Abstract
By the mid-twentieth century (thus following the ‘Modern Synthesis’ in evolutionary biology), the behavioural sciences offered only the sketchy beginnings of a scientific literature documenting evidence for cultural inheritance in animals—the transmission of traditional behaviours via learning from others (social learning). By contrast, recent decades have seen a massive growth in the documentation of such cultural phenomena, driven by long-term field studies and complementary laboratory experiments. Here, I review the burgeoning scope of discoveries in this field, which increasingly suggest that this ‘second inheritance system’, built on the shoulders of the primary genetic inheritance system, occurs widely among vertebrates and possibly in invertebrates too. Its novel characteristics suggest significant implications for our understanding of evolutionary biology. I assess the extent to which this second system extends the scope of evolution, both by echoing principal properties of the primary, organic evolutionary system, and going beyond it in significant ways. This is well established in human cultural evolution; here, I address animal cultures more generally. The further major, and related, question concerns the extent to which the consequences of widespread animal cultural transmission interact with the primary, genetically based inheritance systems, shaping organic evolution.
Keywords: social learning, traditions, culture, cultural evolution, evolutionary biology, gene–culture coevolution
1. Introduction
1.1. A second inheritance system
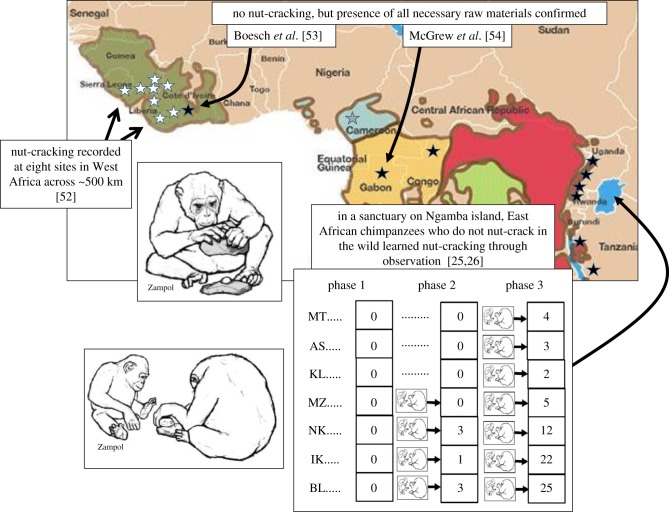
To introduce the concept of a ‘second inheritance system’ [1] discussed in this review, consider the example of chimpanzees (Pan troglodytes verus), who across a swathe of far-West Africa use natural hammer materials to crack open a variety of nuts [2,3] (figure 1). Together with the use of other tools to harvest additional resources, this skill provides a sufficient proportion of the nutritional and energy intake during the dry season, when the favoured fruit diet of the apes is depleted, to suggest it plays a crucial adaptive role in the successful occupation of the habitats involved [10,11]. However, the behaviour is not present in the repertoire of chimpanzees across the greater part of their range, which extends to Tanzania and Uganda in the east [5]; nor does it occur even within the range of the verus subspecies to the east of the great Sassandra river in Ivory Coast [6], despite studies establishing that appropriate nuts and hammer materials are available there and at other sites where the technique is absent [6,7] (figure 1). Such variation thus appears to defy explanation by either environmental or genetic differences, suggesting instead that the behaviour, once invented, has been inherited not via genetics but instead spread by a second inheritance system: observational learning. This would make nut-cracking a local ‘cultural variant’ [5,12], or tradition, analogous to regional variations in human percussive technologies.
Figure 1.
Evidence for nut-cracking as a cultural variant in chimpanzees. Nut-cracking using natural hammer materials is spread across a large area of West Africa (white stars) yet absent elsewhere (dark stars) [3–5], despite independent studies confirming availability of raw materials [6,7]. Experiments with East African chimpanzees show they can acquire nut-cracking through observational learning [8,9]; see text for details. (Online version in colour.)
Convergent evidence is invaluable to test such inferences. These include differences in seasonal preferences for hammer materials documented even between neighbouring chimpanzee communities that are living in similar habitats and subject to much genetic mixing [13,14]. Further support comes from a suite of experimental investigations in both wild and captive communities [8,15]. In one of the latter, East African chimpanzees, who do not naturally crack nuts, were studied in an isolated island sanctuary and either exposed to an expert nut-cracking model or, in a baseline control group, saw no model [8]. Nut-cracking developed in a majority of those who viewed the model but not in the control individuals, and after both groups were exposed to the expert nutcracker, all began to crack nuts, with increasing frequency as their skill grew (figure 1).
In this example of nut-cracking, there are thus multiple convergent sources of evidence assuring the conclusion that social learning underlies the occurrence of the behaviour, such that the regional variations in Africa are fundamentally cultural. It is this social learning that provides chimpanzees with a ‘second inheritance system’ whereby they may benefit from the inventions of rare individuals, that may thus spread across and between communities, a capacity further diffusion experiments with captive apes have confirmed [16,17]. Archaeological excavation at one nut-cracking site has revealed that such beneficial behaviour has been transmitted for at least 4300 years [18] (figure 2), making it an evolutionarily significant phenomenon.
Figure 2.

Nut-cracking continuity confirmed by archaeology. Christophe Boesch holds material evidence of nut-cracking excavated to a time-depth of at least 4300 years ago, corresponding to that currently produced by chimpanzees on the surface here in the Taï Forest [18]. Photo credit: Julio Mercader. (Online version in colour.)
I earlier promoted the expression ‘second inheritance system’ to refer to such a process in the context of the issue of Nature reporting the chimpanzee genome [1], where the term seemed an obvious counterpoint to what is assumed to be the evolutionarily prior, or ‘primary’ system that relies on genetic inheritance, the ‘secondary system’ being built on the shoulders of that pre-existing primary system. This assumption seems reasonable insofar as, despite increasing demonstrations of the widespread occurrence of social learning in varied animal taxa (reviewed below), the phenomenon remains to be reported in the vast majority of living forms, including fungi, plants and most of the major phyla and classes of animals, both unicellular and multi-cellular, all of which display dependence on the primary system.
Labelling social learning the ‘second’ inheritance system might be questioned in the context of other conceptualizations and discussions of non-genetic forms of inheritance [19–25] including those reviewed in this present themed issue. For example, individuals inherit environments resulting from the activities of their parents or others (ecological inheritance), and these may be substantial contributions, as in beavers' dams and lodges, or termite mounds [19,20]. Nevertheless, Odling-Smee et al. ([20, p. 181]) suggest that ecological inheritance ‘more closely resembles the inheritance of territory or property than it does the inheritance of genes’, the former thus not transmitting information in the same sense that genes do. By contrast, social learning does transmit information (as in the case of genes, between generations; but in addition via horizontal transmission within biological generations, discussed further below). In that sense, social learning merits being seen as a second, significantly analogous information transmission system. Genetic and cultural transmission are therefore commonly seen as particularly comparable, as in Dawkins' concept of gene-like cultural ‘memes’ [26,27] as well as other, non-memetic conceptualizations [28,29]. What these systems share is the crucial Darwinian ‘algorithm’ of replication (of either genes or memes, that have the power to shape behaviour in particular ways), and hence repeated cycles of variation and selection on the resulting consequences, that can produce long-term transmission and evolution. This will be discussed further in §4. Beaver dams will evolve only if the behaviour required to recreate them is transmitted over generations through information carried in genes or culture, or both. Darwin himself [30] highlighted the similarities between the evolution of living systems and the cultural evolution of phenomena like human languages, each dependent on the inter-generational inheritance of information. We shall return to such comparative issues concerning cultural and other forms of inheritance and evolution in further discussions below.
1.2. The extension of biology through culture
The creation of the modern, neo-Darwinian evolutionary synthesis occurred most potently in the period 1936–1947 [31,32]. As noted above, the parallels between biological/genetic and (human) cultural evolution were already well recognized before this time; however, the notion of culture among non-human animals was not yet in the frame. The first stirring of attention to such phenomena began to appear only later, in the 1950s and 1960s, in such reports as the diffusion of milk-bottle opening by tits [33], socially learned dialects in songbirds [34] and the spread of foraging novelties in Japanese macaques [35]. Since then, the evidence for animal social learning and culture has grown exponentially, as long-term field studies (which in primates, for example, were achieved in only the last 50 years or so) matured, and increasingly sophisticated experimental and other methodological approaches developed [36]; such evidence now extends to all major classes of vertebrate and several invertebrate taxa too, overviewed in §3. However, these broad manifestations of a second inheritance system, its evolutionary implications and core terminologies have remained strikingly absent from the indexes, glossaries and content of many prominent authors' treatises on evolution, several quite recent [32,37–40]. Where there is treatment, it is typically minimal, referring, for example, to a few restricted topics like birdsong and ape tool use [41]. The common exception is human culture, which has seemed to be an evident analogue in some respects to bio-genetic evolution [37], picked out by concepts such as memes [26] or that of the last ‘major evolutionary transition’ [42]. Even proponents of an extended evolutionary theory linking niche construction, biological evolution and culture have often focused treatment of culture on the human variety [19]. A major aim of this paper is thus to explore how the reams of increasingly pervasive evidence of animal culture may contribute to contemporary extensions of the scope of biology and evolutionary theory.
1.3. Major questions
Set against the background briefly reviewed above, §§3–5 deal with three major questions. In §3, I ask how widespread in the animal kingdom, and thus potentially influential in shaping the nature of evolution, are social learning and culture? The evidence is now sufficiently voluminous that only selected studies can earn mention, but I aim to indicate the nature and variety in the types of evidence now accumulated, and its distribution across major animal taxa. Next, in §4, comes a question I believe has not been addressed in any depth to date in the case of non-human animal cultures: the extent to which properties of the second inheritance system reflect those of the primary, genetic system, and the way these different processes shape evolution. We then examine some of the principal ways in which cultural evolution extends biology by introducing new evolutionary phenomena. Section 5 finally explores types of answer to the important question of how the primary and secondary systems may interact [19–23,25].
2. Definitions
Social learning is essentially learning from others, which provides the foundation of our second inheritance system. More formally, it has been defined as ‘learning that is influenced by observation of, or interaction with, another animal (typically a conspecific) or its products' [43]. This last phrase acknowledges that individuals may learn from the effects of what others achieve, as when it was shown that birds would learn to open bottle tops from the torn holes that others left behind [44]. Note, however, that ‘observation’ needs to be interpreted broadly to include the auditory as well as the visual channel.
By no means all that is acquired by social learning is further transmitted to others such that a larger result that we may recognize as ‘culture’ appears. Much that is socially learned, such as which tree is currently in fruit, will be ephemeral rather than giving rise to a tradition. According to one oft-cited if minimal definition, a tradition is ‘a distinctive behaviour pattern shared by two or more individuals in a social unit, which persists over time and that new practitioners acquire in part through socially aided learning’ [45, p. xiii].
Culture is more variously defined than tradition. Many authors in the biological sciences treat ‘culture’ simply as a synonym for ‘tradition’ as defined above, and for the purposes of this review that will be adequate. However, it is important to recognize that some authors offer additional criteria for referring to culture [46], such as the existence of multiple, diverse traditions that constitute what we call a ‘culture’ in the human case [47].
In this article, we are finally concerned with evolution—and the second inheritance system invoked by the existence of social learning and traditions arguably does not yet constitute evolution. Although Jablonka & Lamb [23, p. 158] defined cultural evolution as a ‘change, in time, in the nature and frequency of socially transmitted preferences, patterns or products in a population’, this may not satisfy all readers, who expect ‘evolution’ to entail some progressive elaboration, perhaps coupled with tree-like branching diversity, corresponding to the richness of evolved and evolving organic nature. Jablonka & Lamb's definition requires only change over time. However, it should be remembered that some changes we generally accept as ‘evolutionary’ involve a reduction in complexity, such as loss of sight in subterranean animals and the loss of limbs in certain reptiles and cetaceans. Standard definitions of evolution include ‘change in the properties of groups of organisms over the course of generations’ [41, p. 2] that often lead ‘to erratic change and to diversification, rather than progress toward any particular end point’ [40, p. 10]. Clearly, human cultures often echo the pervasive cumulative complexity and branching diversification that characterizes organic evolution as a whole, but not always; and in §4, we need to address the senses in which non-human animal culture (henceforth ‘animal culture’) fit and extend the scope of the evolution of forms of life.
3. Animal cultures
Discoveries about animal social learning, traditions and cultures have accelerated over recent decades, and the present century alone has already spawned a literature far too voluminous to detail here. A recent survey of publications in the field for 2012–2014 alone recorded publications from over 100 research groups working on 66 species, spanning a great variety of mammals, birds, fish and insects both in the field and laboratory [48]. A survey of specifically ‘cultural diffusion’ animal experiments identified 30 in 2009–2015, compared with 33 for the whole period 1972–2008 reported earlier, and included eight different species of primates, plus other mammals, birds and insects [49].
Accordingly, the overview that follows cannot provide a comprehensive account, but rather is designed to illustrate through selected examples (i) the diversity of taxonomic groups implicated; (ii) the diversity of functional contexts involved and (iii) the range of observational and experimental approaches and kinds of evidence that have contributed. All these converge to underline the pervading role of culture across a broad span of animal life.
Discussion of this growing corpus of findings could be organized in various ways, such as by types of tradition or patterns of cultural transmission. Here, I opt instead to focus on selected taxonomic groups, in part, because the scope and significance of cultural phenomena appear to vary much in different taxa, as is well illustrated in the contrasts between the first two, primates and cetaceans, that have been the subjects of extensive recent research attention [50,51].
3.1. Primates
Following a number of field researchers' attempts to chart the growing evidence that chimpanzee behaviour varies significantly in different communities, coupled with circumstantial evidence of observational learning, a collaboration of workers from nine field sites achieved a more definitive survey [5]. An initial set of 65 potential cultural variants were consensually defined and were then coded for their prevalence at different sites, allowing 39 to be recognized as putative cultural variants, common in at least one community yet absent in at least one other, and not apparently explicable by genetic or environmental variations. Locally common variants were found to display unique profiles, thus suggested to be ‘cultures’ differentiated by their array of constituent traditions, and exhibiting a rich variety that spans foraging and food processing techniques, tool use, grooming, sexual and other social elements. Many other local behavioural variants have been published since, several reviewed in [52] but not yet systematically collated.
This approach has since been used as a template for similar surveys for other apes, first orangutans [53] and most recently gorillas [54], identifying 24 and 23 cultural variants, respectively. One of the sequelae to the orangutan study included additional direct measures of genetic and ecological variations across the sites [55] and found neither of these predicted the distribution of the 24 putative cultural variations. In the New World, spider monkeys have been studied in the same way [56], identifying 22 different traditions. The same concept has been applied to the very specific and apparently functionless behaviour of provisioned Japanese macaques' ‘stone-handling’, identifying 39 variant forms that match the criterion of being customary at some, yet absent at other, locations [57].
While a fruitful, arguably essential, first step in charting the potential cultural repertoire of a species, this ‘exclusion’ method of ruling out genetic and ecological explanations is of course vulnerable to failing to identify such factors, for example, where the environmental effects are subtle. However, these exploratory studies have provided the foundations for suites of other approaches that have provided convergent and supportive evidence. These can be illustrated for chimpanzees, the best studied case, in the following: (i) neighbouring communities in the wild, that experience genetic cross-mixing and live in similar habitats, have also now been shown to differ in both foraging and social behaviour patterns [13,14,58]; (ii) neighbouring communities in the same sanctuary, that appear environmentally and genetically homogeneous, have also developed cultural differences in foraging and social behaviour [59,60]; (iii) intra-group spread of recognized innovations has been documented [61,62]; (iv) inter-group transfer has resulted in the spread of foraging techniques from one community to another [63]; (v) male–female differences in juveniles' attention to maternal techniques predicted later relative competence [64] (moreover, documentation of juvenile orangutans’ close ‘peering’ at complex manipulative techniques has recently been shown to be associated with multiple effects predicted from its hypothesized role in skill learning [65]); finally, (vi) ‘diffusion experiments' in which alternative techniques to deal with the same foraging problem are seeded in individuals in captive groups have demonstrated both intra-group and inter-group diffusion [16,17]. Interestingly, when communities that habitually use sticks for foraging and others that do not (yet do make and use leaf sponges) were presented with the same problem of extracting honey from vertical holes in wood, the first applied sticks but the latter applied only leaf sponges, that were inherently less effective [66], an effect the authors attribute to conservatism in their existing cultural cognition.
In sum, there is weighty convergent evidence from a welcome diversity of methodological approaches for the reality of multiple-tradition cultures in chimpanzees and several other primate genera, inherited through social learning, and affecting many areas of these animals’ lives [67].
3.2. Cetaceans
Perhaps surprisingly, given how difficult it can be to observe the behaviour of whales and dolphins compared to primates, this second mammalian group has also provided a wealth of evidence for culture that has recently received comprehensive book-length evaluation. Whitehead & Rendell, greatly updating their influential review of 2001, conclude pithily that ‘culture, we believe, is a major part of what the whales are’ [51, p. 17].
One reason that such conclusions can be reached in ocean-based research is that unlike primates such as chimpanzees, cetaceans display complex vocalizations—‘songs’—that can vary much in both space and time. Whales may also undertake very long annual migrations between breeding and feeding grounds, to which they are often very faithful. Both of these phenomena can be well recorded at sea and have provided evidence for cultural transmission. For example, humpback and bowhead whale songs have been documented to change progressively over years, yet at any one time, songs are shared across whole populations, implying cultural diffusion of each change [68,69]. Clearly, these differences cannot be genetic, and indeed the humpback population song off Australia has been observed to change so rapidly, it has been described as a ‘cultural revolution’ as much as a case of gradual evolution [70]. Most remarkably, such radically new songs have been documented crossing the Pacific Ocean in progressive east-shifting waves over successive years [71] (figure 3).
Figure 3.

Diffusion of whale songs in space and time. Different humpback whale songs, here colour-coded for simplicity, were first recorded near East Australia and passed in successive waves across the Pacific Ocean (after [51], modified with additions after [71], with permission of Chicago University Press).
Turning to the learning of migratory routes, the young of both humpback and right whales have been observed to follow their mothers from low-latitude breeding grounds to distant feeding grounds, sometimes following her back the next year, and continue to adhere to the particular maternal migration routes for the remainder of their lives [72,73]. Analyses of the mitochondrial DNA that passes only through the female line have shown, moreover, that individuals may continue to associate with their maternal kin while following these socially inherited routes [73].
Evidence concerning the social learning of other behaviour such as foraging techniques is naturally more elusive in the ocean, but for one behaviour, ‘lob-tail feeding’, in which a humpback smacks its tail on the surface, apparently facilitating the compression of a ball of prey fish before cooperative capture, the earliest occurrences were recorded, followed by charting of its spread to hundreds of other whales over a period of 27 years [74]. The diffusion of the technique was shown to spread along social networks, consistent with a process of cultural transmission.
A very extensive range of other evidence ranging across dolphin tool use, putative teaching and cultural coevolution with human maritime hunters is surveyed in [51]. Potentially, the most culturally differentiated behavioural repertoires in cetaceans—and indeed among non-human species at large—are those of killer whales, or orcas. Orcas have evolved into a range of ‘ecotypes’ that are differentiated by a suite of characteristics attributed to cultural transmission, notably foraging strategies, migratory habits and song repertoires. Some populations specialize in hunting seals, others specialize in fish and there are also significant variants within these two main categories [51,75]. For obvious reasons, cetacean research cannot match the power of experimental studies with primates outlined above, but in the case of orcas, we do have recent experimental evidence that they, like dolphins tested before them [76], have an impressive capacity for social learning, extended to learning a ‘Simon-says’, ‘Do-as-I-do’ game and imitating bodily actions, even of human trainers [77]. Until humans reached Antarctica, killer whales and sperm whales, the cetaceans that have offered the most evidence to date for cultural repertoires, were the most widespread species on the planet, possibly as a result of their cultural sophistications [51].
Interestingly, these different cultural complexes are often sympatric, with the important implication for their evolutionary biology that migration between them becomes difficult [51,75]. If you are in a culture characterized by hunting fish schools and a cluster of other migratory and vocal dimensions, it becomes less easy to transfer into a culture that specializes in a set of alternative behaviours, like the techniques needed to catch seals. The possibility is raised that vocal repertoires even function to actively differentiate such population units, a phenomenon familiar as ‘symbolic marking’ of one's culture in humans [51,78]. Potential consequences for such major biological matters as speciation are discussed further in §§5.3 and 5.4.
3.3. Birds
The two major domains of song and migration available to cetacean researchers also provide avian data on cultural transmission, again differing interestingly from the corpus of primate research. As with cetaceans, the primary evidence for the social learning of migratory routes is circumstantial—the documentation of juveniles initially travelling with parents or other experienced birds on their first migrations, then subsequently remaining faithful to the routes travelled. For example, great bustards were found to travel as families on their initial migration to the wintering grounds, with females subsequently migrating along with other females too, whereas males progressively integrated into male parties and flew with them [79]. The authors interpreted these association patterns and the habitual routes resulting as evidence of the ‘social transmission of migratory patterns' [79], paralleling the evidence for cetaceans [51]. Such initial travel of juveniles with experienced others and establishing long-term fidelity to the routes flown appear to be not uncommon patterns in migratory birds [79], indicating a potentially very significant realm of cultural transmission insofar as around 40% of the approximately 10 000 species of birds are migratory.
However, we know that even isolate juvenile birds of many species show restlessness directed at the normal migratory orientation of their population and thus appears independent of social learning; some, of which young cuckoos are perhaps the most marked example, migrate successfully without any parental guidance. The field would therefore benefit if the circumstantial and correlational data of early co-migration were complemented by experimental manipulations, such as cross-fostering (an approach well suited for animals where clutches of eggs can simply be swapped [80]). In the absence of such studies, we can fortunately turn to successful efforts to imprint young geese, swans and cranes onto micro-lite aircraft, which are subsequently flown to guide them over routes that they have then adopted in later migrations [81] (figure 4). The planes effectively act as surrogate migratory parents, demonstrating cultural transmission of migratory routes [81].
Figure 4.

Micro-lite aircraft to which juvenile birds imprint have acted as surrogate parents to demonstrate migratory routes, confirming a role for cultural transmission as birds adopt the routes in later seasons. Cover illustration of whooping cranes in the study of Mueller et al. [81] with permission of American Association for the Advancement of Science. (Online version in colour.)
An allied effect is birds’ use of ‘public information’ on the breeding success of others to select their own future breeding habitat. Transferring flycatcher nestlings between nests enabled researchers to show that initially resident birds were more likely to migrate to other habitats on reduction in either the quantity or quality of chicks they could locally observe, whereas potential immigrants with less access to such information were positively influenced by the local quantity but not quality of chicks [82].
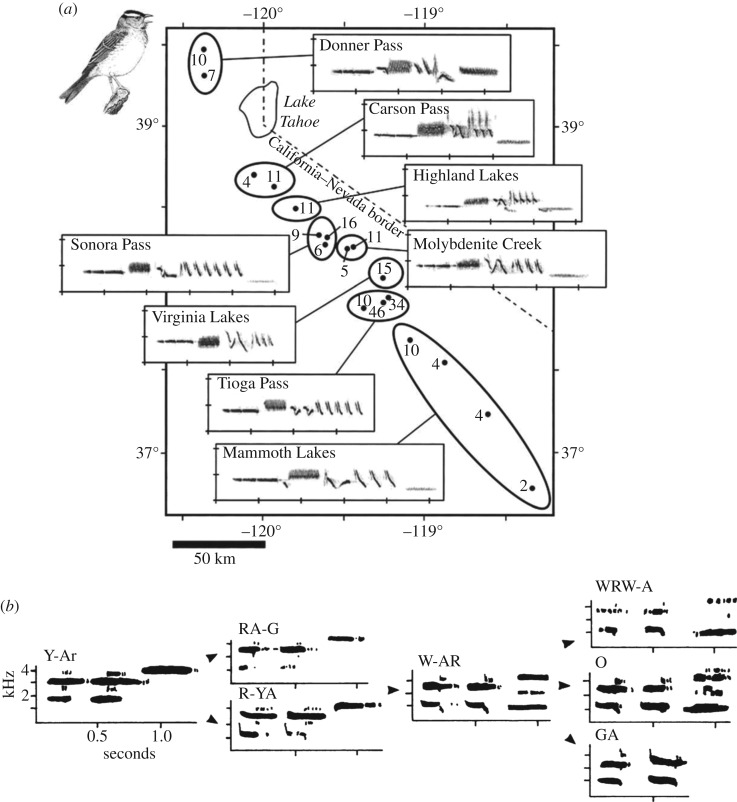
The most substantial and long-standing corpus of research on cultural transmission in birds concerns song learning [34,83,84]. Laboratory studies have demonstrated that songs are socially learned from existing singers in numerous species of songbirds, so social learning is thought to underlie both regional variations typically referred to as ‘dialects’ [34,83], and the ways in which songs may change from year to year while being shared within a local population [83,84], echoing this phenomenon in cetaceans, noted earlier in this article. Regional dialects were documented early in birdsong research [34] and have since been demonstrated in numerous species, the most recent book-length collation [83] listing over 84 different studies reporting and analysing patterns of birdsong dialects. An illustrative example is shown in figure 5a [85] and a case of changes over time in figure 5b [86]. However, the species studied to date can stand only as minimal representatives of the approximately 4000 different species of song-bird that accordingly may also display these kinds of manifestations of cultural transmission. In the reference listing of [83], the term ‘cultural evolution’ occurs repeatedly. We postpone to §4 consideration of the justifications that may be required for applying this expression.
Figure 5.
(a) Regional dialect differences illustrated by sonograms of white-crowned sparrows ([83], modified from [85] with additions, with permission of Cambridge University Press); (b) behavioural change over time through ‘cultural mutations’ in the socially inherited songs of saddlebacks [86].
The variations in birdsong resulting from these processes are not thought to enhance dealing with local ecological circumstances, any more than do differences in human languages around the globe, although there is evidence of adaptation in the sound qualities themselves to transmission properties of different habitats [87]. By contrast, cultural transmission of migratory routes appears functional in guiding naive individuals to critical locations that are often distant and may be inherently difficult to discover by oneself. Other instances of adaptive outcomes delivered through cultural transmission come from a variety of other methodological approaches and behavioural domains. One such approach has been cross-fostering across closely related species, such as great tits and their smaller relatives, blue tits [80]. When birds came to feed their own chicks, individuals that had been reared by the other species tended to reveal the preferences of their foster-parents, with the smaller blue tits offering larger prey than normal and the great tits offering smaller prey. The authors conclude that ‘the fact that young birds learn from their foster-parents, and use this experience later when subsequently feeding their own offspring, suggests that foraging behaviour can be culturally transmitted over generations in the wild’ (p.969). Similar effects on sexual imprinting, alarm calls and song have also been reported by this research team [80].
Cultural transmission of foraging techniques has been revealed in a different approach which involved ‘seeding’ alternative behaviours to extract food from hoppers in different communities of great tits within a marked population of which over 400 individuals participated [88]. The two alternative foraging behaviours initially demonstrated by pairs of trained community members spread differentially and with high fidelity in the respective communities, continuing into the following season and even evidencing the enhanced dispositions to copy others that characterize social conformity.
3.4. Fish
A famous quip in 2003 declared that there was stronger evidence for culture in fish than in primates [89]. The rationale was that it had been possible to translocate not only individuals but whole communities between sites (not feasible with, for example, either chimpanzees or whales), revealing that in French grunts, these novices soon followed and adopted existing preferences of resident fish for travel routes between resting and foraging sites [90]. Conversely, when bluehead wrasse were translocated only once residents were removed from their habitual mating sites, the incomers established new sites; and they then maintained them over a further 12 years of study [91]. Complementary laboratory studies with guppies were able to further show that naive fish put with those who already learned to swim one of two alternative routes would first shoal with and then later independently display the preference they socially inherited in this way [92]; moreover, repeated additions of naive fish and removal of experienced ones until all the original models had gone showed the tradition would be sustained across these simulated generations [93]. This was even the case when fish were adopting the less efficient of two optional routes [93]. In nature, such a maladaptive bias may eventually be overturned, but its existence, alongside other indicators of conformist dispositions even in such experiments of limited duration, demonstrates how potent a force social learning can be in animals like these [94].
The greater bulk of such findings in fish appear to be associated with such route following, but there is some, more limited evidence for a role of social learning in anti-predator behaviour, aggressive interactions and mate choice [95]. Relatively few studies have addressed whether particular behaviour patterns might be socially learned, but there is suggestive experimental evidence that it can play a role in archer fishes' shooting of prey such as insects above the water surface [96]. Perhaps the best evidence of behavioural copying in fish comes from experiments in which models were trained to enter a vertical tube to forage, an unusual behaviour that naive fish failed to show until they observed the models, when they acquired the technique [97]. Once again, repeated addition of naive fish and removal of experienced fish successfully simulated inter-generational transmission.
3.5. Insects
There is some scattered evidence for social learning in other invertebrates [98], but for insects, the evidence has in recent years become substantial and compelling [99,100]. The celebrated phenomenon of the honeybee dance is itself a case of social information transfer, although the way in which the information about distant foraging sites is coded in the configuration of the dance means it is more commonly classed as communication, and could arguably be thought of as a form of coded teaching.
More akin to the forms of social learning in other taxa reviewed above is observational learning, where bees have been shown to use the public information of which flowers other bees are preferentially feeding on [100,101]. Likewise, fruit flies chose egg-laying sites preferred by a majority of experienced flies they interacted with, even when the interaction itself took place spatially separated from the laying sites and thus likely depended on olfactory cues of the preferred medium [102].
In this latter study, second-order observers who interacted with flies that had been through their first social learning experiences tended to adopt these first-order learner's preferences, thus providing minimal evidence for transmission across cultural ‘generations’, although the arbitrary preference for one medium over the other soon faded as individual exploration overrode the socially acquired preferences. A more thorough demonstration of such cultural diffusion, this time in bumblebees, involved not a preference for one of two arbitrary trained options, but instead the adoption of a quite challenging behavioural technique very rarely achieved by naive bees presented with the problem [103]. The novel behaviour involved pulling a string to drag an artificial flower from under a cover, thence to drink from it, and this procedure was introduced stepwise to models who when proficient were introduced singly into interactions with a colony of naive bees, in controlled pairwise encounters. In contrast with the near-absence of string-pulling in naive bees, a majority of those to whom observational learning was available went on to master the technique; moreover, acquisition of the technique spread across up to three cultural ‘generations’ of experienced/naive pairs, eventually becoming distributed across two-thirds of each seeded colony before the experiment was terminated.
A capacity for social learning may thus be widespread in insects and some other invertebrates too; moreover, as in this experiment, the potential for cultural diffusion has been demonstrated in at least some insects. What remains unclear is the implications of these laboratory findings for insect life in the wild. In bumblebees, for example, only queens may survive through a high-latitude winter, so any seasonally achieved ‘wisdom of the hive’ suggested by experiments like that described above [103] will perish. This means the scope for substantial cultural inheritance and evolution in invertebrates remains unclear. It could well exist in colonies with more continuity, such as those of tropical ants and termites with their sometimes gigantic and cumulatively built nests, but this has yet to be subjected to intensive research from this perspective.
4. Culture: both a second inheritance system and second evolutionary system?
As we have seen above, the evidence for social learning is now both strong and widespread across a range of both vertebrate and invertebrate species. There is also widespread evidence for the existence of abilities to sustain repeated cultural transmission, such as in the bumblebee experiments reviewed immediately above. In short, a vast research effort in the last half century has revealed the operation of this second inheritance system to be a widely pervading phenomenon among animals.
But does this second inheritance system generate a second or further forms of evolution also? Not necessarily. What is socially learned may be useful but only temporarily so—such as what is currently a good food patch to exploit [47]. Even where cultural transmission occurs repeatedly so that a tradition is formed, this may have a limited lifetime such that the likelihood of any evolutionary change is accordingly constrained. As discussed above, the scope for cultural evolutionary change may be restricted in this way for insects, by contrast with the evidence of nut-cracking in chimpanzees extending back over several millennia [18].
4.1. Parallels between animal culture and organic evolution
Given such potential for long-term cultural evolutionary change, we can make comparisons with a prior analysis of the ways in which human culture echoes the core principles of organic evolution as set out by Darwin in the Origin [28]. Mesoudi et al. [28] listed these principles as variation, inheritance, competition/selection, adaptation, geographical differentiation, convergent evolution and changes of function. I now consider each of these from the perspective of ‘animal culture’—I believe the first time such questions have been explored.
4.1.1. Variation and inheritance
Inheritance is here a given, instantiated in plentiful evidence of social learning as reviewed above. The element of variation in the contents of behaviours that are culturally transmitted has also been extensively documented in realms of behaviour that include local repertoires of bird [83] and cetacean [68] song. However, those involve variation between spatially separate populations. For competitive selection to operate, variants need to compete in the same space, so intra-group variation documented in, for example, styles of chimpanzee termite fishing [64] and vervet monkey food-cleaning techniques [104] would be more relevant, as would adjacent cultural variants like those attributed to killer whales [51,75]. The interaction of these two factors, variation and cultural inheritance, can give rise to evolution in the limited sense of change over time, via processes of drift created by factors such as imperfect inter-generational copying. Geographical divergence in birdsong dialects, for example, appears to be commonly explicable at least in part to ‘mutations’ [86] in copying, so that as song repertoires become geographically distant from each other they evolve different forms [83,85].
4.1.2. Competition and selection
When we add to variation and inheritance the third core feature of the Darwinian process, selection among elements that are in some sense in competition with each other, we may get not only evolutionary change but some degree of elaboration of the feature at stake, as we see in the broader evolution of increasingly diverse and often more complex organic life forms. Such processes of selection are predicted to shape the outcomes to be adaptations to their respective niches.
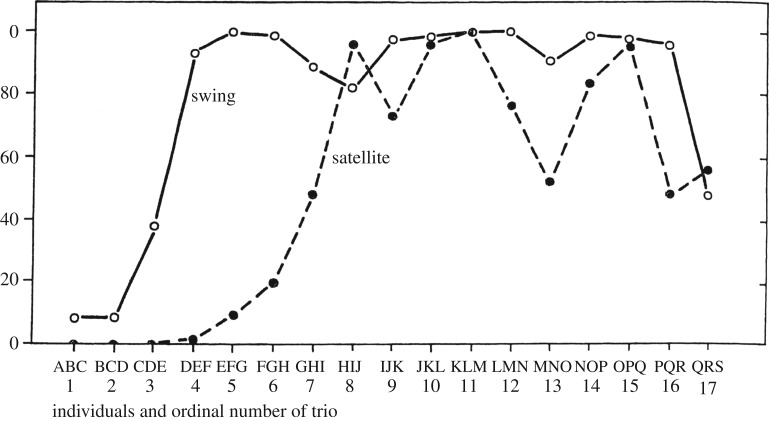
However, such evolutionary change is often slow and may even include long periods of stasis. Accordingly, some of our best documented examples of ‘evolution in action’ are consequences of short-term environmental perturbation by humans, as in the classic text-book case of peppered moths evolving to show dark camouflage during the sooty industrial revolution, then paler morphs again as the environment was cleansed. Perhaps the kind of cultural changes we can be alert for on this count may be particularly likely to arise from human interventions, including experimental ones. For example, Menzel et al. [105] exposed three juvenile chimpanzees to two different objects that the youngsters were disinclined to approach. Then at two-month intervals, one individual was replaced by a fresh one, so that trios three steps apart had totally different compositions. Nevertheless, habituation rose across generations and eventually stabilized (figure 6). Thus, bolder behaviour developed in some juveniles (see discussion of ‘guided variation’ below), competed with shyness, and was selected for because the objects were not dangerous; these shifts were then inherited via social learning, so that the eventual habitual steady state represents a simple case of cultural evolution via variation, competition, selection and (cultural) inheritance. A counterpart was recorded in the wild when two chimpanzees from a community already habituated to human observers migrated to a newly studied community and through these same processes of variation, competition, selection and cultural transmission produced a measurable enhancement in the pace of habituation underway there [106]. Such changes and processes appear to have received little explicit empirical investigation to date [67], but the examples above suggest that both observational and experimental investigation is feasible and now deserves to be pursued more systematically.
Figure 6.
An example of Darwinian cultural change? Three young chimpanzees, ABC, were exposed to two initially alarming objects, ‘swing’ and ‘satellite’. Every two months, one chimpanzee was replaced by a naive one, as indicated in the sequence BCD, CDE and so on. Adaptive bolder approaches were socially inherited and over time came to dominate, resulting in a culture of common contact with the objects [105].
4.1.3. Adaptation
These chimpanzee examples exemplify adaptation because the objects and observers were not in fact dangerous, so habituation was beneficial. Three different categories of adaptation facilitated by cultural transmission can be noted, with illustrative examples. First, adaptation may be to the physical environment: for example, long-tailed macaques exploiting coastal shores in Thailand use a variety of stone tools to smash certain shellfish and prise others off rocks at low tide, opening up possibilities in this intertidal niche otherwise inaccessible to them [107]. Such adaptive significance will likely apply for many animals that learn about tool use by observation, but are not, of course, limited only to tool use. Second, sexual selection may mould behaviours that thence come to be adaptations enhancing mating and reproductive success. For example, there is evidence from several species of birds that greater male song repertoires are preferred by females and confer enhanced reproductive success [83]. Third, socially transmitted behaviours may be adaptations to social life. For example, among white-faced capuchins, a range of intimate social customs arose and diffused across groups, including acts risky and likely costly to participants, such as plucking hair and placing fingers in the other's nostrils and eye sockets, which were thought to serve a bonding function [108].
4.1.4. Accumulation of modifications
In the human case, we are confronted with manifestations of cumulative culture across innumerable aspects of human life, from languages to technologies to institutions, with the cumulation often occurring along with differentiation in different regions [109–111]. This has created cultural evolutionary trees that echo what is familiar in the realm of organic evolution—the ‘tree of life’. In the case of animal cultures, we see such differentiation in examples like the birdsong dialects discussed above. However, as noted above, this appears to involve only evolutionary change, rather than cumulation of the kind that generates increasingly complex or elaborate forms that build on ancestral ones. Indeed, many authors state that cumulative culture is what distinguishes humans from other animals [109–111].
Others interpret some of the evidence for animal culture as potentially or actually demonstrating some cumulative build-up, even if on a small scale compared with human achievements. For example, Jablonka and co-workers [23,112] suggest that Japanese macaques' famous sweet potato washing tradition evolved through stages involving shifts from stream to sea, seasoning items in the salt water, swimming and fish-eating: ‘through the accumulation of social transmitted variations over time, the macaques have acquired a new life style’ [112, p. 99]. Many of the tool-sets used by wild chimpanzees may likewise reflect cumulative build-up, such as the making of deep tunnels to subterranean termite nests using stout sticks, followed by fishing the termites out using long stems brought to the site that are first prepared by oral and manual processing to have more efficient brush-tips [113]. To researchers familiar with chimpanzees, it seems difficult to imagine that this behavioural complex, applied to the extremely opaque problem of harvesting deeply subterranean prey, has come about other than by stepwise elaboration over long periods. However, in most such cases, we cannot check such inferences via the kinds of archaeological or historical records that chart cumulative culture in our own species. An alternative approach is to bring the question into the laboratory and create the conditions for cumulative change, well pioneered in human experiments [114]. This has now begun to be explored in non-human species, particularly in chimpanzees, with evidence to date indicating only minimal foundations of cumulation at best [115–117].
4.1.5. Geographical distributions
Organic evolutionary differentiation, for example, between subspecies, tends to be associated with geographical spacing, including differences between islands like those of the Galapagos. So too for human cultures, with variance in aspects of material and linguistic culture correlating with both geographical and genetic separation [118]. Likewise measures of cultural variance and geographical distance were found to covary strongly in gorillas [54] and differences in birdsong dialects on ‘islands’, whether separated by water or other barriers, are well documented [83].
4.1.6. Convergent evolution
Cases of convergent organic evolution are predicted and evidenced where similar combinations of the key Darwinian forces discussed above are at play in geographically dispersed locations. Again, parallels in human culture abound, from important cases like handwriting to more trivial ones like the neotenization of teddy bears and Mickey Mouse [28]. The use of stones to smash open food items presents a nice primate example, having appeared and spread locally in small populations of chimpanzees in Africa, capuchin monkeys in Brazil and long-tailed macaques in Thailand [119].
4.1.7. Change of function
Changes of function in organic evolution such as the fish swimbladder evolving into lungs are again paralleled by numerous examples in human material culture, so much so that Basalla [120] concluded his extensive survey by suggesting that few major human technologies were originally designed for the function they eventually came to serve. Few cases of animal culture have been tracked for long enough to hope to detect such changes, but the Japanese macaque saga indicates that this can occur, if on a small scale, when cleaning the sweet potatoes gave way to biting and salting them in the sea [112]. Boesch [121] presents evidence that the leaf-clipping display of chimpanzees has evolved to serve different functions such as courtship and play initiation in different regional communities.
4.2. Cultural transmission creates new forms of evolution
Culture extends evolutionary biology not only through these analogies with organic evolution but also by incorporating additional dimensions of inheritance and evolution. This has been well recognized in the sphere of human culture, elucidated in some detail in the works of Boyd & Richerson [122,123]. In the present focus on animal culture, I discuss two issues they highlighted: the nature of the transmission processes, and the way in which these processes may be fine-tuned by selective information processing.
4.2.1. Forms of inheritance
Analyses of human cultural evolution have noted that in addition to vertical, parent-to-offspring social transmission paralleling genetic transmission, cultural transmission may be horizontal within a generation, or oblique, via non-kin across generations [122,123]. In species where parental care occurs and especially where it is intense or extended, there is plentiful evidence for vertical inheritance, for which the great apes with their long period of mother–infant dependence provide good examples [67]; moreover as in the human case [67], a trend may be common for substantial initial learning from parents, followed by horizontal and oblique learning from a widening array of other conspecifics who may display more varied forms of expertise [65]. Horizontal and oblique transmission are often not distinguished, but plentiful evidence of them is available from diffusion experiments in a wide range of species [49], from primates [16] to birds [88] and bees [103], as well as through non-interventive studies in the wild, such as those described above for whales [74] and apes [62]. These processes can provide much faster dispersion of beneficial innovations than can genetic evolution; the latter study, for example, traced the diffusion of an innovation in tool use in a chimpanzee community across a period of 6 days [62].
Genetic inheritance occurs in packages transmitted at conception, even though activation may later be contingent on environmental factors; by contrast, social learning can operate often with considerable flexibility through the lifetime, and refine adaptation further through individual-level learning processes, sometimes in cycles of social and asocial learning (like practice) [8]. This provides what has been called ‘guided variation’ [122], which unlike random genetic mutation can steer learning to greater adaptation within the lifetime. In turn, such improvements can be inherited through social learning from others, in a Lamarckian-like fashion [23,112].
4.2.2. Fine-tuning via ‘transmission biases’
Further selective fine-tuning is offered through a variety of ways in which social learning can be sensitive to context, variously called transmission biases [122], directed social learning [124] or social learning strategies [125]. Rendell et al. ([126], figure 1) distinguish as many as 23 such potential biases. For space limitations, I here illustrate only a handful of examples from five of these, where most empirical evidence exists ([36], table 8.1). ‘When’ social learning strategies are those conditional on circumstances. A critical illustration of ‘when asocial learning is costly’ is learning about predators from others rather than through risky personal experience, experimental evidence of which ranges from fish [127] to primates [128], and an illustration of ‘when uncertain' is naive rats’ but not experienced rats' disposition to learn diet preferences from others [129]. ‘Who’ strategies represent rules of whom to best learn from. These include conformity or ‘copy the majority’, which takes various forms illustrated by route choice in fish [130], foraging method in birds [88] and apes [131] and diet choice in monkeys [132]. ‘Copy success’ ranges from nest site choice in birds [82] to foraging sites in apes [133]. Finally, there are biases in ‘what’ to preferentially learn about, from songbirds learning song from conspecifics rather than heterospecifics [134] to learning about natural predator types rather than random objects in birds [135] and primates [128]. All these biases give the second inheritance system a continuing adaptive finesse beyond that provided by the primary genetically based system.
5. Interplay between cultural and organic evolutionary systems
5.1. Functional traditions may enhance fitness
Many behaviour patterns that are acquired through cultural transmission appear likely to be beneficial to the biological fitness of the animals concerned, which is what shapes evolution. This includes cases of foraging knowledge and skills, predator avoidance, tool use, migratory routes and song. Indeed, in a majority of cases, repeated episodes of cultural transmission are likely to be maintained because the outcomes are functionally significant. For example, many forms of tool use in primates permit the exploitation of embedded food sources otherwise unavailable to the tool-users and relevant competitors; there is evidence that without this extension to their niche, chimpanzees would be significantly limited in the marginal habitats they use [10]. Taking another example from a very different domain of behaviour, birdsong has been shown to function in both territory defence against competitors, and the attraction of mates; for example, larger song repertoires in great reed warblers can result in higher breeding success, sometimes extending to extra-pair copulations [83]. Where culturally transmitted behaviour makes up a significant proportion of a species' repertoire, it may thus provide a critical contribution to an individual's fitness. Of course, this is not straightforward to rigorously test, but now begs for more concerted quantitative analysis.
5.2. Sexual selection
In songbirds, there is evidence that aspects of culturally transmitted songs provide an important basis for female selectivity in mate choice, with variables such as song complexity providing honest, difficult to fake information about a male's quality, correlated with factors such as the adequacy of their breeding territory and food supply [83]. Such sexual selection may hence shape not only further cultural evolution in this respect but also associated aspects of organic evolution, such as the enhanced neurogenesis that has been found to be associated with song repertoire size [136] and that may also be correspondingly associated with females' ability to recognize and evaluate such song qualities. Similar phenomena may well be associated with cetacean song, but here there is an unsurprising lack of the kind of experimental finesse that has been possible in the avian studies.
5.3. Behavioural drive, cultural drive and organic evolution
The hypothesis that behavioural innovation may allow animals to invade or construct a new niche-space, that then exerts new selective pressures that further shape future organic evolution, has been explored in varied ways from a theoretical perspective over the last century, from early ideas that became known as the Baldwin effect, to genetic assimilation and the concept of behavioural drive [47,137–139]. Empirical evidence for such effects has remained sparse, by contrast, but this is arguably unsurprising, given the nature of the phenomena, with organic evolutionary consequences slow to emerge compared with the lifetimes of scientists, and evidence of the initial proposed behavioural innovations often simply buried in the past. However, empirical investigations exist, a striking one being that first explored by Wilson and co-workers [137,138] in demonstrating an almost perfect correlation of r = 0.97 between relative brain size (i.e. corrected for body size) and the rate of change in measures of organic evolution across taxa ranging from reptiles to mammals (and see [140] for a substantial related avian study).
Where the initial behavioural drive is delivered by the kinds of cultural processes discussed in the present review, the phenomenon can be called ‘cultural drive’ [138] and with a particular focus on human culture has been discussed further under the rubric of ‘cultural niche construction’ [19].
What of the non-human animal case? As a potential case of such cultural drive, Whitehead & Rendell [51] present the case of killer whales ecotypes outlined above. These authors present compelling evidence that these are based on culture and that this has had correlated effects in anatomy, such as in the stronger jaw structures of those populations that focus on the more substantial prey of seals. However, if genetic variations are now responsible for the jaw differences, can we be confident the same is not true of the other, behavioural differences? We know from experimental studies in birds such as the Galapagos woodpecker finches that use twigs to fish for insect prey, social learning is not essential [141], a scenario difficult to experimentally address in killer whales, concerning their predatory expertise. However, unlike the case of the finches, experimental evidence of more general bodily imitation has been published for the killer whales [77]. Whitehead & Rendell review a variety of other arguments that in this example, we are looking at the primary effects of cultural transmission, and associated organic adaptations.
Another potential effect of cultural transmission occupying a substantial role in a species' adaptations to its ecological niche is in the organic, notably neuronal, underpinnings of the requisite learning abilities [136]. A ‘Cultural Intelligence Hypothesis’ was initially put forward to explain the marked encephalization of great apes [47,142], but is equally relevant to other taxa in which culture plays a particularly prominent role, such as cetaceans [51]. Testing this hypothesis is challenging, but there is evidence that Sumatran orangutans, that are more social and apparently culturally rich than their Bornean counterparts, also exhibit the superior asocial learning abilities predicted [143,144], and have brain sizes typically 2%–12% larger [144].
Humans likely constitute a special case of such a hypothesis, on the basis that the growing reliance of evolving humans on cultural transmission explains the hyper-development of human cognition, cultural transmission processes and tripling in brain size compared with other apes [110,145]. There is a large literature on this anthropocentric and often controversial topic [110,111,145–149], but this is principally focused on comparisons of our own species with only our closest ape relatives, whereas this review aspires to survey a broad range of species, so does not further address the subject here.
5.4. Culturally driven genetic differentiation and speciation
A corollary of scenarios like that of the killer whales outlined above suggests that as such ecotypes diverge culturally, differentiation in population genetics may follow (culture leads, genes follow) or genes and culture may co-evolve in double helical fashion, driven either by drift or by active selection (as in the case of stronger jaws in seal-hunting killer whales) [51,75]. Effects interpreted in this way are now becoming increasingly common in the cetacean literature [150,151] and a parallel case, supported by modelling, has been made that cultural processes can generate multi-level structured societies of the kind found in sperm whales [152]. Compelling cases of gene–culture coevolution are increasingly well established in the case of human culture [153,154], but these often rest on firm historical evidence of the critical cultural change, such as in the famous instance of dairy farming and lactose tolerance. Such relatively direct evidence of cultural history is more difficult to obtain in the proposed animal cases, but genomic analyses are becoming increasingly sophisticated in the inferences they can offer about the role of factors such as population bottlenecks, drift and selection, and are producing compelling cases for culture being key in these evolutionary effects [75,150,151].
Related hypotheses and empirical data have also been developed in the avian birdsong literature [83], with the added bonus of experimental evidence of the role of selection. Thus, Grant & Grant [155] showed experimentally that populations of a finch species from different islands in the Galapagos chain responded with significantly different levels of intensity to songs sufficiently different to their own, leading the authors to conclude they are ‘well advanced along the path of speciation’ [155, p. 545], full speciation thus being a logical and plausible ultimate scenario following from such findings. Elsewhere, in discussing the question of how songs and responses to them diverge in such apparently incipient species, these authors discuss five different factors likely involved, an analysis too elaborate to summarize here, but which highlights the scope of the evolutionary processes now being explored [155,156].
To date, the study of the effects of culture on genetic and organic differentiation between populations appears limited to ecotypes and songs in cetaceans, and birdsong; it has not been reported in the only other taxon—primates—in which there is sufficient evidence of cultural variance to warrant exploration of this phenomenon, although as in some songbirds [84], there is evidence that in chimpanzees, as seems predictable [118], greater cultural and genetic variance is correlated across greater degrees of geographical separation [157].
6. Conclusion
As reviewed in §3 of this article, recent decades have witnessed an accelerated accumulation of evidence of social learning in animals, including both vertebrates and invertebrates (principally insects). The effects of such learning may be transient, but repeated and sustained cultural transmission has also been documented in many vertebrate species, generating long-standing traditions. These cover a diverse range of types of behaviour including migration, vocal communication, tool use, and foraging and predator avoidance techniques. Because these involve a (second) form of inheritance, display inter-individual variance and can shape adaptation (often more quickly than can genetic change), they deserve to be integrated into contemporary understanding of the scope of biology and the phenomenon at its core, evolution. Yet classic texts, even quite recent ones, show stark omissions in this regard [30,31,36–39,41].
In §4 of this article, I therefore pursued what I believe is the first exploration in non-human species of the extent to which, for the moment setting aside attention to genetic and organic evolution, animal culture displays key properties of evolutionary systems, as human culture has been amply shown to do. Some empirical evidence of each of eight such properties can be offered, even though these often pale in comparison with what we see in the case of human culture. It is to be hoped this exploration nevertheless focuses some research effort on such matters, and on the ways in which the prevalence of culture introduces new evolutionary characteristics such as horizontal transmission and adaptive transmission biases.
In then further exploring the scope of gene–culture interaction in the animal case, I suggest the most important conclusion is that cultural processes may shape adaptation in significant ways, thereby changing the dynamics of evolution at a broader level. In some cases, this may shape genetic and organic differentiation between culturally variant populations, perhaps even leading to speciation. Empirical investigation of such processes is inherently difficult because of such factors as the long timescales that may be involved, but with the accumulated discoveries of animal culture now building through decades of research, this exciting prospect begs further attention.
Acknowledgements
For comments on manuscript drafts, I am grateful to Lucy Aplin, Emma Carroll, Douglas Futyuma, Elli Leadbeater, Alex Mesoudi, Rüdiger Riesch, Luke Rendell, Peter Slater and Hal Whitehead.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Whiten A. 2005. The second inheritance system of chimpanzees and humans. Nature 437, 52–55. ( 10.1038/nature04023) [DOI] [PubMed] [Google Scholar]
- 2.Boesch C, Boesch H. 1983. Optimisation of nut-cracking with natural hammers by wild chimpanzees. Behaviour 83, 265–286. ( 10.1163/156853983X00192) [DOI] [Google Scholar]
- 3.Joulian F. 1996. Comparing chimpanzee and early hominid techniques. In Modelling the early human mind (eds Mellars P, Gibson K), pp. 173–190. Cambridge, UK: McDonald Institute for Archaeological Research. [Google Scholar]
- 4.Carvalho S, McGrew W. 2010. The origins of the Oldowan: why chimpanzees are still good models for technological evolution in Africa. In Stone tools and fossil bones (ed. Domínguez-Rodrigo M.), pp. 201–221. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685. ( 10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 6.Boesch C, Marchesi P, Marchesi N, Fruth B, Joulian F. 1994. Is nutcracking in wild chimpanzees a cultural behaviour? J. Hum. Evol. 26, 325–338. ( 10.1006/jhev.1994.1020) [DOI] [Google Scholar]
- 7.McGrew WC, Ham RM, White LJT, Tutin CEG, Fernandez M. 1997. Why don't chimpanzees in Gabon crack nuts? Int. J. Primatol. 18, 353–374. ( 10.1023/A:1026382316131) [DOI] [Google Scholar]
- 8.Whiten A. 2015. Experimental studies illuminate the cultural transmission of percussive technology in Homo and Pan. Phil. Trans. R. Soc. B 370, 20140359 ( 10.1098/rstb.2014.0359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall-Pescini S, Whiten A. 2008. Social learning of nut-cracking behaviour in East African sanctuary-living chimpanzees (Pan troglodytes schweinfurthii). J. Comp. Psychol. 122, 186–194. ( 10.1037/0735-7036.122.2.186) [DOI] [PubMed] [Google Scholar]
- 10.Yamakoshi G. 1998. Dietary responses to food scarcity of wild chimpanzees at Bossou, Guinea: possible implications for ecological importance of tool use. Am. J. Phys. Anthropol. 106, 283–295. ( 10.1002/(SICI)1096-8644(199807)106:3%3C283::AID-AJPA2%3E3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 11.Whiten A. 2006. The significance of socially transmitted information for nutrition and health in the great ape clade. In Social information transmission and human biology (eds Wells JCK, Laland KN, Strickland SS), pp. 118–134. London, UK: CRC Press. [Google Scholar]
- 12.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. 2001. Charting cultural variation in chimpanzees. Behaviour 138, 1489–1525. ( 10.1163/156853901317367717) [DOI] [Google Scholar]
- 13.Luncz LV, Mundry R, Boesch C. 2012. Evidence for cultural differences between neighbouring chimpanzee communities. Curr. Biol. 22, 922–926. ( 10.1016/j.cub.2012.03.031) [DOI] [PubMed] [Google Scholar]
- 14.Luncz LV, Boesch C. 2014. Tradition over trend: neighboring chimpanzee communities maintain differences in cultural behaviour despite frequent immigration of adult females. Am. J. Primatol. 76, 649–657. ( 10.1002/ajp.22259) [DOI] [PubMed] [Google Scholar]
- 15.Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matasuzawa T. 2003. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim. Cogn. 6, 213–223. ( 10.1007/s10071-003-0183-x) [DOI] [PubMed] [Google Scholar]
- 16.Whiten A, Horner V, de Waal FBM. 2005. Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737–740. ( 10.1038/nature04047) [DOI] [PubMed] [Google Scholar]
- 17.Whiten A, Spiteri A, Horner V, Bonnie KE, Lambeth SP, Schapiro SJ, de Waal FBM. 2007. Transmission of multiple traditions within and between chimpanzee groups. Curr. Biol. 17, 1038–1043. ( 10.1016/j.cub.2007.05.031) [DOI] [PubMed] [Google Scholar]
- 18.Mercader J, et al. 2007. 4,300-Year-old chimpanzee sites and the origins of percussive stone technology. Proc. Natl Acad. Sci. USA 104, 3043–3048. ( 10.1073/pnas.0607909104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laland KN, Odling-Smee FJ, Feldman MW. 2000. Niche construction, biological evolution and cultural change. Behav. Brain Sci. 23, 131–175. ( 10.1017/S0140525X00002417) [DOI] [PubMed] [Google Scholar]
- 20.Odling-Smee FJ, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 21.Danchin E, Giraldeau L-A, Cezilly F. 2008. Behavioural ecology. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Danchin E, Charmentier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. 2011. Beyond DNA: integrating inclusive inheritances into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486. ( 10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 23.Jablonka E, Lamb M. 2014. Evolution in four dimensions. Genetic, epigenetic, behavioural and symbolic variation in the history of life. Cambridge, MA: MIT Press. [Google Scholar]
- 24.Lamm E. 2014. Inheritance systems. In The Stanford encyclopedia of philosophy (ed. Zalta EN.). See http://plato.stanford.edu/archives/win2014/entries/inheritance-systems/. [Google Scholar]
- 25.Laland KN, Uller T, Feldman MW, Sterelny K, Muller GB, Moczek A, Jablonka E, Odling-Smee J. 2015. The extended evolutionary synthesis: its structure, assumptions and predictions. Proc. R. Soc. B 282, 20151019 ( 10.1098/rspb.2015.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawkins R. 1976. The selfish gene. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Aunger R. (ed.). 2000. Darwinizing culture: the status of memetics as a science. Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Mesoudi A, Whiten A, Laland KN. 2004. Is human cultural evolution Darwinian? Evidence reviewed from the perspective of ‘The Origin of Species'. Evolution 58, 1–11. ( 10.1111/j.0014-3820.2004.tb01568.x) [DOI] [PubMed] [Google Scholar]
- 29.Mesoudi A, Whiten A, Laland KN. 2006. Towards a unified science of cultural evolution. Behav. Brain Sci. 29, 329–383. ( 10.1017/S0140525X06009083) [DOI] [PubMed] [Google Scholar]
- 30.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: Murray. [Google Scholar]
- 31.Huxley JS. 1942. Evolution, the modern synthesis. London, UK: Allen & Unwin. [Google Scholar]
- 32.Mayr E. 1982. The growth of biological thought: diversity, evolution and inheritance. Cambridge, MA: Harvard University Press. [Google Scholar]
- 33.Fisher J, Hinde RA. 1949. The opening of milk bottles by birds. Br. Birds 42, 347–357. [Google Scholar]
- 34.Marler P, Tamura M. 1964. Song ‘dialects’ in three populations of white-crowned sparrows. Science 146, 1483–1486. ( 10.1126/science.146.3650.1483) [DOI] [PubMed] [Google Scholar]
- 35.Kawai M. 1965. New acquired pre-cultural behaviour of the natural troop of Japanese monkeys on Koshima Islet. Primates 2, 1–30. ( 10.1007/BF01666109) [DOI] [Google Scholar]
- 36.Hoppitt W, Laland KN. 2013. Social learning: an introduction to mechanism, models and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 37.Maynard-Smith J. 1975. The theory of evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 38.Mayr E. 2002. What evolution is. London, UK: Weidenfield and Nicholson. [Google Scholar]
- 39.Ridley M. 2004. Evolution, 3rd edn Cambridge, MA: Blackwell. [Google Scholar]
- 40.Barton N, et al. 2007. Evolution. Cold Spring Harbor, NY: Cold Spring Harbor. [Google Scholar]
- 41.Futuyama D. 2013. Evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 42.Maynard-Smith J, Szathmary E. 1995. The major transitions in evolution. Oxford, UK: Freeman. [Google Scholar]
- 43.Heyes CM. 1994. Social learning in animals: categories and mechanisms. Biol. Rev. 69, 207–231. ( 10.1111/j.1469-185X.1994.tb01506.x) [DOI] [PubMed] [Google Scholar]
- 44.Sherry DF, Galef BG. 1984. Cultural transmission without imitation: milk bottle opening by birds. Anim. Behav. 32, 937–938. ( 10.1016/S0003-3472(84)80185-2) [DOI] [Google Scholar]
- 45.Fragaszy DM, Perry S (eds). 2003. The biology of traditions: models and evidence. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 46.Whiten A, Hinde RA, Laland KN, Stringer CB. 2011. Culture evolves. Phil. Trans. R. Soc. B 366, 938–948. ( 10.1098/rstb.2010.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whiten A, van Schaik CP. 2007. The evolution of animal ‘cultures’ and social intelligence. Phil. Trans. R. Soc. B 362, 603–620. ( 10.1098/rstb.2006.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galef BG, Whiten A. 2017. The comparative psychology of social learning. In APA handbook of comparative psychology (ed. Call J.). Washington, DC: American Psychological Association. [Google Scholar]
- 49.Whiten A, Caldwell CA, Mesoudi A. 2016. Cultural diffusion in humans and other animals. Curr. Opin. Psychol. 8, 15–21. ( 10.1016/j.copsyc.2015.09.002) [DOI] [PubMed] [Google Scholar]
- 50.Whiten A. 2012. Social learning, traditions and culture. In The evolution of primate societies (eds Mitani J, Call J, Kappeler P, Palombit R, Silk J), pp. 681–699. Chicago, IL: Chicago University Press. [Google Scholar]
- 51.Whitehead H, Rendell L. 2015. The cultural lives of whales and dolphins. Chicago, IL: Chicago University Press. [Google Scholar]
- 52.Whiten A. 2010. Coming of age for cultural Panthropology. In The mind of the chimpanzee (eds Lonsdorf E, Ross S, Matsuzawa T), pp. 87–100. Chicago, IL: Chicago University Press. [Google Scholar]
- 53.van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. 2003. Orangutan cultures and the evolution of material culture. Science 299, 102–105. ( 10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- 54.Robbins MM, et al. 2016. Behavioural variation in gorillas: evidence of potential cultural traits. PLoS ONE 11, e0160483 ( 10.1371/journal.pone.0160483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krützen M, Willems EP, van Schaik CP. 2011. Culture and geographic variation in orangutan behavior. Curr. Biol. 21, 1808–1812. ( 10.1016/j.cub.2011.09.017) [DOI] [PubMed] [Google Scholar]
- 56.Santorelli CJ, Schaffner CM, Campbell CJ, Notman H, Pavelka MS, Weghorst JA, Aureli F. 2011. Traditions in wild spider monkeys are biased towards the social domain. PLoS ONE 6, e16863 ( 10.1371/journal.pone.0016863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leca J-B, Gunst N, Huffman MA. 2007. Japanese macaque cultures: inter- and intra-troop behavioral variability of stone-handling patterns across 10 groups. Behaviour 144, 251–281. ( 10.1163/156853907780425712) [DOI] [Google Scholar]
- 58.Nakamura M, Uehara S. 2004. Proximate factors of different types of grooming hand-clasp in Mahale chimpanzees: implications for chimpanzee social customs. Curr. Anthropol. 45, 108–114. ( 10.1086/381007) [DOI] [Google Scholar]
- 59.Van Leeuwen EJC, Cronin KA, Haun SBM. 2014. A group specific arbitrary tradition in chimpanzees. Anim. Cogn. 17, 1421–1425. ( 10.1007/s10071-014-0766-8) [DOI] [PubMed] [Google Scholar]
- 60.Rawlings B, Davila-Ross M, Boysen ST. 2014. Semi-wild chimpanzees open hard-shelled fruits differently across communities. Anim. Cogn. 17, 891–899. ( 10.1007/s10071-013-0722-z) [DOI] [PubMed] [Google Scholar]
- 61.Hobaiter C, Byrne RW. 2010. Able-bodied wild chimpanzees imitate a motor procedure used by a disabled individual to overcome handicap. PLoS ONE 5, e11959 ( 10.1371/journal.pone.0011959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hobaiter C, Poiset T, Zuberbuhler K, Hoppitt W, Gruber T. 2014. Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 12, e1001960 ( 10.1371/journal.pbio.1001960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Malley RC, Wallauer W, Murray C, Goodall J. 2012. The appearance and spread of ant fishing in the Kasekela chimpanzees of Gombe: a possible case of intercommunity cultural transmission. Curr. Anthropol. 53, 650–670. ( 10.1086/666943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lonsdorf EV, Pusey EA, Eberly L. 2004. Sex differences in learning in chimpanzees. Nature 428, 715–716. ( 10.1038/428715a) [DOI] [PubMed] [Google Scholar]
- 65.Schuppli C, Meulman EJM, Forss SIM, Aprilinayati F, van Noordwijk MA, van Schaik CP. 2016. Observational learning and socially induced practice of routine skills in immature orangutans. Anim. Behav. 119, 87–98. ( 10.1016/j.anbehav.2016.06.014) [DOI] [Google Scholar]
- 66.Gruber T, Muller MN, Strimling P, Wrangham R, Zuberbuhler K. 2009. Wild chimpanzees rely on cultural knowledge to solve an experimental honey acquisition task. Curr. Biol. 19, 1846–1852. ( 10.1016/j.cub.2009.08.060) [DOI] [PubMed] [Google Scholar]
- 67.Whiten A. 2017. How culture extends evolutionary biology in the great apes. Proc. Natl Acad. Sci. USA 114 ( 10.1073/pnas.1620733114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Payne K, Tyack P, Payne R. 1983. Progressive changes in the songs of humpback whales: a detailed analysis of two seasons in Hawaii. In Communication and behavior of whales (ed. Payne R.), pp. 9–57. Boulder, CO: Westview Press. [Google Scholar]
- 69.Tervo OM, Parks SE, Christoffersen MF, Miller LE, Kristensen RM. 2011. Annual changes in the winter song of bowhead whales (Balena mysticetus) in Disko Bay, Western Greenland. Mar. Mamm. Sci. 27, e241–e252. ( 10.1111/j.1748-7692.2010.00451.x) [DOI] [Google Scholar]
- 70.Noad MJ, Cato DH, Bryden MM, Jenner MN. 2000. Cultural revolution in whale songs. Nature 404, 537 ( 10.1038/35046199) [DOI] [PubMed] [Google Scholar]
- 71.Garland EC, Goldizen AW, Rekdah ML, Constantine R, Garrigue C, Daescher Hauser N, Poole MM, Robbins J, Noad MJ. 2011. Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 21, 687–691. ( 10.1016/j.cub.2011.03.019) [DOI] [PubMed] [Google Scholar]
- 72.Baker CS, Palumbi SR, Lambertsen RH, Weinrich MT, Calambokidies J, O'Brien SJ. 1990. Influence of seasonal migration on geographic distribution of mitochondrial DNA haplotypes in humpback whales. Nature 344, 238–240. ( 10.1038/344238a0) [DOI] [PubMed] [Google Scholar]
- 73.Valenzuela LO, Sironi M, Rowntree VJ, Seger J. 2009. Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalena australis). Mol. Ecol. 18, 782–791. ( 10.1111/j.1365-294X.2008.04069.x) [DOI] [PubMed] [Google Scholar]
- 74.Allen J, Weinrich M, Hoppitt W, Rendell L. 2013. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488. ( 10.1126/science.1231976) [DOI] [PubMed] [Google Scholar]
- 75.Reisch R, Barrett-Lennard LG, Ellis GM, Ford JKB, Deecke VB. 2012. Cultural traditions and the evolution of reproductive isolation: ecological speciation in killer whales? Biol. J. Linn. Soc. 106, 1–17. ( 10.1111/j.1095-8312.2012.01872.x) [DOI] [Google Scholar]
- 76.Whiten A. 2001. Imitation and cultural transmission in apes and cetaceans. Behav. Brain Sci. 24, 359–360. ( 10.1017/S0140525X01603960) [DOI] [Google Scholar]
- 77.Abramson JZ, Hernandez-Loreda V, Call J, Colmenares F. 2013. Experimental evidence for action imitation in killer whales (Orcinus orca). Anim. Cogn. 16, 11–22. ( 10.1007/s10071-012-0546-2) [DOI] [PubMed] [Google Scholar]
- 78.Cantor M, Whitehead H. 2013. The interplay between social networks and culture: theoretically and among whales and dolphins. Phil. Trans. R. Soc. B 368, 149–165. ( 10.1098/rstb.2012.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palacín C, Alonso JC, Alonso JA, Magaña M, Martin CA. 2011. Cultural transmission and flexibility of partial migration patterns in a long-lived bird, the great bustard Otis tarda. J. Avian Biol. 42, 301–308. ( 10.1111/j.1600-048X.2011.05395.x) [DOI] [Google Scholar]
- 80.Slagsvold T, Wiebe KL. 2011. Social learning in birds and its role in shaping foraging niches. Phil. Trans. R. Soc. B 366, 969–977. ( 10.1098/rstb.2010.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mueller T, O'Hara RB, Converse SJ, Urbanek RP, Fagan WF. 2013. Social learning of migratory performance. Science 341, 999–1002. ( 10.1126/science.1237139) [DOI] [PubMed] [Google Scholar]
- 82.Doligez B, Danchin E, Colbert J. 2002. Public information and breeding habitat selection in a wild bird population. Science 297, 1168–1170. ( 10.1126/science.1072838) [DOI] [PubMed] [Google Scholar]
- 83.Catchpole CK, Slater PJB. 2008. Bird song: biological themes and variations, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 84.Riebel K, Lachlan RF, Slater PJB. 2015. Learning and cultural transmission in chaffinch song. Adv. Study. Behav. 47, 181–227. ( 10.1016/bs.asb.2015.01.001) [DOI] [Google Scholar]
- 85.MacDougall-Shackleton E, MacDougall-Shackleton SA. 2001. Cultural and genetic evolution in mountain white-crowned sparrows: song dialects associated with population structure. Evolution 55, 2568–2575. ( 10.1111/j.0014-3820.2001.tb00769.x) [DOI] [PubMed] [Google Scholar]
- 86.Jenkins PF. 1978. Cultural transmission and song patterns of dialect development in a free-living bird population. Anim. Behav. 26, 50–78. ( 10.1016/0003-3472(78)90007-6) [DOI] [Google Scholar]
- 87.Brumm H, Naguib M. 2009. Environmental acoustics and the evolution of bird song. Adv. Study Behav. 40, 1–33. ( 10.1016/S0065-3454(09)40001-9) [DOI] [Google Scholar]
- 88.Aplin L, Farine DR, Morand-Ferron J, Cockburn A, Thornton A, Sheldon BC. 2015. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538–541. ( 10.1038/nature13998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laland KN, Hoppit WJE. 2003. Do animals have culture? Evol. Anthropol. 12, 150–159. ( 10.1002/evan.10111) [DOI] [Google Scholar]
- 90.Helfman GS, Schultz ET. 1984. Social transmission of behavioural traditions in a coral reef fish. Anim. Behav. 32, 379–384. ( 10.1016/S0003-3472(84)80272-9) [DOI] [Google Scholar]
- 91.Warner RR. 1988. Traditionality of mating-site preferences in a coral fish. Nature 335, 719–721. ( 10.1038/335719a0) [DOI] [Google Scholar]
- 92.Laland KN, Williams K. 1997. Shoaling generates social learning of foraging information in guppies. Anim. Behav. 53, 1161–1169. ( 10.1006/anbe.1996.0318) [DOI] [PubMed] [Google Scholar]
- 93.Laland KN, Williams K. 1998. Social transmission of maladaptive information in the guppy. Behav. Ecol. 9, 493–499. ( 10.1093/beheco/9.5.493) [DOI] [Google Scholar]
- 94.Pike TW, Kendal JR, Rendell L, Laland KN. 2010. Learning by proportional observational in a species of fish. Behav. Ecol. 20, 238–244. [Google Scholar]
- 95.Laland KN, Atton N, Webster MM. 2011. From fish to fashion: experimental and theoretical insights into the evolution of culture. Phil. Trans. R. Soc. B 366, 958–968. ( 10.1098/rstb.2010.0328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schuster S, Wohl S, Griebsh M, Klostermeier I. 2006. Animal cognition: how archer fish learn to down rapidly moving targets. Curr. Biol. 16, 378–383. ( 10.1016/j.cub.2005.12.037) [DOI] [PubMed] [Google Scholar]
- 97.Stanley EL, Kendal RL, Kendal JR, Grounds S, Laland KN. 2008. The effects of group size, rate of turnover and disruption to demonstration on the stability of foraging traditions in fish. Anim. Behav. 75, 565–572. ( 10.1016/j.anbehav.2007.06.014) [DOI] [Google Scholar]
- 98.Fiorito G, Scotto P. 1992. Observational learning in Octopus vulgaris. Science 256, 545–547. ( 10.1126/science.256.5056.545) [DOI] [PubMed] [Google Scholar]
- 99.Leadbeater E, Chittka L. 2007. Social learning in insects—from miniature brains to consensus building. Curr. Biol. 17, R703–R713. ( 10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 100.Grüter C, Leadbeater E. 2015. Insights from insects about adaptive social information use. Trends Ecol. Evol. 29, 177–184. ( 10.1016/j.tree.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 101.Worden BD, Papaj DR. 2005. Flower choice copying in bumblebees. Biol. Lett. 1, 504–507. ( 10.1098/rsbl.2005.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Battesti M, Merino C, Joly D, Mery F. 2012. Spread of social information and dynamics of social transmission within Drosophila groups. Curr. Biol. 22, 309–313. ( 10.1016/j.cub.2011.12.050) [DOI] [PubMed] [Google Scholar]
- 103.Alem S, Perry CJ, Zhu X, Loukola OJ, Ingraham T, Søvik E, Chittka L. 2016. Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol. 14, e1002564 ( 10.1371/journal.pbio.1002564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van de Waal E, Bshary R, Whiten A. 2014. Wild vervet monkey infants acquire the food-processing variants of their mothers. Anim. Behav. 90, 41–45. ( 10.1016/j.anbehav.2014.01.015) [DOI] [Google Scholar]
- 105.Menzel EW, Davonport RK, Rogers CM. 1972. Protocultural aspects of chimpanzees' responsiveness to novel objects. Folia Primat. 17, 161–170. ( 10.1159/000155425) [DOI] [PubMed] [Google Scholar]
- 106.Samuni L, Mundry R, Terkel J, Zuberbuhler K, Hobaiter C. 2014. Socially learned habituation to human observers by wild chimpanzees. Anim. Cogn. 17, 997–1005. ( 10.1007/s10071-014-0731-6) [DOI] [PubMed] [Google Scholar]
- 107.Gumert MD, Malaivijitnond S. 2012. Marine prey processed with stone tools by burmese long-tailed macaques (Macaca fascicularis aurea) in intertidal habitats. Am. J. Phys. Anthropol. 149, 447–457. ( 10.1002/ajpa.22143) [DOI] [PubMed] [Google Scholar]
- 108.Perry S, et al. 2003. Social conventions in white-face capuchins monkeys: evidence for behavioral traditions in a neotropical primate. Curr. Anthropol. 44, 241–268. ( 10.1086/345825) [DOI] [Google Scholar]
- 109.Pagel M. 2012. Wired for culture: the natural history of human communication. London, UK: Allen Lang. [Google Scholar]
- 110.Henrich J. 2015. The secret of our success: how culture is driving human evolution, domesticating our species, and making us smarter. Princeton, NJ: Princeton University Press. [Google Scholar]
- 111.Tennie C, Call J, Tomasello M. 2009. Ratcheting up the ratchet: on the evolution of cumulative culture. Phil. Trans. R. Soc. B 364, 2405–2415. ( 10.1098/rstb.2009.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Avital E, Jablonka E. 2000. Animal traditions: behavioural inheritance in evolution. Cambridge, UK: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- 113.Sanz C, Call J, Morgan D. 2009. Design complexity in termite-fishing tools of chimpanzees (Pan troglodytes). Biol. Lett. 5, 293–296. ( 10.1098/rsbl.2008.0786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caldwell CA, Millen A. 2008. Studying cumulative cultural evolution in the laboratory. Phil. Trans. R. Soc. B 363, 3529–3539. ( 10.1098/rstb.2008.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marshall-Pescini S, Whiten A. 2008. Chimpanzees (Pan troglodytes) and the question of cumulative culture: an experimental approach. Anim. Cogn. 11, 449–456. ( 10.1007/s10071-007-0135-y) [DOI] [PubMed] [Google Scholar]
- 116.Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. 2012. Identification of the social and cognitive processes underlying human cumulative culture. Science 335, 1114–1118. ( 10.1126/science.1213969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davis SJ, Vale GL, Schapiro SJ, Lambeth SP, Whiten A. 2016. Foundations of cumulative culture in apes: improved foraging efficiency through relinquishing and combining behaviours in chimpanzees (Pan troglodytes). Sci. Rep. 6, 35953 ( 10.1038/srep35953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hewlett BS, De Silvestri A, Guglielmino CR. 2002. Semes and genes in Africa. Curr. Anthropol. 43, 313–321. ( 10.1086/339379) [DOI] [Google Scholar]
- 119.Whiten A. 2013. Archaeology meets primate technology. Nature 498, 303–305. ( 10.1038/498303a) [DOI] [PubMed] [Google Scholar]
- 120.Basalla G. 1988. The evolution of technology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 121.Boesch C. 2012. Wild cultures: a comparison between chimpanzee and human cultures. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 122.Boyd R, Richerson P. 1985. Culture and the evolutionary process. Chicago, IL: Chicago University Press. [Google Scholar]
- 123.Richerson P, Boyd R. 2005. Not by genes alone. How culture transformed human evolution. Chicago, IL: Chicago University Press. [Google Scholar]
- 124.Coussi-Korbel S, Fragaszy DM. 1995. On the relation between social dynamics and social learning. Anim. Behav. 50, 1441–1450. ( 10.1016/0003-3472(95)80001-8) [DOI] [Google Scholar]
- 125.Laland KN. 2004. Social learning strategies. Learn. Behav. 32, 4–14. ( 10.3758/BF03196002) [DOI] [PubMed] [Google Scholar]
- 126.Rendell L, Fogarty L, Hoppitt WJE, Morgan TJH, Webster M, Laland KN. 2011. Cognitive culture: theoretical and empirical insights into social learning strategies. Evolution 63, 534–548. ( 10.1016/j.tics.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 127.Kelley JL, Evans JP, Ramnarine IW, Magurran AE. 2003. Back to school: can antipredator in guppies be enhanced by social learning? Anim. Behav. 65, 655–662. ( 10.1006/anbe.2003.2076) [DOI] [Google Scholar]
- 128.Mineka S, Cook M. 1988. Social learning and the acquisition of snake fear in monkeys. In Social learning: psychological and biological perspectives (eds Galef BG, Jr, Zentall T.), pp. 51–73. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- 129.Galef BG. 2009. Strategies for social learning: testing predictions from formal theory. Adv. Study Behav. 39, 117–151. ( 10.1016/S0065-3454(09)39004-X) [DOI] [Google Scholar]
- 130.Pike T, Laland KN. 2010. Conformist learning in nine-spined sticklebacks’ foraging decisions. Biol. Lett. 6, 466–468. ( 10.1098/rsbl.2009.1014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haun DB, Rekers Y, Tomasello M. 2012. Majority-biased transmission in chimpanzees and human children, but not orangutans. Curr. Biol. 22, 727–731. ( 10.1016/j.cub.2012.03.006) [DOI] [PubMed] [Google Scholar]
- 132.van de Waal E, Borgeaud C, Whiten A. 2013. Potent social learning and conformity shape a wild primate's foraging decisions. Science 340, 483–485. ( 10.1126/science.1232769) [DOI] [PubMed] [Google Scholar]
- 133.Vale GL, Flynn EG, Lambeth SP, Schapiro SJ, Kendal RL. 2011. Public information use in chimpanzees (Pan troglodytes) and children (Homo sapiens). J. Comp. Psychol. 128, 215–223. ( 10.1037/a0034420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marler P, Peters S. 1977. Selective vocal learning in a sparrow. Science 198, 591–521. ( 10.1126/science.198.4316.519) [DOI] [PubMed] [Google Scholar]
- 135.Curio E, Ernst U, Vieth W. 1978. Cultural transmission of enemy recognition: one function of mobbing. Science 202, 899–901. ( 10.1126/science.202.4370.899) [DOI] [PubMed] [Google Scholar]
- 136.Nottebohm F, Kasparian S, Pandazis S. 1981. Brain space for a learned task. Brain Res. 213, 99–109. ( 10.1016/0006-8993(81)91250-6) [DOI] [PubMed] [Google Scholar]
- 137.Wyles JS, Kunkel JG, Wilson AC. 1983. Birds, behavior and anatomical evolution. Proc. Natl Acad. Sci. USA 80, 4394–4397. ( 10.1073/pnas.80.14.4394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wilson AC. 1985. The molecular basis of evolution. Sci. Am. 253, 148–157. ( 10.1038/scientificamerican1085-164) [DOI] [PubMed] [Google Scholar]
- 139.Bateson PPG. 2004. The active role of behavior in evolution. Biol. Philos. 19, 283–298. ( 10.1023/B:BIPH.0000024468.12161.83) [DOI] [Google Scholar]
- 140.Sol D, Stirling DG, Lefebvre L. 2005. Behavioral drive or behavioral inhibition in evolution: subspecific diversification in Holocene passerines. Evolution 59, 2669–2677. ( 10.1111/j.0014-3820.2005.tb00978.x) [DOI] [PubMed] [Google Scholar]
- 141.Tebbich S, Taborsky M, Fessl B, Blomqvist D. 2001. Do woodpecker finches acquire tool use by social learning? Proc. R. Soc. Lond. B 268, 2189–2193. ( 10.1098/rspb.2001.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Schaik CP, Burkart JM. 2011. Social learning and evolution: the cultural intelligence hypothesis. Phil. Trans. R. Soc. B 366, 1008–1116. ( 10.1098/rstb.2010.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burkart J, Schübiger MN, van Schaik CP. 2017. The evolution of general intelligence. Behav. Brain Sci. ( 10.1017/S0140525X16000959) [DOI] [PubMed] [Google Scholar]
- 144.Forss SIF, Willems E, Call J, van Schaik CP. 2016. Cognitive differences between orangutan species: a test of the cultural intelligence hypothesis. Sci. Rep. 6, 30516 ( 10.1038/srep30516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tomasello M. 1999. The cultural origins of human cognition. Cambridge, MA: Harvard University Press. [Google Scholar]
- 146.Herrman E, Call J, Hernandez-Loreda MV, Hare B, Tomasello M. 2007. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366. ( 10.1126/science.1146282) [DOI] [PubMed] [Google Scholar]
- 147.Whiten A, Erdal D. 2012. The human socio-cognitive niche and its evolutionary origins. Phil. Trans. R. Soc. B 367, 2119–2129. ( 10.1098/rstb.2012.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morin O. 2015. How traditions live and die. Oxford, UK: Oxford University Press. [Google Scholar]
- 149.Whiten A. 2017. Social learning and culture in child and chimpanzee. Ann. Rev. Psychol. 68, 129–154. ( 10.1146/annurev-psych-010416-044108) [DOI] [PubMed] [Google Scholar]
- 150.Carroll EL, Baker CS, Watson M, Alderman R, Bannister J, Gaggiotti OE, Cröcke DR, Pateneude N, Harcourt R. 2015. Cultural traditions across a migratory network shape the genetic structure of southern right whales around Australia and New Zealand. Sci. Rep. 5, 16182 ( 10.1038/srep16182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Foote AD, et al. 2016. Genome-culture co-evolution promotes rapid divergence of killer whale ecotypes. Nat. Commun. 7, 11693 ( 10.1038/ncomms11693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cantor M, Shoemaker LG, Cabrai RB, Flores CO, Varga M, Whitehead H. 2015. Multilevel animal societies can emerge from cultural transmission. Nat. Commun. 6, 8091 ( 10.1038/ncomms9091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laland KN, Odling-Smee J, Myles S. 2010. How culture shaped the human genome: bringing genetics and the human sciences together. Nat. Rev. Genet. 11, 137–148. ( 10.1038/nrg2734) [DOI] [PubMed] [Google Scholar]
- 154.Richerson PJ, Boyd R, Henrich J. 2010. Gene-culture co-evolution in the age of genomics. Proc. Natl Acad. Sci. USA 107(Suppl. 2), 8985–8992. ( 10.1073/pnas.0914631107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grant BR, Grant PR. 2002. Simulating secondary contact in allopatric speciation: an empirical test of premating isolation. Biol. J. Linn. Soc. 76, 545–556. ( 10.1046/j.1095-8312.2002.00076.x) [DOI] [Google Scholar]
- 156.Grant PR, Grant BR. 2002. Adaptive radiation of Darwin's finches. Am. Sci. 90, 130–139. ( 10.1511/2002.2.130) [DOI] [Google Scholar]
- 157.Langergraber KE, et al. 2010. Genetic and cultural similarity in chimpanzees. Proc. R. Soc. B 277, 408–416. ( 10.1098/rspb.2010.1112) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.