally conveyed and transmitted from experienced to inexperienced individuals.
Making the stone tools themselves presents a series of challenges that are particularly methodologically complex and difficult to convey relative to other forms of tool use [19,60,78]. One must examine the core for shapes and patterns in the rock, selecting the specific site to strike the core to create the best flake. Then one has to support the core in a certain way to get a clean strike, have a specific grip on, and swing, the hammer in a specific manner. Once a flake has been detached, one has to repeat the process, but now with a modified core and a new set of possibilities: new shape of the core, new options for striking surfaces. Some authors [82] have suggested that this process of production of stone tools creates a spread of debris in the environment which in itself acts as a form of niche construction. Behavioural transmission of knapping information from experienced to novice tool makers, in the context of this lithic production landscape, necessitates a level of instruction and/or social facilitation that is multi-modal and fairly distinctive relative to other forms of tool-use acquisition across a range of species [19,60,78,82]. Hiscock [81] argues for a ‘niche of lithic production’ in Homo that involved the development of learning environment(s) that had substantive social, material and ecological feedback loops that facilitated the creation and transmission of increasingly complex stone-manipulation processes.
Stout and colleagues [80] and Hecht and colleagues [83] recently demonstrated that learning to make Oldowan tools stimulates patterns of activity in the visual cortex and other areas of the brain, suggesting that the act of tool making affects the way certain neurological pathways respond to stimuli. This suggests that learning to make stone tools may have initiated a specific feedback process between Homo neurobiological development and behavioural activity. The areas where overall tool-making activity had the clearest effects were the supra-marginal gyrus in the parietal lobe and right inferior frontal gyrus of the prefrontal cortex (areas associated with planning complex actions, advanced cognition, and possibly the development of skills in language) [80,83]. Stout & Khreisheh [84] report that experienced contemporary stone toolmakers show increases in activity in the supra-marginal gyrus in their parietal lobe when making tools. They also found that other individuals watching the tool makers could experience some increases in activity in that brain area as well—the action of tool making, and the watching, imitating and sharing information about tool making, can set up and expand the activity and dynamics of particular areas in the brain.
Perception, cognition, neurobiology and collaboration in information transfer and environmental exploitation are entangled in a material and physiological feedback loop implicated in the creation and use of stone tools [60,81]. This process is difficult to model under the standard evolutionary approach where the focus is often on the relative fitness value of behaviours or traits, and models rely on natural selection as the key evolutionary force. Previous work relying on developing models of the fitness benefits of making stone tools (e.g. [85,86]) can miss some of the critical social components of the tool-making process that are likely to be implicated in specific and significant evolutionary changes in Homo. Adding niche construction, and a central role for behavioural inheritance and apprentice-style learning [60] as causal factors, alongside selection and genetic inheritance, offers a more robust model [54,81].
Hiscock [81] argues that the early hominin niche of lithic production involved the development of highly scaffolded learning environment(s). That is to say, such environments are supported by the social group and developed in the context of specific modified landscapes (created via earlier tool production and use). This support facilitates the creation and transmission of increasingly complex stone manipulation processes (see [54,60]). The social transmission of such knowledge, and the potential for the accumulation of technologies, results in access to new, or a greater range of, resources within the local environment. As a result, scaffolded learning environments, and the transmission of knowledge and the potential for expanded resource exploitation they bring, form social, material and ecological feedback loops, which must be considered to understand human evolution. It is this context of tool production (one that is simultaneously social, material and ecological) that forefronts the role of niche constructive feedback relationships at individual and group levels constituting a core early component of the human niche. In examining such a context, the EES (specifically the processes niche construction and behavioural inheritance) offers additional theoretical pathways, and an expanded potential for modelling, extending the range of explanatory options beyond the standard evolutionary approach.
Rather than seeing this outcome (complex tool making) primarily as the result of selection focusing on particular behavioural or physiological traits it is likely that increases in brain size/neural connectivity and cognitive complexity, the extended childhood period, enhanced communication capabilities and the plasticity of brain development emerge and are interlaced via feedback systems among neurobiology, innovation, instruction/learning and increased and diversified ‘tool’ use in the genus Homo [77,87,88]. This implicates multiple systems of inheritance involving a ratcheting up of information transfer, niche construction, substantive developmental plasticity associated with learning time and neurological development, and selection acting on specific combinations of traits in a dynamic system operating at multiple levels (genic, organismal and group/population).
EES approaches to this particularly dynamic suite of relationships can assist in developing better models for the reconfiguration of, and patterns of plasticity in, different cognitive and sensorimotor processes (and the potentially related neurobiological structures) implicated in the system of stone tool making [83,88,89]. As such, specific areas of focus might range from aspects of a mirror neuron system, to specific canonical neurons, to specific structures such as Broca's area, the inferior frontal gyrus, or even the whole parietal cortex [89,90]. Thinking through these systems via the EES, with its inclusion of niche construction and developmental plasticity as evolutionarily causal processes, in the context of the fossil and archaeological records in dialogue with neurobiological and behavioural analyses in living populations might enable the construction of hypotheses about neural reactivity and function related to the processes of stone tool manufacture and use that can be extrapolated to earlier Homo [83,84,87,89]. Such hypotheses may provide target foci for studies of genetic and epigenetic activation and regulation which can offer insight into the rates and timings of significant neuro-genomic–epigenomic events in the evolution of our genus associated with tool-use/creation processes [88–90].
The EES, with the inclusion of niche construction, key roles for ecological inheritance and multiple modes of inheritance, and an emphasis on developmental processes, offers a more dynamic suite of modelling options for connecting individual feedback systems (at the cognitive, sensorimotor and, potentially, neurological level) with subgroup- and community-level systems (behavioural and instructional) and their material records [44,54,91]. This is a more nuanced approach than a focus exclusively on fitness values and a strict separation of ultimate and proximate processes influencing the capacities to make tools, and at the same time it complexifies attempts that seek to focus on the potential fitness values of the tools as traits themselves.
Cultural innovation and accumulation associated with the interaction between tool creation/use and neurological systems involve high-intensity and broad bandwidth information transfer increasing across the Pleistocene [64,87,88]. Such niche constructing patterns facilitate the potential for rapid and dramatic changes via patterns of feedback interactions between behaviour, local ecologies, the manipulation and alteration of material items, and the development of neural architecture. Viewing these patterns as in the context of a suite of evolutionary processes, not just selection, offers a more robust and dynamic context in laying the groundwork for the development of a ‘language-ready brain’ and the emergence of ubiquitous symbolic, cultural behaviour in the genus Homo [54,61,65,84,87].
5.2. Case 2: warfare
Groups of monkeys occasionally fight over fruiting trees; members of different ant colonies will occasionally encounter one another and engage in physical conflicts; and many other types of animals engage in intergroup conflicts, but rarely (if ever) are they planned, organized and lethal with regularity. Humans, unlike most other organisms, engage in warfare [91]. One of the most basic definitions of warfare is: organized lethal violence by members of one group against members of another group [92]. Here ‘organized’ refers to premeditated, coordinated, collective action. Importantly, this definition differentiates war from homicide (single events where an individual is killed by another). Human warfare involves the use of specific tools, and can have a long temporal duration with multiple battles (actual physical conflict events) with lethal outcomes between the two (or more) warring groups. Even in this very broad definition there are very few organisms aside from humans who exhibit such behaviour.
Some (but not all) communities of chimpanzees engage in border patrols and occasional intergroup lethal attacks; however, there remains much contention about the nature and structure of the coordination, the premeditation and the patterns of occurrence across chimpanzee distribution of such behaviour [93–96]. Wolves have also been reported to engage in high levels of intra- and intergroup conflict, with lethal outcomes; however, there is little evidence of consistent large-scale and long-term premeditation, coordination and consistent intraspecific lethality across wolf populations [97]. Across other species, be they primates, canids or cetaceans, the instances of intergroup conflict with lethal outcomes rarely conform to the behavioural pattern of human warfare wherein combatants in institutionalized wars do not fight primarily because they are aggressive or over individual grievances or for specific obtainable resources. Humans largely engage in warfare primarily because of perceptions, beliefs, training and the role in society they occupy [98,99].
Across the Pleistocene Homo brain size and complexity increased and an extreme extended childhood period and increasingly complex social structures developed [100]. Such structures, and their concomitant ecological and technological outcomes, eventually altered the landscape of human evolution in especially distinctive ways, resulting in increasing group sizes, higher population densities and more diverse patterns of social interactions; human groups got larger and more structurally complex [74]. One outcome of this increasingly complex social group living is the development of the human capacity for warfare. Warfare as a species-wide behavioural complex is distinctive to humans (for at least the last approximately 10–14 000 years) [101].
Broadly accepted evidence of warfare coincides with a suite of particular demographic and behavioural patterns including increased densities and group sizes, resource storage, increased intra- and intergroup stratification and sedentism/agriculture [91,101]. However, there is much disagreement about the origins of warfare and whether or not it has deeper roots in the Pleistocene; in short, whether prehistoric interpersonal violence can be considered warfare, a topic that is highly debated [91,96,101,102]. However, there is little debate on the point that the development of organized warfare has been central to recent human evolution [91]. Understanding the emergence of warfare, as an organized and premeditated act, can only be achieved by understanding how inherited technologies and ideas likely formed feedback loops that then influenced the evolution of societies. The standard evolutionary approach does not incorporate such feedback loops and reciprocal causal processes due to its reliance on selection as the sole architect of adaptive processes and its steadfast distinction between proximate and ultimate causation. The expansion of potentially relevant processes by the EES allows for the modelling of complex feedback loops and the inclusion of reciprocal causation chains enabling a more full engagement with human cultural, ecological, economic and political processes.
Looking across the skeletal evidence of the genus Homo from the Pleistocene only 11 of the 447 sites, or approximately 2.5%, have fossils that show evidence of serious trauma [102,103]. The overall database used to assess this pattern includes the remains from at least 2605 individuals, and of these only 58, or approximately 2%, show any evidence of traumatic violent injury [101–103]. Thus, approximately 98% of the fossil evidence for the genus Homo between approximately 2 million years ago and approximately 15 000 years ago show no sign of traumatic, lethal violence [91,101].
This is not to say that violence did not occur, as there is some evidence of violent trauma in the Pleistocene [103,104]. A cranium from Sima de los Huesos dating to about 430 000 years ago has two depression fractures on the frontal bone (forehead) [105], the Shanidar 3 Neandertal, from Iraq, has a cut rib, and an Upper Paleolithic Homo sapiens from Sunghir 1 has a damaged neck vertebrae. Maba 1 (from China) and Dolní Věstonice 11/12 (in the Czech Republic) have healed damage that also might be good examples of interpersonal violence [103,104,106]. However, this small dataset does not offer much insight for the pre-Holocene period (before approximately 8–14 000 years ago) regarding the occurrence of inter-individual violence as a result of intergroup lethal conflict. In fact, given the current fossil datasets, there is no clear evidence of coordinated intergroup lethal violence in the Pleistocene fossil record and insufficient data to argue that inter-individual conflict was a major cause of mortality for most Pleistocene Homo populations [101,104].
Recent reviews [106] demonstrate that in the Pleistocene–Holocene transition (approximately 14 000 to 8000 years ago) violent trauma, at what appears to be a group level, becomes more common in the archaeological record and that during the Holocene Neolithic (8–4000 years ago) substantially more human skeletal remains show signs of violent trauma [101,104]. Individuals with identifiable violent injuries become increasingly common at Neolithic sites and this has been interpreted as the result of organized and lethal conflict between groups [102,107].
The earliest solid evidence of intergroup lethal violence comes from two sites: Jebel Sahaba in Northern Sudan dating to approximately 14 000 and 12 000 years ago and Nataruk, a site west of Lake Turkana (Kenya), dating to approximately 9–10 000 years ago [108,109]. At Jebel Shaba roughly 40% of the 59 bodies are interpreted as showing evidence of traumatic violence [109]. At Nataruk 27 individuals, and 12 full bodies were discovered. Ten of the 12 complete skeletons show signs of lethal violent trauma [108]. A similar case comes from Ukraine and the sites of Voloshkoe and Vasilyevka, dating between 12 000 and 10 035 years ago ([110], see also [111,112]). In each of these cases the humans killed were foragers in particularly rich local ecosystems. Interestingly, these sites also date to a period of rapid climate change [110]. A suggestive hypothesis is that survival pressure and an inequality in access to the best locales and resources primed groups for violent conflict [104]. However, despite these examples, most archaeological sites of the time period do not show signs of intergroup, lethal violence. Up until about 7500 years ago, clear evidence of larger scale, coordinated lethal violence between humans is relatively rare, but by 6–7000 years ago there is a steady increase in the density of unambiguous evidence of coordinated, relatively large-scale killing [92,101,104,112].
Numerous studies demonstrate that the emergence of more complex societies, sedentism, increasing demographic pressures and inequality are correlated with the appearance of evidence of intergroup lethal violence and warfare [101,102,104,113]. While there has been some facile comparisons between these patterns and the general notion of territoriality and conflict in other organisms, the patterns, pace, characteristics, scale and intensity of the human expansion at this time period and its concomitant association with increased evidence of warfare is currently seen as a distinctive process relative to territorial conflict in other species (see overviews of this discussion in [91,101,114]).
When agricultural settlements become common in the Holocene they are accompanied by increasingly strong evidence for group identity, increased storage capacities, enhanced physical and social obligation to place, the potential for increased/more complex trading relationships between groups, and increased inequality [111,115]. The expansion of the human niche to include domestication and sedentism created new ecologies, expanding the opportunities and incentive (pay-offs) for violence [111,115]. Hierarchies in status, wealth and power and the control and management of larger surpluses of food, and the division of land and other protectable resources, created ecologies and altered patterns of gene flow and material exchange [91,92]. These restructured the fitness implications of conflict behaviour, and lethal violence, and increased options and incentives for conflict, greed, distrust and violence. Bowles and Choi [115] note the coincidence of sedentism, agriculture and storage practices with the emergence of symbolic and behavioural processes associated with the concepts of ‘property’. They argue that this produced specific patterns of ecological and symbolic inheritance and novel opportunities, and pay-offs, for collaboration and conflict between human groups.
Key to the ability to conduct warfare that involves the use of specific tools, and has long temporal duration with multiple battles and outcomes between the two (or more) warring groups, is the capacity to develop an extreme sense of shared community, social coordination and a will to engage in highly risky behaviour for the community. How do human groups maintain such a sense of cohesion and coordination concomitant with the increased stratification of individuals and roles emerging in societies at this time? The tendency (or even the capacity) to engage in warfare is unlikely to be a specific trait, or even a suite of physiological or behavioural traits, that can be targeted via direct selection, as there is little, none or a negative correlation between participation in warfare and fitness (direct or inclusive) across populations where it has been assessed [114,116].
One mechanism to facilitate the emergence of a human capacity to engage in warfare that is evident in the archaeological record (and in the ethnographic record) is the development of symbolic identities and ritual practices creating and reinforcing group identities (and ideologies), which can be a central feature in behavioural and symbolic inheritances [117], and social niche construction [44].
Given the core role of cooperation in human evolution, a sense of group identity that could be co-opted and deployed to get individuals to engage in warfare is likely to be very old in the human lineage, dating to at least the middle Pleistocene [88,100,118]. However, increasing role differentiation in groups and the development of clans and lineages who leave material and symbolic evidence is much more recent. The earliest material evidence for symbolic behaviour that can be attributed to group identity construction dates to at least approximately 2–300 000 years ago, and becomes much more common by 45–80 000 years ago, still well before any evidence of warfare [119]. However, it is not until the terminal Pleistocene that we see evidence in archaeological sites of all of these variables coming together alongside sedentism, increasing stratification, consistent storage practices, and the emergence of specific symbolic and behavioural processes associated with the concepts of property [101].
It appears that at some point in the terminal Pleistocene/early Holocene a suite of social, perceptual, behavioural and ecological facets of the human niche coincided in multiple regions resulting in a critical juncture in human history; the development of new human ecologies wherein inherited landscapes and materials involved symbolic identities and structured ecosystems of ownership, inequity and increasing group sizes. Add to this the emergence of institutionalized differences within and between groups and the increasing collective complexity manifest in the increasing specialization and diversity of societal roles and one can see that the template for a broader emergence of warfare is present [91,101,115,120–122]. Given the scenario laid out here, selection-only models for the emergence of such systems fall short, as do facile comparisons with non-human animal conflict patterns [101,104,113,123,124]. The diversity of evolutionary processes mutually interacting in the EES offer a greater toolkit to assess and model the core evidence for the emergence of warfare. The physical remains that emerge around, and subsequent to, the early evidence of warfare such as the construction of defensive structures and landscapes, surpluses of food and other goods, trade relationships, material evidence of strong group identities, higher density residential structures, structural inequalities, and the diverse and symbolically complex skeletal evidence of large-scale, coordinated lethal conflict, offer robust focal points to be incorporated into testable models that combine selection, niche construction, symbolic inheritance and other evolutionary forces in the EES.
For most of human history lethal violence probably took the form of homicides from revenge killings, fights over mates and domestic disputes [113,114]. In such disputes one, or a few, individuals were targeted. But the social, ecological and perceptual changes in human niches across the terminal Pleistocene and Holocene provided the context for the emergence of incentive and justification for group-level violence without identifying specific individuals as the targets [114,121]. Humans made the mental shift from individual-on-individual violence to the possibility of perceiving another group as ‘the enemy’, creatively de-humanizing them [114]. The EES offers a richer and more effective evolutionary framework with which to assess and model such changes.
6. Looking forward
In light of the aforementioned complexity in human nature(s), human evolution can only be properly understood by modelling the development of the evolution of social complexity, including cultural and demographic processes well as changing morphologies. These processes interface with multiple sets of feedback loops that structure the trajectory of human evolution.
The webs of action and perception, memory and history, items and ideas, which humans are entangled in are a dynamic and fundamental constituent of a human niche. A niche that is simultaneously constructed by, and constructing of, human experience and thus highly evolutionarily relevant [6]. Human action and perception are as evolutionarily relevant as human genes, bones and muscles, and we need a body of theory that reflects this complexity [125]. The current state of human societal complexity requires effective models that can account for both ‘the many scales of behaviour of a system and the interplay between environmental and system properties and their dynamic behaviour patterns’ [122].
There is little doubt that the standard evolutionary approach has brought us a long way in assessing and understanding human lineage distinctiveness and offered substantive insight into the processes of human evolution, and thus into human natures. However, as we gather increasingly rich data from human histories, cultures, ecologies and behaviour it is obvious that we need a more integrative toolkit [24,122]. The EES offers critical components of such a toolkit in that it:
retains the fundaments of evolutionary theory, but differs in its emphasis on the role of constructive processes in development and evolution, and reciprocal portrayals of causation. In the EES, developmental processes, operating through developmental bias, multiple modes of inheritance and niche construction, share responsibility for the direction and rate of evolution, the origin of character variation and organism–environment complementarity [8].
Human natures as both a question and an answer has been and will continue to be a topic of scholarly inquiry. In order to achieve a better template for integration across diverse fields we need an evolutionary theory that both best reflects the data available and provides the intellectual tools to weave together narratives of meaning that are robust enough to withstand scientific analyses but not be constrained to always shrink complexity to an explicitly selectionist explanation. A core challenge to this integration is the development of a heuristic that includes an evolutionary framework that engages with the complexity of human systems and recognizes the fluid and entangled interfaces between individuals, groups and community-level dynamics across cultural landscapes and evolutionary time scales. Such an approach must be able to take both biology and social histories into equal account, without necessarily collapsing one into the other. Instead of thinking of human biological and cultural processes as distinct, we need to see them as intertwined and integrated in our quests to understand human nature(s). Contemporary evolutionary theory, as epitomized in the EES, offers us strong options for such attempts.
Acknowledgements
I wish to thank Susan Anton, Melinda Zeder, Tim Lewens, Polly Wiessner, Tim Ingold, Robert Sussman, Kim Sterelny, Jeffery Peterson, Celia Deane-Drummond and Marc Kissel for their influence on the themes and content in this article and the organizers of the ‘New trends in evolutionary biology: biological, philosophical and social science perspectives’, co-sponsored by the Royal Society and the British Academy, for their kind invitation to participate. I also thank the editor of Interface Focus and two anonymous reviewers for substantial and efficient critiques and commentary on earlier versions of this article. Agustin Fuentes is responsible for 100% of the development and writing of this article.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
This research was in part funded by grants from the John Templeton Foundation and support from the office of the Dean of the College of Arts and Letters at the University of Notre Dame.
References
- 1.Fuentes A, Visala A. 2017. Verbs, bones, and brains: interdisciplinary perspectives on human nature. Notre Dame, IN: University of Notre Dame Press. [Google Scholar]
- 2.Downes S, Machery E. 2013. Arguing about human nature: contemporary debates. London, UK: Routledge. [Google Scholar]
- 3.Fuentes A, Visala A. 2016. Conversations on human nature. London, UK: Routledge. [Google Scholar]
- 4.Laland K, Brown G. 2017. The social construction of human nature. In Why we disagree about human nature (eds T Lewens, B Hannon). Oxford, UK: Oxford University Press. [Google Scholar]
- 5.van Schaik CP. 2016. The primate origins of human nature. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 6.Fuentes A. 2016. The extended evolutionary synthesis, ethnography, and the human niche: toward an integrated anthropology. Curr. Anthropol. 57, S13–S26. ( 10.1086/685684) [DOI] [Google Scholar]
- 7.Scott-Phillips TC, Laland KN, Shuker DM, Dickins TE, West SA. 2013. The construction perspective: a critical appraisal. Evolution 68, 1231–1243. ( 10.1111/evo.12332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laland KN, Uller T, Feldman MW, Sterelny K, Müller GB, Moczek A, Jablonka E, Odling-Smee J. 2015. The extended evolutionary synthesis: its structure, assumptions and predictions. Proc. R. Soc. B 282, 20151019 ( 10.1098/rspb.2015.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayr E. 1961. Cause and effect in biology. Science 134, 1501–1506. ( 10.1126/science.134.3489.1501) [DOI] [PubMed] [Google Scholar]
- 10.Mayr E. 1993. Proximate and ultimate causations. Biol. Philos. 8, 93–94. ( 10.1007/BF00868508) [DOI] [Google Scholar]
- 11.Dewsbury D. 1999. The proximate and the ultimate: past, present, and future. Behav. Process. 46, 189–199. ( 10.1016/S0376-6357(99)00035-2) [DOI] [PubMed] [Google Scholar]
- 12.Laland KN, Sterelny K, Odling-Smee FJ, Hoppitt W, Uller T. 2011. Cause and effect in biology revisited: is Mayr's proximate-ultimate dichotomy still useful? Science 334, 1512–1516. ( 10.1126/science.1210879) [DOI] [PubMed] [Google Scholar]
- 13.Laland KN, Odling-Smee J, Hoppitt W, Uller T. 2012. More on how and why: cause and effect in biology revisited. Biol. Philos. 28, 719–745. ( 10.1007/s10539-012-9335-1) [DOI] [Google Scholar]
- 14.Read D. 2012. How culture makes us human: primate evolution and the formation of human societies. Walnut Creek, CA: Left Coast. [Google Scholar]
- 15.Richerson P, Boyd R. 2005. Not by genes alone: how culture transformed human evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 16.Kaplan H, Hill K, Lancaster J, Hurtado M. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. ( 10.1002/1520-6505(2000)9:4%3C156::AID-EVAN5%3E3.0.CO;2-7) [DOI] [Google Scholar]
- 17.Kuzawa CW, Bragg JM. 2012. Plasticity in human life history strategy. Curr. Anthropol. 53, S369–S382. ( 10.1086/667410) [DOI] [Google Scholar]
- 18.Whitten A, Hinde RA, Stringer CB, Laland KN. 2012. Culture evolves. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Sanz CM, Call J, Boesch C. 2014. Tool use in animals: cognition and ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Vale GL, Dean L, Whiten A. 2016. Culture in nonhuman animals. In Wiley International Encyclopedia of Anthropology (ed. A Fuentes). Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- 21.Foote AD, et al. 2016. Genome-culture coevolution promote rapid divergence of killer whale ecotypes. Nat. Commun. 7, 11693 ( 10.1038/ncomms11693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewens T. 2015. Cultural evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Lock M. 2015. Comprehending the body in the era of the epigenome. Curr. Anthropol. 56, 151–177. ( 10.1086/680350) [DOI] [Google Scholar]
- 24.Fuentes A, Wiessner P. 2016. Reintegrating anthropology: from inside out. Curr. Anthropol. 57(Suppl. 13), S3–S12. ( 10.1086/685694) [DOI] [Google Scholar]
- 25.Hrdy SB. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA: Harvard University Press. [Google Scholar]
- 26.Barkow JH, Cosmides L, Tooby J (eds). 1992. The adapted mind: evolutionary psychology and the generation of culture. New York, NY: Oxford University Press. [Google Scholar]
- 27.Barrett L, Robin D, John L. 2002. Human evolutionary psychology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 28.Barrett L, Pollett TV, Stulp G. 2014. From computers to cultivation: reconceptualizing evolutionary psychology. Front. Psychol. 5, 867 ( 10.3389/fpsyg.2014.00867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richerson P, et al. 2016. Cultural group selection plays an essential role in explaining human cooperation: a sketch of the evidence. Behav. Brain Sci. 39, 55 ( 10.1017/S0140525X1400106X) [DOI] [PubMed] [Google Scholar]
- 30.Mesoudi A, Whiten A, Laland K. 2006. Towards a unified science of cultural evolution. Behav. Brain Sci. 29, 329–347. ( 10.1017/S0140525X06009083) [DOI] [PubMed] [Google Scholar]
- 31.Henrich J. 2011. A cultural species: how culture drove human evolution. Psychol. Sci. Agenda 25 See http://www.apa.org/science/about/psa/2011/11/human-evolution.aspx. [Google Scholar]
- 32.Andersson C, Törnberg A, Törnberg P. 2014. An evolutionary developmental approach to cultural evolution. Curr. Anthropol. 55, 154–174. ( 10.1086/675692) [DOI] [PubMed] [Google Scholar]
- 33.Gettler LT. 2016. Becoming DADS: considering the role of cultural context and developmental plasticity for paternal socioendocrinology. Curr. Anthropol. 57(Suppl. 13), S38–S51. ( 10.1086/686149) [DOI] [Google Scholar]
- 34.Shenk MK, Towner MC, Voss EA, Alam N. 2016. Consanguineous marriage, kinship ecology, and market transition. Curr. Anthropol. 57(Suppl. 13), S167–S180. ( 10.1086/685712) [DOI] [Google Scholar]
- 35.Jordan B, Davis-Floyd R. 1992. Birth in four cultures: a crosscultural investigation of childbirth in Yucatan, Holland, Sweden, and the United States. Walnut Creek, CA: Waveland. [Google Scholar]
- 36.Downey G. 2016. Being human in cities: phenotypic bias from urban niche construction. Curr. Anthropol. 57(Suppl. 13), S52–S64. ( 10.1086/685710) [DOI] [Google Scholar]
- 37.Downey G, Lende DH. 2012. The encultured brain: introduction to neuroanthropology. Cambridge, MA: MIT Press. [Google Scholar]
- 38.Bogin B, Scheffler C, Hermanussen M. 2017. Global effects of income and income inequality on adult height and sexual dimorphism. Am. J. Hum. Biol. 29, e22980 ( 10.1002/ajhb.22980) [DOI] [PubMed] [Google Scholar]
- 39.Glowacki L, Wrangham R. 2015. Warfare and reproductive success in a tribal population. Proc. Natl Acad. Sci. USA 112, 348–353. ( 10.1073/pnas.1412287112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith EA. 2000. Three styles in the evolutionary analysis of human behavior. In Adaptation and human behavior: an anthropological perspective (eds Cronk L, Chagnon N, Irons W), pp. 27–46. New York, UK: Aldine de Gruyter. [Google Scholar]
- 41.Flinn MV, Quinlan R, Coe K, Ward CV. 2007. Evolution of the human family: cooperative males, long social childhoods, smart mothers, and extended kin networks. In Family relationships: an evolutionary perspective (eds Salmon CA, Shackleford TK), pp. 16–38. Oxford, UK: Oxford University Press. [Google Scholar]
- 42.Andersson C, Read D. 2016. The evolution of cultural complexity: not by the treadmill alone. Curr. Anthropol. 57, 261–286. ( 10.1086/686317) [DOI] [Google Scholar]
- 43.Foley RA. 2016. Mosaic evolution and the pattern of transitions in the hominin lineage. Phil. Trans. R. Soc. B 371, 20150244 ( 10.1098/rstb.2015.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kendal J. 2012. Cultural niche construction and human learning environments: investigating sociocultural perspectives. Biol. Theory 6, 241–250. ( 10.1007/s13752-012-0038-2) [DOI] [Google Scholar]
- 45.Laland K, Kendall J, Brown G. 2007. The niche construction perspective: implications for evolution and human behavior. Evol. Psychol. 5, 51–66. ( 10.1556/JEP.2007.1003) [DOI] [Google Scholar]
- 46.Hinde RA. 1976. Interactions, relationships and social structure. New York, NY: McGraw-Hill Book Company. [Google Scholar]
- 47.Lewontin RC. 1983. Gene, organism, and environment. In Evolution from molecules to men (ed. Bendall DS.), pp. 273–285. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 48.Oyama S, Griffiths PE, Gray RD. 2001. Cycles of contingency: developmental systems and evolution. Cambridge, MA: MIT Press. [Google Scholar]
- 49.Bateson P, Gluckman P. 2011. Plasticity, robustness, development and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 50.Anton SC, Potts R, Aiello LC. 2014. Evolution of early Homo: an integrated biological perspective. Science 345, 1236828 ( 10.1126/science.1236828) [DOI] [PubMed] [Google Scholar]
- 51.Haile-Selassie Y, Melillo SM, Su DF. 2016. The Pliocene hominin diversity conundrum: do more fossils mean less clarity? Proc. Natl Acad. Sci. USA 113, 6364–6371. ( 10.1073/pnas.1521266113 ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimbel WH, Villmoare B. 2016. From Australopithecus to Homo: the transition that wasn't. Phil. Trans. R. Soc. B 371, 20150248 ( 10.1098/rstb.2015.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ackermann RR, Mackay A, Arnold ML. 2015. The hybrid origin of ‘modern’ humans. Evol. Biol. 43, 1–11. ( 10.1007/s11692-015-9348-1) [DOI] [Google Scholar]
- 54.Fuentes A. 2015. Integrative anthropology and the human niche: toward a contemporary approach to human evolution. Am. Anthropol. 117, 302–315. ( 10.1111/aman.12248) [DOI] [Google Scholar]
- 55.Steffen W, Grinevald J, Crutzen P, McNeil J. 2011. The Anthropocene: conceptual and historical perspectives. Phil. Trans. R. Soc. A 369, 842–867. ( 10.1098/rsta.2010.0327) [DOI] [PubMed] [Google Scholar]
- 56.Malone NM, Fuentes A, White FJ. 2012. Variation in the social systems of extant hominoids: comparative insight into the social behaviour of early hominins international. J. Primatol. 33, 1251–1277. ( 10.1007/s10764-012-9617-0) [DOI] [Google Scholar]
- 57.Tomasello M. 2014. The natural history of human thinking. Cambridge, UK: Harvard University Press. [Google Scholar]
- 58.Whiten A, Erdal D. 2012. The human socio-cognitive niche and its evolutionary origins. Phil. Trans. R. Soc. B 367, 2119–2129. ( 10.1098/rstb.2012.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enquist M, Ghirlanda S, Jarrick A, Wachtmeister CA. 2008. Why does human culture increase exponentially? Theor. Popul. Biol. 74, 46–55. ( 10.1016/j.tpb.2008.04.007) [DOI] [PubMed] [Google Scholar]
- 60.Sterelny K. 2012. The evolved apprentice: how evolution made humans unique. Cambridge, MA: MIT Press. [Google Scholar]
- 61.Arbib MA. 2011. From mirror neurons to complex imitation in the evolution of language and tool use. Annu. Rev. Anthropol. 40, 257–273. ( 10.1146/annurev-anthro-081309-145722) [DOI] [Google Scholar]
- 62.Scott-Phillips TC. 2015. Nonhuman primate communication, pragmatics, and the origins of language. Curr. Anthropol. 56, 56–80. ( 10.1086/679674) [DOI] [Google Scholar]
- 63.Wake DB, Hadley EA, Ackerly D. 2009. Biogeography, changing climates, and niche evolution. Proc. Natl Acad. Sci. USA 106, 19 631–19 636. ( 10.1073/pnas.0911097106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deacon T. 1997. The symbolic species. London, UK: Penguin Press. [Google Scholar]
- 65.Deacon T. 2016. On human (symbolic) nature: how the word became flesh. In Embodiment in evolution and culture (eds Fuchs T, Tewes C), pp. 129–149. Tübingen, Germany: Mohr Siebeck. [Google Scholar]
- 66.Fuentes A. 2014. Human evolution, niche complexity, and the emergence of a distinctively human imagination. Time Mind 7, 241–257. [Google Scholar]
- 67.Kuhn SL. 2014. Signaling theory and technologies of communication in the Paleolithic. Biol. Theory 9, 42–50. ( 10.1007/s13752-013-0156-5) [DOI] [Google Scholar]
- 68.Lombard M. 2012. Thinking through the Middle Stone Age of sub-Saharan Africa. Quat. Int. 270, 140–155. ( 10.1016/j.quaint.2012.02.033) [DOI] [Google Scholar]
- 69.Mithen SJ. 2006. Overview and response to reviewers of The Singing Neanderthals. Camb. Archaeol. J. 16, 97–112. ( 10.1017/S0959774306000060) [DOI] [Google Scholar]
- 70.Rossano MJ. 2009. Ritual behavior and the origin on modern cognition. Camb. Archaeol. J. 19, 243–256. ( 10.1017/S0959774309000298) [DOI] [Google Scholar]
- 71.van Huyssteen JW. 2006. Alone in the world? Human uniqueness in science and theology. Grand Rapids, MI: Eerdmans Publishing. [Google Scholar]
- 72.Kuhn SL, Hovers E. 2014. Alternative pathways to complexity: evolutionary trajectories in the Middle Paleolithic and Middle Stone Age: an introduction to supplement 8. Curr. Anthropol. 54, S176–S182. ( 10.1086/673501) [DOI] [Google Scholar]
- 73.Sterelny K, Hiscock P. 2014. Symbols, signals, and the archaeological record. Biol. Theory 9, 1–3. ( 10.1007/s13752-013-0154-7) [DOI] [Google Scholar]
- 74.Gamble C, Gowlett J, Dunbar RIM. 2011. The social brain and the shape of the Paleolithic. Camb. Archeol. J. 21, 115–136. ( 10.1017/S0959774311000072) [DOI] [Google Scholar]
- 75.Sterelny K. 2014. A Paleolithic reciprocation crisis: symbols, signals, and norms. Biol. Theory 9, 65–77. ( 10.1007/s13752-013-0143-x) [DOI] [Google Scholar]
- 76.Rutz C, St Clair JJH. 2012. The evolutionary origins and ecological context of tool use in New Caledonian crows. Behav. Processes 89, 153–165. ( 10.1016/j.beproc.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 77.Iriki A, Taoka M. 2012. Triadic (ecological, neural, cognitive) niche construction: a scenario of human brain evolution extrapolating tool use and language from the control of reaching actions. Phil. Trans. R. Soc. B 367, 10–23. ( 10.1098/rstb.2011.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaesen K. 2012. The cognitive bases of human tool use behavioural and brain. Sciences 35, 203–262. [DOI] [PubMed] [Google Scholar]
- 79.Harmand S, et al. 2015. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature 521, 310–315. ( 10.1038/nature14464) [DOI] [PubMed] [Google Scholar]
- 80.Stout D, Hecht E, Khreisheh N, Bradley B, Chaminade T. 2015. Cognitive demands of Lower Paleolithic toolmaking. PLoS ONE 10, e0121804 ( 10.1371/journal.pone.0121804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hiscock P. 2014. Learning in lithic landscapes: a reconsideration of the hominid ‘toolmaking’ niche. Biol. Theory 9, 27–41. ( 10.1007/s13752-013-0158-3) [DOI] [Google Scholar]
- 82.Davidson I, McGrew WC. 2005. Stone tools and the uniqueness of human culture. J. R. Anthropol. Inst. 11, 793–817. ( 10.1111/j.1467-9655.2005.00262.x) [DOI] [Google Scholar]
- 83.Hecht EE, Gutman DA, Khreisheh N, Taylor SV, Kilner J, Faisal AA, Bradley BA, Chaminade T, Stout D. 2014. Acquisition of Paleolithic toolmaking abilities involves structural remodeling to inferior frontoparietal regions. Brain Struct. Funct. 220, 2315–2331. ( 10.1007/s00429-014-0789-6) [DOI] [PubMed] [Google Scholar]
- 84.Stout D, Khreisheh N. 2015. Skill learning and human brain evolution: an experimental approach. Camb. Archaeol. J. 25, 867–875. ( 10.1017/S0959774315000359) [DOI] [Google Scholar]
- 85.Kohn M, Mithen S. 1999. Handaxes: products of sexual selection? Antiquity 73, 518–526. ( 10.1017/S0003598X00065078) [DOI] [Google Scholar]
- 86.Nowell A, Chang ML. 2009. The case against sexual selection as an explanation of handaxe morphology. Paleoanthropology 2009, 77–88. [Google Scholar]
- 87.Stout D, Chaminade T. 2012. Stone tools, language and the brain in human evolution. Phil. Trans. R. Soc. B 367, 75–87. ( 10.1098/rstb.2011.0099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grove M, Coward F. 2008. From individual neurons to social brains. Camb. Archaeol. J. 18, 387–400. ( 10.1017/S0959774308000437) [DOI] [Google Scholar]
- 89.Stout D. 2012. Stone toolmaking and the evolution of human culture and cognition. In Culture evolves (eds Whitten A, Hinde RA, Stringer CB, Laland KN), pp. 197–214. Oxford, UK: Oxford University Press. [Google Scholar]
- 90.Gallese V, Lakoff G. 2005. The brain's concepts: the role of the sensory-motor system in conceptual knowledge. Cogn. Neuropsychol. 22, 455–479. ( 10.1080/02643290442000310) [DOI] [PubMed] [Google Scholar]
- 91.Fry DP. (ed.). 2013. War, peace, and human nature. Oxford, UK: Oxford University Press. [Google Scholar]
- 92.Ferguson B. 2008. War before history. In The ancient world at war (ed. D'Souza P.), pp. 15–27. London, UK: Thames and Hudson. [Google Scholar]
- 93.Watts DP, Muller M, Amsler SJ, Mbabazi G, Mitani JC. 2006. Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am. J. Primatol. 68, 161–180. ( 10.1002/ajp.20214) [DOI] [PubMed] [Google Scholar]
- 94.Stumpf RM. 2011. Chimpanzees and Bonobos: inter- and intraspecies diversity. In Primates in perspective (eds Campbell C, Fuentes A, MacKinnon KC, Bearder S, Stumpf R), pp. 340–356, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 95.Boesch C, Crockford C, Herbinger I, Wittig R, Moebius I, Normand E. 2008. Intergroup conflicts among chimpanzees in Tai National Park: lethal violence and the female perspective. Am. J. Primatol. 70, 519–532. ( 10.1002/ajp.20524) [DOI] [PubMed] [Google Scholar]
- 96.Wilson ML. 2013. Chimpanzees, warfare and the invention of peace. In War, peace, and human nature (ed. Fry DP.), pp. 361–388. Oxford, UK: Oxford University Press. [Google Scholar]
- 97.Mech LD, Boitani L. 2003. Wolves: behavior, ecology and conservation. Chicago, IL: University of Chicgao Press. [Google Scholar]
- 98.Ferguson BR. 2011. Born to live: challenging killer myths. In Origins of altruism and cooperation (eds Sussman RW, Cloninger RC), pp. 249–270. Developments in Primatology series, vol. 36, part 2: Progress and prospects. New York, NY: Springer. [Google Scholar]
- 99.Fuentes A. 2012. Race, monogamy and other lies they told you: busting myths about human nature. Berkeley, CA: UC Press. [Google Scholar]
- 100.Anton SC, Snodgrass J. 2012. Origin and evolution of genus Homo: new perspectives. Curr. Anthropol. 53, 479–496. ( 10.1086/667692) [DOI] [Google Scholar]
- 101.Kim N, Kissel M. In press. Emergent warfare and peacemaking in our evolutionary past. London, UK: Routledge.
- 102.Haas J, Piscitelli M. 2013. The prehistory of warfare: misled by ethnography. In War, peace, and human nature (ed. Fry DP.), pp. 168–190. Oxford, UK: Oxford University Press. [Google Scholar]
- 103.Kissel M, Piscatelli M. Submitted. Violence in Pleistocene populations: introducing a new skeletal database of modern humans to test theories on the origins of warfare.
- 104.Ferguson BR. 2013. Pinkers list: exaggerating prehistoric war mortality. In War, peace and human nature (ed. Fry D.), pp. 112–131. New York, NY: Oxford University Press. [Google Scholar]
- 105.Sala N, Arsuaga JL, Pantoja-Pérez A, Pablos A, Martínez I, Quam RM, Gómez-Olivencia A, María Bermúdez de Castro J, Carbonell E. 2015. Lethal interpersonal violence in the Middle Pleistocene. PLOS ONE 10, e0126589 ( 10.1371/journal.pone.0126589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gómez J, Verdú M, González-Megías A, Méndez-Gomez M. 2016. The phylogenetic roots of human lethal violence. Nature 538, 233–237. ( 10.1038/nature19758) [DOI] [PubMed] [Google Scholar]
- 107.Debra M, Harrod R. 2015. Bioarchaeological contributions to the study of violence. Yearb. Phys. Anthropol. 156, 116–145. ( 10.1002/ajpa.22662) [DOI] [PubMed] [Google Scholar]
- 108.Lahr, et al. 2016. Inter-group violence among early Holocene hunter-gatherers of West Turkana, Kenya. Nature 529, 394–398. ( 10.1038/nature16477) [DOI] [PubMed] [Google Scholar]
- 109.Wendorf R. (ed.). 1968. The prehistory of Nubia. Dallas, TX: Southern Methodist University Press. [Google Scholar]
- 110.Lillie MC. 2004. Fighting for your life? Violence at the Late-glacial to Holocene transition in Ukraine. In Violent interactions in the Mesolithic: evidence and meaning (ed. Roksandic M.), pp. 89–96. British Archaeological Reports International Series 1237. Oxford, UK: Archaeopress. [Google Scholar]
- 111.Pinhasi R, Stock J (eds). 2011. Human bioarcheology of the transition to agriculture. New York, NY: John Wiley & Sons. [Google Scholar]
- 112.Wild EM, et al. 2004. Neolithic massacres: local skirmishes or general warfare in Europe? Radiocarbon 46, 377–385. ( 10.1017/S0033822200039680) [DOI] [Google Scholar]
- 113.Fry DP, Söderberg P. 2013. Lethal aggression in mobile forager bands and implications for the origins of war. Science 341, 270 ( 10.1126/science.1235675) [DOI] [PubMed] [Google Scholar]
- 114.Fry D. 2007. Beyond war: the human potential for peace. Oxford, UK: Oxford University Press. [Google Scholar]
- 115.Bowles S, Choi J. 2013. Coevolution of farming and private property during the early Holocene. Proc. Natl Acad. Sci. USA 110, 8830–8835. ( 10.1073/pnas.1212149110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beckerman S, Erickson PI, Yost J, Regalado J, Jaramillo L, Sparks C, Iromenga M, Long K. 2009. Life histories, blood revenge, and reproductive success among the Waorani of Ecuador. Proc. Natl Acad. Sci. USA 106, 8134–8139. ( 10.1073/pnas.0901431106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jablonka E, Lamb M.. 2005. Evolution in four dimensions: genetic, epigenetic, behavioral, and symbolic variation in the history of life. Cambridge, MA: MIT Press. [Google Scholar]
- 118.Fuentes A. 2004. It's not all sex and violence: integrated anthropology and the role of cooperation and social complexity in human evolution. Am. Anthropol. 106, 710–718. ( 10.1525/aa.2004.106.4.710) [DOI] [Google Scholar]
- 119.Kissel M, Fuentes A. 2017. Semiosis in the Pleistocene. Camb. Archaeol. J., 1–16. ( 10.1017/S0959774317000014) [DOI] [Google Scholar]
- 120.Bowles S. 2009. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324, 1293–1298. ( 10.1126/science.1168112) [DOI] [PubMed] [Google Scholar]
- 121.Fuentes A. 2013. Cooperation, conflict, and niche construction in the genus Homo. In War, peace, and human nature (ed. Fry D.), pp. 78–94. Oxford, UK: Oxford University Press. [Google Scholar]
- 122.Bar-Yam Y. 2002. Complexity rising: from human beings to human civilization, a complexity profile. In The encyclopedia of life support systems. Oxford, UK: United Nations. [Google Scholar]
- 123.Pinker S. 2011. The better angels of our nature. New York, NY: Viking. [Google Scholar]
- 124.Gat A. 2015. Proving communal warfare among hunter-gatherers: the quasi-Rousseauan error. Evol. Anthropol. 24, 111–126. ( 10.1002/evan.21446) [DOI] [PubMed] [Google Scholar]
- 125.Ingold T. 2004. Beyond biology and culture: the meaning of evolution in a relational world. Soc. Anthropol. 12, 209–221. ( 10.1017/S0964028204000291) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.