Abstract
The Modern Synthesis led to fundamental advances in understandings of human evolution. For human palaeontology, a science that works from ancestral phenotypes (i.e. the fossil record), particularly important have been perspectives used to help understand the heritable aspects of phenotypes and how fossil individuals might then be aggregated into species, and relationships among these groups understood. This focus, coupled with the fragmentary nature of the fossil record, however, means that individual phenotypic variation is often treated as unimportant ‘noise’, rather than as a source of insight into population adaptation and evolutionary process. The emphasis of the extended evolutionary synthesis on plasticity as a source of phenotypic novelty, and the related question of the role of such variation in long-term evolutionary trends, focuses welcome attention on non-genetic means by which novel phenotypes are generated and in so doing provides alternative approaches to interpreting the fossil record. We review evidence from contemporary human populations regarding some of the aspects of adult phenotypes preserved in the fossil record that might be most responsive to non-genetic drivers, and we consider how these perspectives lead to alternate hypotheses for interpreting the fossil record of early genus Homo. We conclude by arguing that paying closer attention to the causes and consequences of intraspecific phenotypic variation in its own right, as opposed to as noise around a species mean, may inspire a new generation of hypotheses regarding species diversity in the Early Pleistocene and the foundations for dispersal and regional diversification in Homo erectus and its descendants.
Keywords: Homo erectus, phenotypic variation, human biology, developmental plasticity, speciation
1. Introduction
The Modern Synthesis led to fundamental advances in our understandings of human evolution. For human palaeontology, a science that works predominantly with the phenotypes of our distant ancestors (i.e. the fossil record), perspectives used to help understand the heritable aspects of traits have been particularly important. These approaches influence how fossil individuals might then be aggregated into species and relationships among these groups understood. A related but separate thread has focused on understanding the environmental contexts of the fossil remains and thereby the natural selective forces that acted on phenotypes at different times during human evolutionary history. In this paper, we discuss how the modern synthesis, and its debated revision in the extended evolutionary synthesis (EES), influences how we interpret and use fossil phenotypes. We also consider how new (or newly highlighted) perspectives in the evolutionary process might provide novel insights into old puzzles in the study of fossil Homo.
The early recognition by Mayr of speciation as a process related to interbreeding groups of organisms (and their relative isolation; i.e. the biological species concept) catalysed important revisions of how human fossils were categorized. These revisions would influence the questions posed of the human fossil record. In a now famous paper, Mayr [1] revised and reduced the more than eight genera of fossil hominins broadly used at the time (including Australopithecus and Homo) to just one genus (Homo) with three species (H. sapiens, H. erectus and H. transvaalensis). The argument was meant to bring palaeoanthropology into the norm of other fields of biology, and while overly extreme in its course, formal recognition of greater levels of synonymy allowed for consideration of questions about the biology, dispersal and habits of ancestral hominin species that were not the focus when the fossils were considered as regional atomized parts. For example, in a revision that still largely stands, Mayr [1,2] sank at least three genera of Asian fossil hominins (Sinanthropus, Pithecanthropus and Homo) into the single genus and species H. erectus. Although the specialist palaeontologists of the day had routinely stated that most of these genera were likely to be biologically the same [3–5], the formal recognition that a single early hominin existed across Asia led to new questions regarding its central adaptation [6]. The subsequent discovery of older H. erectus in Africa [7] naturally raised the question of what led to its dispersal to Asia [8]. In each instance, a key prerequisite was determining the relationship of new discoveries to previously known species by using aspects of fossil anatomy that were inferred to be heritable (i.e. genetically determined).
While encompassing a broad umbrella of conceptual and methodological advances, the main focus of the modern synthesis was to meld understandings of the genetic basis of evolution (Mendelism) with Darwinian theory [9], and in light of this, a key goal in evaluations of fossil hominins has been discerning the genetically heritable component of fossil phenotypes. Neither Darwin nor the architects of the modern synthesis denied that there were mechanisms other than natural selection that contributed to evolutionary change, nor excluded the existence of non-hereditary contributions to the adult phenotype, but these were (and largely continue to be) understood as having modest importance to long-term evolutionary change [9]. Thus, teasing apart the relationship between quantitative genetics and phenotypic traits has been important for understanding morphological evolution (e.g. [10–12]), and from this intuiting the genetic aspects of phenotypic traits has been a primary focus of palaeontological research agendas (e.g. [13–15]). The cladistics movement, committed to determining phylogenetic relationships from phenotypic character states, was in part a reaction against the merging of taxa by the modern synthesis. Yet it implicitly holds at its core the same principle, that the interesting thing about phenotypes is their ability to reflect genotype. When applied to the fossil record, particularly in the frequent cases in which a single fossil represents the species, these methods have generally resulted in a focus on individual phenotypes as presumed species (genetic) proxies (e.g. [16–20]). This lack of focus on the full breadth of processes by which individual phenotypes are created is a key issue for human palaeontology, a science whose primary database is fossil phenotypes and whose conclusions rest on their interpretations.
Following on the recognition of similar limitations in other areas of evolutionary biology, a sometimes loose array of concepts thought to be either implicitly or explicitly excluded from the modern synthesis have been proposed as evidence for the need for an ‘EES' (e.g. [21]). Although the database of human palaeontology is poorly suited for the study of some of these, several concepts have clear application to studies of human evolution and their methods have started to be incorporated into the discipline. Of particular importance are those discussions related to the construction of phenotypes by altering selective pressures acting on them (e.g. niche construction), the evolution of patterns of development and the ability to evolve (e.g. evo-devo and evolvability), and the non-genetic and epigenetic aspects of phenotype development itself (e.g. developmental biology, phenotypic and developmental plasticity, parental effects, developmental niche construction and other non-genetic forms of inheritance). The idea of niche construction has obvious applicability to recent humans and locating the origin of our particular biocultural pattern has influenced research in human evolution (e.g. [22]). Similarly, studies of integration and evolvability have gained importance within studies of human evolution for their insights into species-level patterns of morphological units, such as the pelvis and thorax in humans, that might evolve in tandem, and which might thereby provide possibilities and/or constrain the direction of evolutionary change in the group (e.g. [23,24]). As a complement to this work, here we focus on the importance of plasticity, and its intergenerational manifestation as parental effects, which we believe has overlooked implications for understanding the skeletal phenotypes at the heart of the palaeontological database.
Developmental plasticity and parental effects have only recently been emphasized in human evolutionary studies (e.g. [25]), although they have a long history of interrogation by other fields (e.g. [10,11,26–30]). Whether these effects are important to long-term evolutionary change remains hotly debated within evolutionary biology more generally, but what is clear is that many phenotypic traits respond to, and come to embody, patterns of experience or in some cases of use and disuse, that can occur through a range of non-genetic adaptive processes (e.g. [31]). These processes of accommodation and adaptation can powerfully shape individual phenotypes [32,33], and thus have an important bearing on our interpretation of the meaning and identity of fossil individuals and their opportunities for survival and therefore potential contributions to evolution. As we argue below, from this perspective individual variation may be understood not only as the basis for the common practice of defining central tendencies to reconstruct taxonomy, but as a storehouse of information on individual adaptive responses to local environments. In addition, because selection acts only on those phenotypes actually expressed in individuals (rather than the range of phenotypes possible from an individual's genotype), non-genetic processes that influence phenotype expression could have substantial influence on the direction and pace of genetic evolution.
Plasticity in phenotypic form has long been recognized as an important source of variation (e.g. [26]) and one that can allow relatively rapid change under certain circumstances (e.g. [27]). For instance, reaction norms have long been used as formal descriptions of how environments interact with underlying genotypes to shape the phenotypes created across environments [26,34]. Lande [27] showed that with a significant and abrupt environmental change, different norms of reaction for plasticity would lead to substantial increases in the levels of plasticity in a population while also facilitating potentially rapid phenotypic change towards the new environmental optimum. A focus on plasticity thus moves us away from viewing novel phenotypes as being slowly cobbled together through random mutations of small phenotypic effect, and provides a mechanism for relatively quick and functionally integrated population-level phenotypic change.1
While phenotypic responses allow novel phenotypes to emerge in response to environmental, rather than genetic, change, this decoupling of phenotypic novelty from underlying genetic novelty has traditionally cast doubts on the long-term evolutionary implications of these changes. However, an emerging perspective builds on the early work of Baldwin [35], Waddington [36,37], Schmalhausen [38] and others to address this issue, and is re-imagining the role of phenotypic plasticity as a pervasive influence on the direction and pace of genetic evolution. Authors such as West-Eberhard, Pigliucci and others note that phenotypic and developmental plasticity—generated from stimuli such as behavioural change, climate or nutritional or environmental stress—is often the original source for novel individual variation (plasticity; [39,40]). Because natural selection can only operate on existing phenotypes, these plasticity-induced trait configurations are the raw variation that is subjected to selection, and that eventually facilitates genetic adaptation.
Environmentally induced phenotypes may ‘lead the way’ to more gradual genetic adaptation through several processes, which have been labelled ‘phenotype-first’ evolution (e.g. [25]). One long-recognized example is genetic assimilation, as classically illustrated by Waddington's [37] fruit fly breeding experiments in which an environmentally induced phenotype was, after multiple generations of exposure, eventually expressed in the absence of the inducing stimulus. West-Eberhard's [39] concept of genetic accommodation recognizes that fixing a trait (reducing plasticity) through genetic assimilation is only one of several possible means by which plasticity-induced phenotypes might lead the way for genetic change. Genetic changes may also be favoured, for instance, if they help generate the plasticity-induced phenotype with fewer costs, or by re-centring the trait around new functional loadings, which can protect the organism's ability to mount a plastic response to future environmental challenges [41].
One example of an application of these principles that has largely unexplored applicability to the hominin fossil record is the ‘flexible-stem’ model of species dispersal [39]. The flexible-stem model proposes that when an ancestral (stem) population is faced with new ecological pressures, the pattern of phenotypic plasticity in the ancestor will constrain the direction and form of phenotypes induced in descendant populations. Support for this model has been demonstrated in living species like stickleback fish, in which the phenotypes of independently evolved freshwater species repeatedly converge on similar phenotypes that mirror environmentally induced developmental variation in the extant ancestral marine population. This work shows that plasticity provided the developmental template from which the freshwater species diverged as they moved into novel environments with different prey types [42]. In addition, subtle genetic differences between the freshwater populations point to partial genetic accommodation of the induced phenotypes. Similarly, among species of spadefoot toads, species diversity has been shown to reflect ancestral larval plasticity interacting with local climactic conditions [43]. These studies of contemporary wild populations show how plasticity facilitates not only short-term survival in novel ecological conditions—which has long been acknowledged—but also guides the direction of longer-term genetic evolution by determining the specific phenotypes that are locally expressed. An explicit focus on plasticity may similarly prove helpful in thinking about and framing expectations for populations of H. erectus, the first hominin to disperse widely (figure 1).
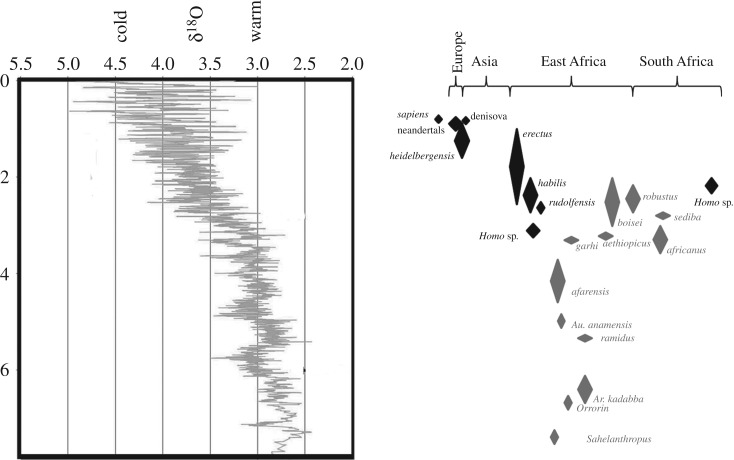
Figure 1.
Geographical and temporal distribution of fossil hominin species discussed. The y-axis represents geological time in millions of years. The diamonds represent hominin species. Except for their length, which indicates approximate duration of a taxon, diamond size has no meaning. Black diamonds are approximate temporal ranges of Homo species. Grey diamonds are Australopithecus or other non-Homo hominins. Homo sp. diamond in South Africa likely represents multiple taxa including H. erectus (for fossil SK 847) and other non-erectus, non-habiline Homo. Homo naledi in South Africa is not figured due to the absence of a geological age at the time the paper was accepted. Homo sapiens is present in all regions and likely originates in Africa, but is shown only in Europe for clarity of figure purposes. Climate curve modified from [158] and based on data from [159].
While the fossil record is admittedly an imperfect database from which to assess the role of plasticity in evolution, we believe that keeping in view this perspective on the importance of variation may inspire new research questions. Here we hope to nudge these discussions forward by using the study of contemporary humans to model the kinds of non-genetic drivers that might contribute to variation in skeletal size and shape of fossil Homo and the kinds of ground-truthing studies that are needed. We then focus on a preliminary analysis of these same signals of variation and plasticity across palaeodemes of H. erectus, and pose testable hypotheses for future work.
2. Modelling non-genetic drivers of variation in the hominin skeleton: a view from contemporary humans
We focus on a few aspects of the human skeleton that have been implicated as important in the origin and evolution of the genus Homo, that are commonly studied in human biology, and that lend themselves to accessible bone proxies in the fossil record. We consider how phenotypes of skeletal size and robusticity and cranial size respond in living humans to changes in behaviour (function), climate and nutritional environment (for additional discussion, see [25]).
(a). Behaviour (function) and plasticity in skeletal development and body size
Skeletal tissue has a repertoire of adaptive processes that allows it to respond to functional loading—including both changes in body mass and activity—during growth and across adulthood (e.g. [31,44]). Increased force or loading on long bones influences the structure of trabecular bone in, for example, the femoral head, and leads to diaphyseal changes that increase strength [45]. During development, exercise and weight bearing increase bone mineral density and thicken the cortices at the expense of medullary volume [46]. After cessation of growth, strain on long bones, whether due to loading or age-related bone loss, leads to a compensatory expansion of diaphyseal diameter via periosteal bone deposition [47].
This capacity for the skeletal system to develop and remodel its structure in response to patterns of loading means that an individual's remains embody a record of these influences and can be generally used to infer other aspects of the phenotype and behaviour, such as body weight and activity patterns. While phenotypes are averages over a lifetime and are not specific enough to map particular activity types for an individual, relative patterns of use and disuse can be inferred. For instance, compared to non-athletes, cricketeers show asymmetrical arm development resulting in a more robust dominant throwing arm (especially in humeral size and shape), whereas robusticity is increased in both arms among swimmers (e.g. [48]). Similarly, disuse atrophy resulting from acquired and congenital conditions that limit mobility leads to limb bone loss, particularly in shaft width but not length (e.g. [49–51]). Palaeoanthropologists have used related signatures to argue for behavioural locomotor differences between, for example, H. habilis and H. erectus [52]. The former is argued to have used more arboreal substrates (perhaps for nesting or refuge) based on relatively stronger upper limb bone cross sections compared with lower limb cross sections, whereas H. erectus shows much stronger lower than upper limbs, suggesting a more committed terrestrial biped.
(b). Skeletal response to climate variables
Climatic and environmental factors, notably temperature, humidity and altitude are also important predictors of human variation in body size and proportions. A key adaptive challenge of migrating to high altitude is coping with the lower oxygen pressure (hypoxia). Although there is evidence for genetic adaptations to hypoxia among populations with long histories of high altitude habitation [53], developmental processes are also important [54,55]. Individuals raised at high altitude have increased pulmonary function, in part as a result of increased lung volume. Skeletal development accommodates this anatomical change by increasing chest dimensions [56], a pattern visible in the skeletal remains of high altitude human populations [57].
Human populations appear to conform to the general expectations of both Bergmann's and Allen's rules that link warmer temperatures to smaller body masses and increased surface area (e.g. longer limbs), respectively [58]. Recent studies in a mouse model have explicitly tested for plasticity mediated by temperature [59], and found a possible mechanism for short-term plastic responses that in theory could lead the way to genetic accommodation (ala [37]). Mice reared in colder environments showed stunting of appendages and shorter tail lengths, following Allen's rule. The mechanism underlying these changes appears to be reduction in the efficiency of vascular delivery of nutrients to the growth plate. Palaeoanthropologists have used related skeletal signatures to argue that Neandertals showed long-term adaptation to cold climates in Europe, but that the first Homo sapiens did not [60]; whereas little signal of climatic adaptation was found in the skeletal remains of H. erectus [61]. There is also skeletal evidence of a correlation between nasal volume and palatal phenotype and climatic differences (especially humidity) across human populations [62].
(c). Differential skeletal response to nutritional deprivation
While climate predicts some aspects of body proportions in humans, a large component of global variation in growth and stature traces to plasticity in response to factors like nutrition and early life exposure to infectious disease [63]. From conception through infancy, growth rate is directly tied to insulin production and thus nutrient availability [64,65]. This leads to increased growth rate and taller adult stature in populations with favourable early life nutrition, and stunting in nutritionally stressed populations. Any source of energy deficit can constrain growth rate at this age, and in many contemporary low income populations nutritionally taxing infectious diseases (e.g. diarrheal illness) are among the most important predictors of growth stunting [66]. Although breastfed infants obtain protective immune factors along with balanced nutritional resources, the weaning transition introduces new sources of nutritional stress. In modern humans, less balanced and less sterile complementary foods are typically introduced at or before six months of age, resulting in weaning stress [67], which can be tracked in subadult skeletal samples [68].
Because the weaning transition occurs during the age of insulin-dependent, nutrition-driven growth, growth faltering that accompanies weaning lingers into adulthood and faltering during this period has a stronger influence on adult stature and body size than stresses encountered during later growth. Studies of marginally nourished contemporary populations typically find that nearly all of the adult height deficits compared with healthy reference standards are already present by 2–3 years of age [69,70]. Although some populations have been shown to experience some degree of ‘catch-up’ growth after infancy [66,71], work on human growth has led to the idea that the prenatal period and infancy represent a critical period in final stature attainment, with much contemporary population variation in adult standing height tracing to the impact of nutrition and hygiene during infancy and early childhood [63,72,73]. By contrast, after 2–3 years of age growth-hormone rather than insulin regulates growth [64] and nutritional deficits after this age slow the pace of both growth and maturation. This link between nutrition and maturity leads to the achievement of a similar adult stature during a longer and slower period of growth and can lead to large, cumulative phenotypic changes across multiple generations (e.g. the gradual decline in menarcheal age from 16–17 years to 12 years since the mid-nineteenth century in some European nations; [63]).
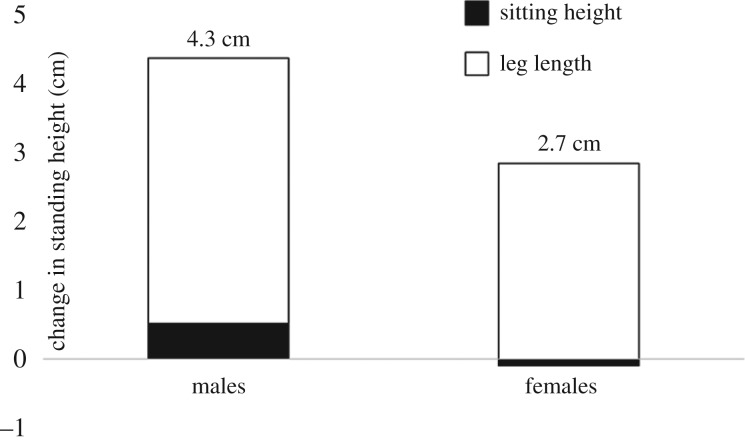
In contemporary human populations, different body segments exhibit differential sensitivity to environmental stress, which can lead to plasticity-induced changes in body or limb proportions. The greater nutritional sensitivity of the limbs, and especially the legs, has been noted repeatedly, which partly reflects the fact that leg growth is fastest during the ages of nutrition-sensitive early growth [55]. This tends to lead to relatively longer legs as nutritional conditions improve. As one example, in post-WWII Japan, adult stature increased dramatically in males and females, an increase thought to reflect improvements in living conditions and dietary change [74]. Nearly, all of this statural increase was accounted for by longer legs; by contrast, trunk length was not appreciably increased (figure 2).
Figure 2.
Secular changes in height in Japan post-WWII. Note that leg length accounts for most of the secular change in height. Adapted from [74].
Although nutrition is a key driver of plasticity in growth and development, non-nutritional factors are also important. In the past two decades, the logic of life-history theory [75] has been extended to help shed light on the impact of psychosocial stressors on plasticity in maturational tempo and reproductive scheduling in humans [76,77]. In contrast to the effects of nutritional stress, which as noted delay maturity, psychosocial stress has been shown to accelerate maturation. Girls raised without a father in the household, who experienced the death of a sibling, or traumatic abuse during early childhood have all been shown to mature earlier than their non- or less-stressed peers [76]. These same stressors also predict an earlier age at first reproduction, higher fertility and shorter lifespan [77,78]. It has been hypothesized that these findings point to an evolved ability to sense cues reflective of extrinsic (unavoidable) mortality and to facultatively accelerate life-history scheduling when the risk of delaying maturity (e.g. of dying before reproducing) is high. The acceleration of maturity in response to these stressors is of the order of several months of acceleration, which is modest in comparison to the effects of nutrition. However, it is presently unclear whether these effects might cumulatively lead to more dramatic maturational change across multiple generations faced with high mortality or related stressful cues, as has been inferred for the effects of nutrition.
In contrast to traits like leg length and stature, human brain growth is considered to be highly buffered and thus canalized, as reflected in smaller responses to nutritional and environmental stress. This begins in utero in the form of ‘brain-sparing’ in which blood flow is distributed away from the periphery to the growing brain under conditions of nutritional or oxygen deficit, resulting in conserved brain size relative to body size at birth [79]. Similarly, it has recently been proposed that the greater sensitivity of the limbs to nutritional stress may represent a postnatal expression of brain sparing [80]. As a result of these and other buffering mechanisms, cranial capacity is expected to vary little in response to factors like nutrition.
Although relatively canalized, there is evidence that the size of specific brain structures relates to their use and disuse during and after development. In rats, for instance, environmental enrichment (i.e. an increase in stimulation) has been shown to increase cortical thickness and weight by roughly 10% in some regions [81,82]. Conversely, in humans, early life stress has been shown to predict a reduced brain volume [83]. Such studies underscore that variation in even relatively canalized traits like cranial capacity will partly reflect changes in environment and experience, and in theory might lead cumulatively to sustained change across multiple generations, as shown for less canalized traits like stature, body proportions or maturational tempo (e.g. [84]).
(d). Environmental effects on future phenotypes: parental effects and epigenetic inheritance
In addition to the effects that expressed phenotypes have on the patterns of selection, there are also a range of pathways by which environmental effects can transcend a single generation to directly influence phenotypes in future generations. Parental effects are emerging as important examples of this in humans and other organisms [85]. Parental effects broadly refer to any effect of the parent on the phenotype or survival of offspring, beyond the direct transmission of genes. We review these examples here not because they are particularly amenable to testing in the fossil record; rather, they reveal yet another way by which plasticity can induce adaptive phenotypes: not in response to environmental experience, but in response to non-genetic cues communicating information about recent ancestral environments.
In well-studied avian examples, the mother responds to environmental stimuli by modifying the nutrient or hormonal constituents deposited in eggs which in turn modify the offspring's development or behaviour and improve its match with the immediate environment [86]. For instance, among house finches faced with novel environments, maternal effects increase pre-selection variance in offspring phenotypes, which has been crucial to the success of this colonizing species. As resource competition increases, western bluebird mothers modify the order of egg-laying to favour offspring with an aggressive, dispersing phenotype [87]. These examples illustrate how mechanisms of developmental plasticity may, in some instances, open up opportunities for the developing offspring not only to adjust to the environment that it experiences itself, but to respond to historical environmental signals transduced through the mother's phenotype.
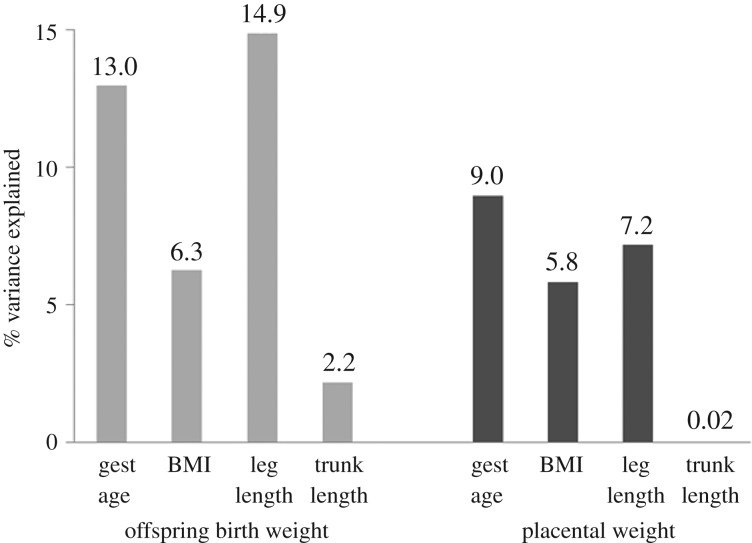
Maternal experience in humans has similarly been shown to have intergenerational effects on diverse aspects of offspring biology. Importantly, nutrition and psychosocial stress—the same factors that drive variation in growth, maturity and body size—seem to have particularly pronounced prenatal impacts. In addition to better-studied influences on metabolism and risk for chronic disease [88], long-term effects include changes in traits like postnatal growth trajectory, adult size, body fat and muscle mass, and strength [89], along with changes in reproductive biology [90,91]. Although the adaptive significance of these effects remains the topic of debate [92–94], it is notable that fetal nutrition does not respond to acute changes in the mother's intake during pregnancy, which may reflect the unreliable signal of short-term, transient conditions. It has instead been argued to reflect a more integrated signal of her past experience that better represents typical local conditions (phenotypic inertia, [89]). For example, nutritional supplementations of pregnant mothers—which represent a substantial but only temporary (e.g. transient) increase above normal intake—typically have modest or even negligible effects on offspring birth weight [95]. By contrast, there is evidence that the mother's nutrition prior to pregnancy and across her own development has important intergenerational impacts on fetal nutrition and growth. As one example, in a study of a low resource population in the rural Philippines, the mother's leg length was among the strongest predictors of offspring placental size and birth weight. By contrast, her trunk length was unrelated to placental size and only weakly related to offspring birth weight. Because leg length is the most nutritionally sensitive component of stature growth, as noted above, these findings suggest that fetal nutrition—and thus the range of downstream phenotypic outcomes set in motion by the gestational nutritional milieu—is linked to the mother's chronic developmental nutritional experiences ([96]; figure 3).
Figure 3.
Percent of variance of offspring birthweight and placental weight explained by maternal variables. Note greatest predictive value of mother's leg length over other variables. Adapted from [96].
(e). Phenotype-first evolution
Collectively, the studies reviewed above illustrate some of the mechanisms by which changes in the behaviours, activities and experiences of individuals can lead to marked and rapid shifts in phenotypes, and in ways that can directly or indirectly leave a trace in the skeletal record. This plasticity in phenotypic expression clearly influences survival and reproduction, but as noted, the EES takes this point one step further to view these environmentally induced phenotypes as the starting points for new directions in genetic evolution [29,39,86]. Because phenotypic and developmental plasticity can lead to novel, adaptively fit phenotypes within a single generation, in such instances selection is expected to operate not on gene variants to produce the new phenotype, but to help generate an already existing phenotype with fewer costs, or with greater efficiency. As examples, this might take the form of altering the regulatory set-points that determine whether a trait is expressed in response to an environmental cue, or by reducing any costly ‘side-effects’ generated by inducing the trait through plasticity [39]. In this sense, short-term adaptive processes within individual lifetimes can have long-term effects on the pace and direction of genetic evolution within a population by helping determine the phenotypes that are expressed and subjected to selection [35,39]. It behoves palaeoanthropology then to consider non-genetic, short-term phenotypic responses as a driver of the phenotypic variation that we study in the fossil record, and as a potentially important influence on longer-term genetic evolutionary trends.
A key problem facing the palaeoanthropologist, however, is connecting the scale of these discussions and experiments with the scale and resolution of the fossil record; a record in which we rarely have complete individuals, large samples or tight time control. Because fossils are usually few, fragmentary and are never discovered with a species name, non-genetic sources of phenotypic variation are potential sources of confusion for our first job, which is to use individual fossil anatomy to establish what (taxonomically) the fossil is. In recent decades, the discipline has developed remarkably clever ways of handling some of the complications arising from the need to reconstruct adult anatomy from immature individuals, fill in missing individuals, missing elements or missing age classes2 (e.g. [97–102]). Yet these techniques all more or less consider individual variation as the messy noise around the central ‘mean’ morphology, which is understood as the thing that is heritable and important for the survival of individuals and populations. This central tendency (the inferred ‘bauplan’ if you will) is considered the thing of importance for understanding adaptation and evolution. We argue that, when sample sizes allow, disaggregating fossil assemblages into local and temporal subsamples (palaeodemes3) is crucial to clarify these local sources of influence on the phenotype and to allow reconstruction of both local and long-term evolutionary processes (e.g. [6,97,105–115]). There is a notable history of pursuing such questions; in the 1960s, for example, Howell [107,108] developed important models about the relationship between Neandertal anatomy and climate that built from local and regional palaeodemes in the context of temporal and environmental clues to understand the then thorny issue of the relationship between Neandertals and modern humans. Nonetheless, variation below the species level has not been a focus of much practical interest in fossil studies because the fossil record is so sparse. Many studies that look at evolvability and integration in extant mammals whose results are in turn used to illuminate the fossil record, for example, often do so at the specific or even generic level to enhance sample size (e.g. [116,117]). We suggest that considering phenotypic variation across populations is an important goal in its own right and a shift in focus that needs continual reinforcement. This shift will ultimately help shape the kinds of questions we consider reasonable to ask of the fossil record, the strength of our conclusions, and perhaps more importantly, the types of data we think about collecting for extant comparative collections (a question to which we will return below).
Here we start to build a theoretical framework around how we might explore intraspecific variation and plasticity in the fossil record of early genus Homo given what is known about plasticity in living organisms, especially humans, and what is hypothesized from phenotype-first evolutionary models. We follow that with some (preliminary) observations against the fossil record. We frame this with respect to questions circulating about early genus Homo, which based on recent fossil finds and new analyses, appears after about 2.5 Ma to contain a diversity of species of variable size and shape (e.g. [118]; figure 1). After about 1.8 Ma, the genus is, at least for a time, represented by just a single taxon, H. erectus, characterized by overall larger body and brain size [119,120]. Around the origin of the genus and the survival and dispersal of H. erectus swirl questions concerning the importance and drivers of larger body and brain size, but also substantial size differences across populations. Based on modern human data, we might expect that plasticity in body size will have been achieved relatively easily as members of fossil Homo moved into novel nutritional niches. Absolutely smaller bodies (especially limbs) but not brains might be the immediate expected response to colder, more resource deprived contexts, and especially when paired with any maturity-accelerating effects of extrinsic mortality due to factors like conspecific violence or higher predator load [25], which in theory could also reduce size by ceasing growth early (i.e. maturing earlier). We note that these factors are expected to result in differences in phenotypic means across subpopulations faced with different ecologies (colder climate, higher extrinsic mortality) and not necessarily as differences in the within-group variation between subpopulations.
Based on a flexible-stem model, we expect that dispersed populations will be limited by the plasticity of the source population with respect to the kinds and extent of the developmental reactions that the descendant populations mounted in the face of novel environments. We would expect that dispersal of several groups from a source population into similar environments will elicit similar responses; for example, repeated movement into low nutrition environments will result in small size in the short term. Additionally, ancestral populations with greater plasticity should be more successful at colonizing novel environments (those that do not match their long-term adaptive homes) because the dispersing population will have more flexible reaction norms over which to respond. Given that more plastic populations should be better able to disperse, we expect that earlier populations in novel environments might vary more than later populations in those same environments (assuming environmental stability). As initial assessments of these ideas, we would consider both differences in absolute size in new environments but also differences in variance. Differences in coefficient of variation (CV), for example, may address questions concerning the amount of variation in a population and may be useful to consider. Below we expand on how these expectations might be assessed in the fossil record.
3. Detecting signals of phenotypic plasticity in the fossil record: a research agenda
Although variation below the species level has not been a focus of much practical interest in fossil studies, because the fossil record is so sparse, certain segments of the record allow the exploration of why fossil variation might exist, or how it might have arisen. Here, we consider possible signatures of plasticity that might be evaluated in the fossil record. We acknowledge that the fossil record cannot at present substantially address many of these questions, but having explicit expectations will allow more rigorous future inquiry.
To recognize phenotypic plasticity in the fossil record, we require fossil samples (palaeodemes) from distinct environments (including climate, parasite/predator load, etc., and nutritional circumstances) that differ in prescribed ways [106]. We would infer that a pattern of differences between samples is potentially consistent with having originated via plasticity if (i) the traits known to be plastic in closely related extant taxa vary between the fossil samples in expected ways relative to inferred differences in nutrition, climate or other relevant influences and (ii) traits known to be less plastic in living groups vary little across these same fossil samples. In addition, based upon the principle that dimorphic traits tend to be costly to build and maintain, conditions like resource stress are expected to differentially influence the sex with the more costly phenotype (in hominins, generally males), leading to a reduction in dimorphism [25].
To approach the question of the source of the phenotype (adaptation achieved via plasticity first and natural selection), as a starting point we assume that, all else equal, the clearer the distinction in a trait between groups and the longer the directional change, the more likely this is to be a case of evolved genetic selection for a characteristic. Alternatively, the more variable the trait across populations of a species and the greater the difference in that variation within a species relative to the variation in other species, the more likely we are to be looking at plasticity-induced variation. In the light of this, several examples illustrate possible signatures that plasticity-led novelty might leave in the fossil record. If in the absence of climate change body size remained the same through time, but in the presence of indicators of extreme climate change both body size and variance increases and then in later sites at that new climate the increase in size but not variance is maintained, we might infer an initial quick variation in size is then winnowed to a phenotypic optimum for that climate—supporting the type of changes proposed by Lande [27]. Plasticity-induced phenotypic novelty might instead relate to other local conditions, food resources or stressors such as predation, which as noted above are important drivers of plasticity in development and size. This would be supported, for example, if body size tended to be larger, and legs proportionately longer than trunk, in local regions where resources were abundant and smaller (and with shorter legs) where resources were scarce. Such studies would require multiple palaeodemes of a species at a particular time and ideally also an ability to track phenotypic changes within single populations as local ecological changes unfold. This would allow evaluation of whether and how much plasticity there is in a species at a particular time (as we do in the extant record) and an assessment of whether those differences are maintained, reduced or exaggerated through time. Plasticity followed by fixity of a character would be one means (albeit not the only means) of supporting a plasticity first model. Even the best represented hominin taxa cannot currently meet these criteria, and the fossil record will always be plagued by issues of scale/time averaging that are only approachable by both innovation in fossil/environmental studies and in extant models.
Thus, pursuing this agenda will require a multipronged research approach that involves nuanced evaluation of both the fossil and extant skeletal records. On the fossil side, we need to pay attention not just to the fossils themselves but to refining how we make inferences regarding their local environment. This means paying attention to issues of microstratigraphy and geochronology, to refine time packets for palaeodemes, but also to issues such as how we infer predator load and resource availability at a locale (e.g. [121,122]). Each of these requires its own set of extant models to ground-truth the linkages between the signal and the resource inferred from it, for example, between predator accumulations of bone and predator load on the landscape, or between presence of shellfish and signatures of aquatic resource use (e.g. [121,123]). Influences of resource scarcity made from environmental, palaeobotanical or faunal proxies should be combined with signals from the fossil hominins themselves concerning their specific resource regimes. For example, isotopic studies of teeth and/or dental calculus [124–126], paired with microwear analyses, may provide several scales of insight regarding individual diet for comparison with archaeobotanical or faunal resource abundance data. Non-specific indicators of individual stress (e.g. linear enamel hypoplasia; [127]) and radiological [128] and histological proxies that assess catch-up growth and growth faltering (e.g. [129,130]), which have proven useful in studies of extant humans (and other mammals), may ultimately provide similar insights into the origins of variation in individual fossils that can likewise be correlated with archaeological resource data.
Putting the focus on how local variation is generated puts added (and different) emphasis on what we ask of (and know about) our extant models. For example, if inferring the heritable bauplan of a species (such as H. erectus) across all the environments and times in which it is found is the main goal, then we may not think that local and regional palaeodeme variation has evolutionary importance or interpretive value. That being the case, then for our comparative skeletal samples we might not record more than the species name. We might record only that we have collected and skeletonized a rhesus macaque, but not other information that may be important to interpreting the origin and function of key phenotypes, such as specific locale, altitude or diet. In much the same way, Darwin did not record the island of origin of many finches [131]. Indeed, as also noted by Plavcan [110], most museum collections do not include locational, climatological, environmental or populational data on recent skeletons, thus impeding our ability to clarify the local drivers of individual ontogenetic variation within these taxa. Thus, on the extant skeletal side of the research agenda, we need to pay attention to how individual skeletal variation relates within demes to local resources and climate, but also behaviour and rank. Currently, too little attention is paid to the linkages between skeletal anatomy, soft tissue anatomy, and behaviour and local context. And while a number of studies of extant primates and non-primate mammals collect some somatometric data (e.g. [132–133]), vanishingly few also collect skeletal data from the same animals [133] or collect somatometric data in a way that might be effectively proxied in a skeletal sample (see bonesandbehavior.org and [133] for some exceptions). For free-ranging colony and wild studies, then, these types of data should be prioritized. This gap between skeletal bodies and their embodied behaviours can and should be closed.
With this as a research agenda, we take a preliminary look at the early evolution of genus Homo and what light the concepts surrounding plasticity and plasticity-first models might throw on the meaning of body size variation.
4. Using developmental plasticity as a frame for understanding intraspecific variation and dispersal in Homo erectus
Homo erectus was the lone survivor of early Homo species diversity and also the first hominin to exhibit extensive range expansion (figure 1). Shortly after the increase in Northern Hemispheric glaciation around 2.5 Ma, multiple, contemporaneous morphs of Homo and Australopithecus are recognized [118,134]. In East Africa, between about 2.1–1.7 Ma three named species of Homo (H. habilis [1.9–1.44 Ma], H. rudolfensis [2.1–1.78 Ma], and H. erectus [1.95–0.9 Ma in Africa; and later worldwide]), all persisted in the same locales over a period of at least a few hundred thousand years [118,134,135]. They differ from one another primarily in the shapes and proportions of their dental arcades, with H. habilis having the most primitive conformation and H. rudolfensis, with its very small anterior teeth and squared anterior arcade, seemingly the most derived.4 All three show overlapping variation in brain and body size. At the same time, multiple species of Australopithecus also occur throughout Africa with at least one, A. boisei, in these same East African locales. H. erectus, who will be the main surviving group, tends towards larger average brains and bodies and smaller average tooth and jaw sizes when species-wide means are considered ([136,137]; table 1) and is the only one to expand outside of Africa.
Table 1.
Size variation in palaeodemes of fossil Homo and archaeological skeletal samples of H. sapiens.
| cranial capacity (cm3) |
femur length (mm) |
|||||
|---|---|---|---|---|---|---|
| palaeodemesa—oldest to youngest (duration) | N | CVb | X | N | CVb | X |
| H. habilis species wide | 6 | 12 | 617 | 3 | 13 | 371 |
| Olduvai (130–150 000 years) | 4 | 8.7 | 654 | 1 | — | 315 |
| Koobi Fora (20 000–200 000 years) | 2 | 9 | 545 | 2 | 0.9 | 398.5 |
| H. erectus species wide | 24 | 20.8 | 937 | 9 | 9.1 8.6 | 434 430 |
| Africa—Koobi Fora (130 000 years) | 3 | 10.4 | 781 | 33 | 6.4 7.9 | 473 462 |
| Georgia—Dmanisi (10 000 years) | 4 | 11.7 | 642 | 1 | — | 386 |
| Indonesia—Sangiran (200 000 years) | 5 | 10.6 | 927 | — | — | — |
| Indonesia—Trinil (unknown) | 1 | — | 940 | 3 | 0.6 | 435 |
| China—Zhoukoudian (120 000 years) | 5 | 10.8 | 1043 | 2 | 6.2 | 395.5 |
| Indonesia—Ngandong (catastrophic) | 6 | 8.9 | 1132 | — | — | — |
| H. neanderthalensis | ||||||
| Western Europe (40 000 years) | 7 | 7.4 | 1449 | 6 | 4.6 | 431 |
| H. sapiens mixed sex and locality (recent/archaeological) | 271 | 11.2 | 1293 | 146 | 7.2 | 429 |
| Spitalfields, UK | 22 | 7.9 | 1242 | 19 | 5.7 | 398 |
| South China | 54 | 9.9 | 1445 | 45 | 6.7 | 425 |
| Murray Valley, Australia | 91 | 8.2 | 1215 | 25 | 7.6 | 440 |
| non-human primates | ||||||
| Macaca mulatta (Cayo Santiago) | 80 | 8.0 | 175 | |||
| M. fuscata (Japan) | 42 | 7.2 | 164 | |||
| Chlorocebus aethiops (Kenya) | 35 | 8.2 | 142 | |||
aPalaeodeme specimens included by taxon; data as per [106] unless otherwise noted. H. habilis: Koobi Fora crania KNM-ER 1805, 1813 and femora perhaps representing unaffiliated early Homo KNM-ER 1472, 1481. Olduvai crania OH 7, 13, 16, 24 and femur OH 62; H. erectus: Koobi Fora crania KNM-ER 42700, 3733, 3883 and femora KNM-ER 736, 737, 1808 based on dimensions of [155] left, [156] right. Dmanisi crania D2282, 2280, 2600, 3444 and femur D4167/3901/4501; Sangiran crania Sangiran 10, 12, 17, 38, IX. Trinil crania Trinil 2 and femora Femur II, III, IV. Zhoukoudian crania, Skull II, III, X, XI, XII and femora Femur I and IV. Ngandong-crania Solo I, V, VI, IX, X, XI. H. neanderthalensis: Western Europe crania Neandertal, La Chapelle, La Quina, Spy 1, 2, Guattari 1, Gibraltar 1 and femora Neandertal, La Ferrassie 1, 2, La Chapelle, St. Cesaire, Spy2. H. sapiens data from [157]. Mixed sex and locality include individuals from the subsamples listed as well as samples without both matched cranial and femur data from North China and Swanport Australia.
bIn a conservative test of our question, we report CVs uncorrected for sample size. Small sample size tends to lower CV values and thus underestimate variation in the fossil palaeodemes relative to the more recent skeletal demes.
Much like recent humans, Homo erectus inhabited environments in equatorial Africa and more temperate Eurasia and as such, considerable work has been framed around understanding what made dispersal possible and what the broad geographical and temporal trends in variation might mean (e.g. [8,138]). Currently, H. erectus has been identified in groups of fossils from continental and Southeast Asia, East and South Africa, the Republic of Georgia but not Europe. Although all of these fossil assemblages show size variation within them, the fossils from Dmanisi, Georgia are by far the smallest in both brains and bodies, and the Asian assemblages are the largest in average brain size (table 1). African fossils are, on average, at the smaller end of the range of Asian assemblages but with well-preserved relatively large bodied individuals (e.g. KNM-ER 1808) as well. The Asian and Southeast Asian assemblages were the first to be discovered (being those referred to in the introduction that Mayr sank into Homo) and partly for this reason, H. erectus was viewed historically as larger brained and bodied (when comparing species means) than the other early Homo taxa (e.g. H. habilis, H. rudolfensis) or Australopithecus. Homo erectus is thus often envisioned as a first step towards modern humans.
In traditional approaches, and perhaps because of our own heavy reliance on cognitive capacities, this shift to larger brains (and bodies) has often implicitly been viewed as one-directional. That is, brain size increase but also to a lesser extent body size increase, are taken as species-specific characters that accumulate with time and allow dispersal. Consequently, foraging shifts that allowed this brain and body size increase have been sought (e.g. [138,139]). These shifts (coupled with an increasing dependence on technology) are in turn understood to play an important role in allowing dispersal of this taxon as opposed to earlier hominins (see [8,136,138,139]). As a result, new finds that did not conform to size expectations, such as those from the Republic of Georgia, have been argued to perhaps represent a different species (e.g. H. habilis) or at least something more primitive than the early H. erectus in Africa [140,141]. And indeed even for those who accepted Dmanisi as H. erectus, the small size (body mass and stature) of the remains posed a bit of a conundrum in terms of dispersal arguments because these first dispersers did not have the large bodies and brains posited to be important to dispersal [8].
More recently, plasticity has been hypothesized to have allowed the dispersal of H. erectus—that is their ability to vary and thrive across environments has been posited to be similar to our own (e.g. [120,136]) and important to their ability to disperse and survive. In this view, the small size of the Dmanisi fossils might be understood as an expected reflection of phenotypic plasticity in a more seasonal, resource limited environment (leading to smaller size) and a predator heavy environment (leading to earlier maturity and thus smaller size). That is, small body size might be viewed as a reflection of specific/immediate local environments rather than an indicator of a long-term, species-defining characteristic that suggests membership in a different species than H. erectus. The site of Dmanisi itself certainly represents an extreme change in temperature to that experienced by any African hominins and could thus fit a model like that of Lande [27], although we have no means of assessing whether the assemblage samples individuals who are among the first generations at the site. Alternatively, a non-genetic phenotypic response might also be posited as a reflecting short-term idiosyncratic response to the local environment (sensu [25,59]). For instance, increases in dietary stress, perhaps due to more seasonal temperate environments combined with rising mortality from predation or other sources, could favour a slower pace of growth, perhaps earlier maturation and a smaller adult size (see also [109]). These adjustments in turn might be cumulative across multiple generations, not unlike the secular trend in height and maturity documented in contemporary human populations experiencing sustained nutritional changes [63]. If these changes persisted for long enough, selection could then operate to either fix the phenotype or to generate it with fewer costs (genetic accommodation). Potentially, we could unravel this a bit further by looking at the relative contributions of body parts to the small size in Dmanisi. If the limbs are disproportionately short relative to the trunk, such a relationship would be consistent with the result of a short-term plastic response to marginal nutrition or energetic stress. Alternatively, more equivalent reduction in both trunk and limbs to overall small size might signal a longer-term genetic accommodation. Here, however, the fossil record thwarts our endeavours as the Dmanisi limbs are short (and this is the source of the size estimates for the group), but the trunk skeleton is insufficiently preserved to yield a statement about trunk height.
Other empirical tests have not provided strong support for the idea that H. erectus is more human-like in its plasticity and accommodation to environments than other members of at least later Homo. Most tests of body size variation have shown H. erectus to be no more variable (as based on CVs) than either earlier or later Homo for whom there are adequate assemblages (e.g. [106,142]), nor than other widely dispersed primate species whose demes live in a variety of climates [106]. For example, table 1 shows CVs for femur length for palaeodemes of H. erectus, Neandertals and recent humans as well as widely dispersed macaques and vervets. All show similar levels of variation except those few palaeodemes that appear anomalously low. However, because the levels of variation in H. erectus (and H. sapiens) were compared to widely dispersed primates (macaques and vervets), it remains possible that widely dispersed taxa exhibit generally greater plasticity than geographically more constrained taxa. If this is the case, then greater plasticity could be inferred for both H. erectus and H. sapiens (and later Homo).
By contrast, brain size seems somewhat more variable in H. erectus palaeodemes than other Homo, including H. sapiens; it also seems more variable than even dimorphic apes (e.g. [143,144]; table 1). All the palaeodemes of H. erectus, regardless of environment or timespan encompassed by the sample, show relatively large CVs for cranial capacity (table 1).5 In addition, the mean values for cranial capacity show large differences between palaeodemes. As a result, the species-wide CV for cranial capacity in H. erectus is about twice that of the species-wide CV for H. habilis or H. sapiens. Correlatively, the population-specific CVs for brain size in recent humans are lower than in H. erectus populations and there is less absolute difference between populations of humans for mean cranial capacity. This result is inconsistent with our expectation that brain size should be less plastic than body size in H. erectus (as is the case in H. sapiens). In a traditional model, one could argue that brain size variation is then too great to be accommodated in a single species and H. erectus should represent several species (e.g. [143,144]).6 We think the hypothesis of multiple species is unlikely based on other anatomical details of the fossils [135,146]. Alternatively, the fossil CVs might be large due to greater time sampling in the palaeodemes than in the recent comparator samples. The H. erectus samples cover similar and often lesser timescales than the other Homo fossil samples which nonetheless have lower CVs than H. erectus palaeodemes. On the other hand, the recent human samples are likely to cull just a few hundreds of years of time and thus possibly span longer periods than the H. erectus from Ngandong but shorter periods than the Dmanisi sample. We consider these two H. erectus palaeodemes to be the most comparable to demes, and hence the most appropriate to test our current hypotheses. Ideally, a palaeodeme captures a small amount of time and hence samples a single population at a single time. In the case of Ngandong, a catastrophic assemblage, this ideal is met. The recent human, Neandertal, and Dmanisi H. erectus samples, while incorporating more time, also meet well the expectations of a palaeodeme. The others are simply the best that can be done given the current fossil record. Nonetheless, we note that limiting the results to just Ngandong and Dmanisi yields similar conclusions as does considering all the H. erectus palaeodemes. Despite this, the latter hypothesis that a temporal difference might be responsible for the elevated H. erectus CVs, deserves attention and may be tested as additional, less time-transgressive samples of H. erectus accrue.
For the present, however, available fossil assemblages for H. erectus seem to point to an unusually high level of variability in cranial capacity, which could either point to a predominant role of genetic selection on these traits, or perhaps more speculatively, to relatively greater plasticity in brain size in this species than what is observed in contemporary humans. Indeed, with a slight tilt in perspective, we might speculate that evidence for elevated CVs in brain size represents a period of heightened variability across closely related populations that could indicate an evolutionary opportunity—a point at which brain size was perhaps particularly labile, opening up the possibility for changes in the relationship between brains and bodies that had persisted for millennia within the hominins ([119], see also [10]). This idea is supported by recent work modelling relative encephalization in primates that finds all hominins to be encephalized relative to apes, but also that H. erectus is more encephalized than other earlier Homo [147]. Of course, greater encephalization could be achieved without changing the underlying lability of either trait by stabilizing (or even negative) selection on body size and directional selection on brain size [148]. A recent study [148] reported similar levels of phenotypic integration (i.e. covariation in this instance) of brain and body size in chimpanzees, other non-human primates, and living humans and argued just this—suggesting that (i) all hominins were likely to show a similar relationship and that (ii) given magnitudes of phenotypic covariation or genetic covariation (based on a living human sample), both the transition between A. afarensis and H. erectus and H. erectus and H. heidelbergensis were likely to be driven by positive selection on brains and either slightly positive or negative selection on bodies rather than a relaxation of integration between the two traits.
This is an important study because it attempts to construct a framework for understanding the covariation in brains and bodies in the fossil record. However, because of the structure of the fossil record and the statistical requirements of those analyses, the study is forced to aggregate fossil remains across multiple sites and time periods and as such loses precisely the view on variation across samples of H. erectus that we argue could be so important and could shed light on the role of plasticity. As a result, that study considers large, somewhat unreal transitions by looking, for example, at the jump between A. afarensis and H. erectus without considering any data from the intervening taxa H. habilis or H. rudolfensis at all (see also [149]). The study conclusions may then be right at a grand level—that brain size selection might drive body size increase from Australopithecus to Homo erectus—and still miss the fact that absolute size differences in both variables across samples of H. erectus do not seem to support the idea that body size and brain size were tightly linked within H. erectus. The similar integration in brain and body size variables across extant taxa suggests that these living taxa have achieved the same relationship, which perhaps represents an optimal relationship at this particular point in time. But it need not mean that this relationship/optimum has always been the case. For this to have been true in the past the phenotypic and genotypic covariation would have to be the same [12] in the fossils as in humans, which is plausible but untested. And whether the relationship was relaxed in the past (perhaps due to plasticity), and then later fixed when the survival premium of trait stability increased, remains plausible, if untestable based upon current data. Heterochronic change, in which the developmental and maturational trajectories of different body traits or systems shift relative to one another [150], represents one mechanism that could lead to alterations in the relationship between brain and body size. Indeed vault shape shows such size and shape dissociation between H. erectus and H. sapiens [151] resulting in paedomorphosis via neoteny, thus raising the possibility that covariation between brain and body size could also have differed between the two taxa.
5. Summary
Based on the current fossil record and the apparently large differences between ‘populations’ of H. erectus in different times and places, we hypothesize that plasticity in both body and brain size were important sources of local variation and dispersal in H. erectus. The large absolute differences in body size across palaeodemes of H. erectus correlate with inferred nutritional and other stressors that result in smaller body size in living humans. The smallest bodied H. erectus occurs in the Dmanisi palaeodeme, situated in a more seasonal climate that, along with dental signals, suggest nutritional stress while also being found in association with taphonomic signals of predator activity (i.e. high extrinsic mortality). Plasticity may have allowed the development of suboptimal (but survivable) phenotypes—such as those at Dmanisi—that could have provided a foothold for survival in less than ideal habitats, while also opening up the possibility of longer-term adaptation and the acquisition of environmentally optimal phenotypes with time.
More speculatively, the nutritional stressors that tend to lead to smaller body size might also be expected to favour reduced metabolic expenditure on the brain, which represents a sizeable energetic burden [152]. Although brain size is generally canalized, there are, as noted, studies showing that environmental factors like stress and deprivation, or social enrichment, can influence brain development and adult size [81,82]. The effect in any given generation is relatively modest, but in theory might accumulate across multiple generations, as is seen for other developmentally plastic traits. That is, plastic short-term responses may yield environmentally altered phenotypes that selection then acted on in the longer term. The current record of H. erectus is too sparse to test these alternative views. Nonetheless these perspectives can provide testable research agendas to pursue—ones that would be overlooked if we were to focus solely on species-wide (or even higher taxonomic level) relationships.
Because H. erectus palaeodemes are widely dispersed across different kinds of environments, it is worth considering how phenotypic variation might relate, for instance, to local cues of extrinsic mortality, via close examination of skeletal variation in widely dispersed extant species. Because we speculate that H. erectus may have represented a point at which brain and body scaling relationships were decoupled, analogous instances in which these relationships might also be loosened, such as the brain size decrease in domesticated breeds, may also yield important datasets [153]. Indeed the similarities and differences between the effects of domestication on brain size variation in different taxa (e.g. wolf to dog versus fox to fox-domesticates or among ungulate taxa) may be particularly relevant (e.g. [153,154]). As noted by Lande ([10], 413) genetic uncoupling of brain and body size (as well as phenotypic uncoupling) could further facilitate encephalization—and domesticates potentially provide evidence to evaluate both.
Here, the flexible-stem model might also lend further structure to our expectations of what we may find in future H. erectus samples in different environments, as well as in the stem sample when more fossils are known. First, if plasticity is a key to allowing dispersal in H. erectus, then we should expect that the stem sample of H. erectus will be quite plastic itself, as its underlying variation provides the substrate for descendant populations to react to novel environments. And we hypothesize that this plasticity should be greater than in other early Homo who did not disperse. Second, we should expect that populations in new environments will also show great variation initially, but less variation through time (assuming stable environmental contexts in the same locale). Third, we should expect new samples in similar contexts to show parallel phenotypic responses to earlier dispersers and that are in line with known patterns of plasticity-induced response in humans and other non-human primates.
Our tests and framework are necessarily preliminary. These questions require additional fossils to test, but also argue for the collection of finer-grained data on phenotype-modifying factors like nutritional substrate, general health and parasite load in relation to skeletal remains that comprise comparative collections. While the fossil record can never provide a complete test of the phenotype-first propositions of the EES, we feel that reorganizing our research agendas towards understanding the causes and consequences of individual variation will drive research on extant skeletal collections where finer-grained data are often available, providing opportunities to address the role of plasticity and other components of the EES in human evolution. We hope that others will share our conviction that this framework holds promise, and that future work will test, refine and improve these ideas.
Acknowledgements
S.C.A. is grateful to Denis Noble, Kevin Laland, John Dupre, Nancy Cartwright and Sir Patrick Bateson, organizers of the joint discussion meeting of the Royal Society and the British Academy ‘New trends in evolutionary biology: biological, philosophical and social science perspectives', 7–9 November 2016 and to the meeting participants and attendees for stimulating papers and discussion. We are grateful for the insights and suggestions of the editors and three anonymous reviewers.
Endnotes
We recognize that Lande's [27] model explicitly stipulates that reaction norms for plasticity are based on pre-existing, underlying genetic architecture.
We note that all of these methods of adjustment are potentially confounded by issues of taphonomic bias (differential sampling) in the representation of individuals in fossil assemblages, an important issue beyond the scope of the present contribution.
Following Simpson [103] demes are local populations that actively interbreed with one another and are thus the smallest reproductive populations of polytypic species. Palaeodemes similarly refer to a ‘local’ population of a fossil taxon that is temporally and geographically restricted fossil groupings that presumably capture the same local influence on past populations that demes do in the extant world [104].
They are recognized as distinct species because their divergent anatomies appear to persist over geological time.
We note that CV is influenced by sample size, with smaller samples yielding lower CVs. In a conservative test, table 1 reports CVs uncorrected for sample size so that greater CV values may indeed suggest robust differences in variation. Sample size adjusted CVs are much greater for the palaeodemes.
Authors' contributions
Both authors designed interpreted data and drafted, revised and approved the article.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Mayr E. 1950. Taxonomic categories in fossil hominids. Cold Spring Harb. Symp. Quant. Biol. 15, 109–118. ( 10.1101/SQB.1950.015.01.013) [DOI] [PubMed] [Google Scholar]
- 2.Mayr E. 1944. On the concepts and terminology of vertical subspecies and species. Common Prob. Genet. Paleontol. Syst. Bull. 2, 11–16. [Google Scholar]
- 3.Weidenreich F. 1940. The torus occipitalis and related structures and their transformations in the course of human evolution. Bull. Geol. Soc. China 19, 479–559. ( 10.1111/j.1755-6724.1939.mp19004005.x) [DOI] [Google Scholar]
- 4.Weidenreich F. 1941. The extremity bones of Sinanthropus pekinensis. Palaeont. Sin Ser D 5, 1–150. [Google Scholar]
- 5.Weidenreich F. 1943. The skull of Sinanthropus pekinensis; a comparative study on a primitive hominid skull. Palaeont. Sin Ser D 10, 1–298. [Google Scholar]
- 6.Antón SC. 2002. Evolutionary significance of cranial variation in Asian Homo erectus. Am. J. Phys. Anthrop. 118, 301–323. ( 10.1002/ajpa.10091) [DOI] [PubMed] [Google Scholar]
- 7.Leakey RE, Walker AC. 1976. Australopithecus, Homo erectus and the single species hypothesis. Nature 261, 572–574. ( 10.1038/261572a0) [DOI] [PubMed] [Google Scholar]
- 8.Antón SC, Leonard WR, Robertson ML. 2002. An ecomorphological model of the initial hominid dispersal from Africa. J. Hum. Evol. 43, 773–785. ( 10.1006/jhev.2002.0602) [DOI] [PubMed] [Google Scholar]
- 9.Huxley J. 2010. Evolution the modern synthesis: the definitive edition. Cambridge, MA: MIT Press. [Google Scholar]
- 10.Lande R. 1979. Quantitative genetic analysis of multivariate evolution, applied to brain: body size allometry. Evolution 33, 402–416. ( 10.1111/j.1558-5646.1979.tb04678.x) [DOI] [PubMed] [Google Scholar]
- 11.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.1111/j.1558-5646.1983.tb00236.x) [DOI] [PubMed] [Google Scholar]
- 12.Cheverud JM. 1988. A comparison of genetic and phenotypic correlations. Evolution 42, 958–968. ( 10.1111/j.1558-5646.1988.tb02514.x) [DOI] [PubMed] [Google Scholar]
- 13.Wood B, Lieberman DE. 2001. Craniodental variation in Paranthropus boisei: a developmental and functional perspective. Am. J. Phys. Anthrop. 116, 13–25. ( 10.1002/ajpa.1097) [DOI] [PubMed] [Google Scholar]
- 14.Collard M, Wood B. 2000. How reliable are human phylogenetic hypotheses? Proc. Natl Acad. Sci. USA 97, 5003–5006. ( 10.1073/pnas.97.9.5003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collard M, Wood B. 2007. Hominin homoiology: an assessment of the impact of phenotypic plasticity on phylogenetic analyses of humans and their fossil relatives. J. Hum. Evol. 52, 573–584. ( 10.1016/j.jhevol.2006.11.018) [DOI] [PubMed] [Google Scholar]
- 16.Strait DS, Grine FE, Moniz MA. 1997. A reappraisal of early hominid phylogeny. J. Hum. Evol. 32, 17–82. ( 10.1006/jhev.1996.0097) [DOI] [PubMed] [Google Scholar]
- 17.Stringer CB. 1987. A numerical cladistic analysis for the genus Homo. J. Hum. Evol. 16, 135–146. ( 10.1016/0047-2484(87)90064-9) [DOI] [Google Scholar]
- 18.Skelton RR, McHenry HM. 1992. Evolutionary relationships among early hominids. J. Hum. Evol. 23, 309–349. ( 10.1016/0047-2484(92)90070-P) [DOI] [Google Scholar]
- 19.Manzi G, Bruner E, Passarello P. 2003. The one-million-year-old Homo cranium from Bouri (Ethiopia): a reconsideration of its H. erectus affinities. J. Hum. Evol. 44, 731–736. ( 10.1016/S0047-2484(03)00061-7) [DOI] [PubMed] [Google Scholar]
- 20.Strait D, Grine FE, Fleagle JG. 2007. Analyzing hominid phylogeny. In Handbook of paleoanthropology, pp. 1781–1806. Berlin, Germany: Springer. [Google Scholar]
- 21.Laland KN, Uller T, Feldman MW, Sterelny K, Mueller GB, Moczek A, Jablonka E, Odling-Smee J. 2015. The extended evolutionary synthesis: its structure, assumptions and predictions. Proc. R. Soc. B 282, 20151019 ( 10.1098/rspb.2015.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuentes A. 2015. Integrative anthropology and the human niche: toward a contemporary approach to human evolution. Am. Anthropol. 117, 302–315. ( 10.1111/aman.12248) [DOI] [Google Scholar]
- 23.Grabowski MW, Polk JD, Roseman CC. 2011. Divergent patterns of integration and reduced constraint in the human hip and the origins of bipedalism. Evolution 65, 1336–1356. ( 10.1111/j.1558-5646.2011.01226.x) [DOI] [PubMed] [Google Scholar]
- 24.Middleton ER. 2015. Ecogeographical influences on trunk modularity in recent humans. PhD thesis, New York University. [Google Scholar]
- 25.Kuzawa CW, Bragg JM. 2012. Plasticity in human life history strategy: implications for contemporary human variation and the evolution of genus Homo. Currr. Anthro. 53, S369–S382. ( 10.1086/667410) [DOI] [Google Scholar]
- 26.Via S, Lande R. 1985. Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.1111/j.1558-5646.1985.tb00391.x) [DOI] [PubMed] [Google Scholar]
- 27.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 28.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Func. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 29.West-Eberhard MJ. 1989. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278. ( 10.1146/annurev.es.20.110189.001341) [DOI] [Google Scholar]
- 30.Stearns SC. 1983. The evolution of life-history traits in mosquitofish since their introduction to Hawaii in 1905: rates of evolution, heritabilities, and developmental plasticity. Am. Zool. 23, 65–75. ( 10.1093/icb/23.1.65) [DOI] [Google Scholar]
- 31.Pearson OM, Lieberman DE. 2004. The aging of Wolff's ‘law’: ontogeny and responses to mechanical loading in cortical bone. Am. J. Phys. Anthrop. 125, 63–99. ( 10.1002/ajpa.20155) [DOI] [PubMed] [Google Scholar]
- 32.Lasker GW. 1969. Human biological adaptability. The ecological approach in physical anthropology. Science 166, 1480–1486. ( 10.1126/science.166.3912.1480) [DOI] [PubMed] [Google Scholar]
- 33.Frisancho AR, Frisancho AR. 1993. Human adaptation and accommodation. Ann Arbor, MI: University of Michigan Press. [Google Scholar]
- 34.Stearns SC. 1992. The evolution of life histories. Oxford, NY: Oxford University Press. [Google Scholar]
- 35.Baldwin JM. 1896. A new factor in evolution. Am. Nat. 30, 441–451. ( 10.1086/276408) [DOI] [Google Scholar]
- 36.Waddington CH. 1942. Canalization of development and the inheritance of acquired characters. Nature 150, 563–565. ( 10.1038/150563a0) [DOI] [PubMed] [Google Scholar]
- 37.Waddington CH. 1953. Genetic assimilation of an acquired character. Evolution 7, 118–126. ( 10.1111/j.1558-5646.1953.tb00070.x) [DOI] [Google Scholar]
- 38.Schmalhausen II. 1949. Factors of evolution: the theory of stabilising selection. Philadelphia, PA: Blakiston. [Google Scholar]
- 39.West-Eberhard MJ. 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 40.Pigliucci M. 2010. Ch. 14. Phenotypic plasticity in evolution. In The extended synthesis (eds Pigliucci M, Mueller GB), pp. 355–378. Cambridge, MA: MIT Press. [Google Scholar]
- 41.Bateson G. 1963. The role of somatic change in evolution. Evolution 17, 529–539. ( 10.1111/j.1558-5646.1963.tb03310.x) [DOI] [Google Scholar]
- 42.Wund MA, Baker JA, Clancy B, Golub JL, Foster SA. 2008. A test of the ‘flexible stem’ model of evolution: ancestral plasticity, genetic accommodation, and morphological divergence in the threespine stickleback radiation. Am. Nat. 172, 449–462. ( 10.1086/590966) [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Mestre I, Buchholz DR. 2006. Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proc. Natl Acad. Sci. USA 103, 19 021–19 026. ( 10.1073/pnas.0603562103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan TM, Ketcham RA. 2005. Angular orientation of trabecular bone in the femoral head and its relationship to hip joint loads in leaping primates. J. Morph. 265, 249–263. ( 10.1002/jmor.10315) [DOI] [PubMed] [Google Scholar]
- 45.Ryan TM, Krovitz GE. 2006. Trabecular bone ontogeny in the human proximal femur. J. Hum. Evol. 51, 591–602. ( 10.1016/j.jhevol.2006.06.004) [DOI] [PubMed] [Google Scholar]
- 46.Bradney M, Pearce G, Naughton G, Sullivan C, Bass S, Beck T, Carlson J, Seeman E. 1998. Moderate exercise during growth in prepubertal boys: changes in bone mass, size, volumetric density, and bone strength: a controlled prospective study. J. Bone Min. Res. 13, 1814–1821. ( 10.1359/jbmr.1998.13.12.1814) [DOI] [PubMed] [Google Scholar]
- 47.Seeman E. 2003. The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol. Metab. Clin. North Am. 32, 25–38. ( 10.1016/S0889-8529(02)00078-6) [DOI] [PubMed] [Google Scholar]
- 48.Shaw Colin N, Stock JT. 2009. Habitual throwing and swimming correspond with upper limb diaphyseal strength and shape in modern human athletes. Am. J. Phys. Anthrop. 140, 160–172. ( 10.1002/ajpa.21063) [DOI] [PubMed] [Google Scholar]
- 49.Richards GD, Antón SC. 1991. Craniofacial configuration and postcranial development of a hydrocephalic child (ca. 2500 BC–500 AD): with a review of cases and comment on diagnostic criteria. Am. J. Phys. Anthrop. 85, 185–200. ( 10.1002/ajpa.1330850207) [DOI] [PubMed] [Google Scholar]
- 50.Giangregorio L, McCartney N. 2006. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J. Spinal Cord Med. 29, 489 ( 10.1080/10790268.2006.11753898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Millis DL, Levine D. 2013. Canine rehabilitation and physical therapy, 2nd edn London, UK: Saunders. [Google Scholar]
- 52.Ruff C. 2009. Relative limb strength and locomotion in Homo habilis. Am. J. Phys. Anthrop. 138, 90–100. ( 10.1002/ajpa.20907) [DOI] [PubMed] [Google Scholar]
- 53.Beall CM, et al. 2010. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl Acad. Sci. USA 107, 11 459–11 464. ( 10.1073/pnas.1002443107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frisancho AR. 1977. Developmental adaptation to high altitude hypoxia. Intl. J. Biometerol. 21, 135–146. ( 10.1007/BF01553707) [DOI] [PubMed] [Google Scholar]
- 55.Frisancho AR. 2007. Relative leg length as a biological marker to trace the developmental history of individuals and populations: growth delay and increased body fat. Am. J. Hum. Biol. 19, 703–710. ( 10.1002/ajhb.20676) [DOI] [PubMed] [Google Scholar]
- 56.Brutsaert TD, Soria R, Caceres E, Spielvoge H, Haas JD. 1999. Effect of developmental and ancestral high altitude exposure on chest morphology and pulmonary function in Andean and European/North American natives. Am. J. Hum. Biol. 11, 383–395. ( 10.1002/(SICI)1520-6300(1999)11:3%3C383::AID-AJHB9%3E3.0.CO;2-X) [DOI] [PubMed] [Google Scholar]
- 57.Weinstein KJ. 2007. Thoracic skeletal morphology and high-altitude hypoxia in Andean prehistory. Am. J. Phys. Anthrop. 134, 36–49. ( 10.1002/ajpa.20619) [DOI] [PubMed] [Google Scholar]
- 58.Leonard WR, Katzmarzyk PT. 2010. Body size and shape: climatic and nutritional influences on human body morphology. In Human evolutionary biology (ed. Muehlenbein MP.), pp. 157–169. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 59.Serrat MA, King D, Lovejoy CO. 2008. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc. Natl Acad. Sci. USA 105, 19 348–19 353. ( 10.1073/pnas.0803319105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holliday TW. 1997. Postcranial evidence of cold adaptation in European Neandertals. Am. J. Phys. Anthrop. 104, 245–258. ( 10.1002/(SICI)1096-8644(199710)104:2%3C245::AID-AJPA10%3E3.0.CO;2-%23) [DOI] [PubMed] [Google Scholar]
- 61.Antón SC. 2007. Climatic influences on the evolution of early Homo? Folia Primatol. 78, 365–388. ( 10.1159/000105150) [DOI] [PubMed] [Google Scholar]
- 62.Noback ML, Harvati K, Spoor F. 2011. Climate-related variation of the human nasal cavity. Am. J. Phys. Anthrop. 145, 599–614. ( 10.1002/ajpa.21523) [DOI] [PubMed] [Google Scholar]
- 63.Eveleth PB, Tanner JM. 1990. Worldwide variation in human growth, p. 397, 2nd edn Cambridge, NY: Cambridge University Press. [Google Scholar]
- 64.Karlberg J. 1989. A biologically-oriented mathematical model (ICP) for human growth. Acta Paediatr. Scand. Suppl. 350, 70–94. ( 10.1111/j.1651-2227.1989.tb11199.x) [DOI] [PubMed] [Google Scholar]
- 65.Lowe LP, et al. 2012. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 35, 574–580. ( 10.2337/dc11-1687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowland MG, Cole TJ, Whitehead RG. 1977. A quantitative study into the role of infection in determining nutritional status in Gambian village children. Brit. J. Nutr. 37, 441–450. ( 10.1079/BJN19770047) [DOI] [PubMed] [Google Scholar]
- 67.Sellen DW. 2007. Evolution of infant and young child feeding: implications for contemporary public health. Annu. Rev. Nutr. 27, 123–148. ( 10.1146/annurev.nutr.25.050304.092557) [DOI] [PubMed] [Google Scholar]
- 68.Wall CE. 1991. Evidence of weaning stress and catch-up growth in the long bones of a central California Amerindian sample. Ann. Hum. Biol. 18, 9–22. ( 10.1080/03014469100001362) [DOI] [PubMed] [Google Scholar]
- 69.Billewicz W, McGregor I. 1982. A birth-to-maturity longitudinal study of heights and weights in two West African (Gambian) villages, 1951–75. Ann. Hum. Biol. 9, 309–320. ( 10.1080/03014468200005811) [DOI] [PubMed] [Google Scholar]
- 70.Martorell R, Shroeder D, Rivera J, Kaplowitz H. 1995. Patterns of linear growth in rural Guatemalan adolescents and children. J. Nutr. 125, 1060–1067. [DOI] [PubMed] [Google Scholar]
- 71.Cameron N, Gordon-Larsen P, Wrchota EM. 1994. Longitudinal analysis of adolescent growth in height, fatness, and fat patterning in rural South African black children. Am. J. Phys. Anthrop. 93, 307–321. ( 10.1002/ajpa.1330930304) [DOI] [PubMed] [Google Scholar]
- 72.Habicht JP, Martorell R, Yarbrough C, Malina RM, Klein RE. 1974. Height and weight standards for preschool children. How relevant are ethnic differences in growth potential? Lancet 1, 611–614. ( 10.1016/S0140-6736(74)92663-4) [DOI] [PubMed] [Google Scholar]
- 73.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. 2008. Maternal & Child Undernutrition Study, G. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357. ( 10.1016/S0140-6736(07)61692-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanner JM, Hayashi T, Preece MA, Cameron N. 1982. Increase in length of leg relative to trunk in Japanese children and adults from 1957 to 1977: comparison with British and with Japanese Americans. Ann. Hum. Biol. 9, 411–423. ( 10.1080/03014468200005951) [DOI] [PubMed] [Google Scholar]
- 75.Charnov E. 1993. Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford, UK: Oxford University Press. [Google Scholar]
- 76.Belsky J, Steinberg L, Draper P. 1991. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Dev. 62, 647–670. ( 10.2307/1131166) [DOI] [PubMed] [Google Scholar]
- 77.Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. 2009. Fundamental dimensions of environmental risk: the impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Hum. Nat. 20, 204–268. ( 10.1007/s12110-009-9063-7) [DOI] [PubMed] [Google Scholar]
- 78.Gettler LT, McDade TW, Bragg JM, Feranil AB, Kuzawa CW. 2015. Developmental energetics, sibling death, and parental instability as predictors of maturational tempo and life history scheduling in males from Cebu, Philippines. Am. J. Phys. Anthrop. 158, 175–184. ( 10.1002/ajpa.22783) [DOI] [PubMed] [Google Scholar]
- 79.Hales C, Barker D. 1992. Type 2 (non-insulin dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35, 595–601. ( 10.1007/BF00400248) [DOI] [PubMed] [Google Scholar]
- 80.Pomeroy E, Stock JT, Stanojevic S, Miranda JJ, Cole TJ, Wells JC. 2012. Trade-offs in relative limb length among Peruvian children: extending the thrifty phenotype hypothesis to limb proportions. PLoS ONE 7, e51795 ( 10.1371/journal.pone.0051795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diamond MC, Krech D, Rosenzweig MR. 1964. The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neurol. 123, 111–120. ( 10.1002/cne.901230110) [DOI] [PubMed] [Google Scholar]
- 82.Bennett EL, Rosenzweig MR, Diamond MC. 1969. Rat brain: effects of environmental enrichment on wet and dry weights. Science 163, 825–826. ( 10.1126/science.163.3869.825) [DOI] [PubMed] [Google Scholar]
- 83.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. 1999. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol. Psychiatry 45, 1271–1284. ( 10.1016/S0006-3223(99)00045-1) [DOI] [PubMed] [Google Scholar]
- 84.Tanner J. 1965. The trend towards earlier physical maturation. In Biological aspects of social problems, (eds Meade JE, Parkes AS), pp. 40–65. New York, NY: Springer. [Google Scholar]
- 85.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 86.Badyaev AV, Uller T. 2009. Parental effects in ecology and evolution: mechanisms, processes and implications. Phil. Trans. R. Soc. B 364, 1169–1177. ( 10.1098/rstb.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duckworth RA. 2009. Maternal effects and range expansion: a key factor in a dynamic process? Phil. Trans. R. Soc. 364, 1075–1086. ( 10.1098/rstb.2008.0294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barker D. 1994. Mothers, babies, and disease in later life. London, UK: BMJ Publishing. [Google Scholar]
- 89.Kuzawa CW. 2005. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am. J. Hum. Biol. 17, 5–21. ( 10.1002/ajhb.20091) [DOI] [PubMed] [Google Scholar]
- 90.Jasienska G, Ziomkiewicz A, Lipson SF, Thune I, Ellison PT. 2006. High ponderal index at birth predicts high estradiol levels in adult women. Am. J. Hum. Biol. 18, 133–140. ( 10.1002/ajhb.20462) [DOI] [PubMed] [Google Scholar]
- 91.Kuzawa CW, McDade TW, Adair LS, Lee N. 2010. Rapid weight gain after birth predicts life history and reproductive strategy in Filipino males. Proc. Natl Acad. Sci. USA 107, 16 800–16 805. ( 10.1073/pnas.1006008107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuzawa C, Quinn E. 2009. Developmental origins of adult function and health: evolutionary hypotheses. Annu. Rev. Anthropol. 38, 131–147. ( 10.1146/annurev-anthro-091908-164350) [DOI] [Google Scholar]
- 93.Wells JC. 2007. Flaws in the theory of predictive adaptive responses. Trends Endocrinol. Metab. 18, 331–337. ( 10.1016/j.tem.2007.07.006) [DOI] [PubMed] [Google Scholar]
- 94.Bateson P, Gluckman P, Hanson M. 2014. The biology of developmental plasticity and the predictive adaptive response hypothesis. J. Physiol. 592, 2357–2368. ( 10.1113/jphysiol.2014.271460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kramer MS, Kakuma R. 2002. Selected Cochrane systematic reviews. Birth 29, 141–142. ( 10.1046/j.1523-536X.2002.00175.x) [DOI] [Google Scholar]
- 96.Chung GC, Kuzawa CW. 2014. Intergenerational effects of early life nutrition: maternal leg length predicts offspring placental weight and birth weight among women in rural Luzon, Philippines. Am. J. Hum. Biol. 26, 652–659. ( 10.1002/ajhb.22579) [DOI] [PubMed] [Google Scholar]
- 97.Lockwood CA, Kimbel WH, Johanson DC. 2000. Temporal trends and metric variation in the mandibles and dentition of Australopithecus afarensis. J. Hum. Evol. 39, 23–55. ( 10.1006/jhev.2000.0401) [DOI] [PubMed] [Google Scholar]
- 98.Aiello LC, Wood B, Key C, Lewis M. 1999. Morphological and taxonomic affinities of the Olduvai ulna (OH 36). Am. J. Phys. Anthrop. 109, 89–110. ( 10.1002/(SICI)1096-8644(199905)109:1%3C89::AID-AJPA8%3E3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 99.Aiello LC, Collard M, Thackeray JF, Wood BA. 2000. Assessing exact randomization-based methods for determining the taxonomic significance of variability in the human fossil record. S. Afr. J. Sci. 96, 179–183. [Google Scholar]
- 100.Villmoare B. 2005. Metric and non-metric randomization methods, geographic variation, and the single-species hypothesis for Asian and African Homo erectus. J. Hum. Evol. 49, 680–701. ( 10.1016/j.jhevol.2005.07.005) [DOI] [PubMed] [Google Scholar]
- 101.Harvati K, Frost SR, McNulty KP. 2004. Neanderthal taxonomy reconsidered: implications of 3D primate models of intra- and interspecific differences. Proc. Natl Acad. Sci. USA 101, 1147–1152. ( 10.1073/pnas.0308085100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McNulty KP, Frost SR, Strait DS. 2006. Examining affinities of the Taung child by developmental simulation. J. Hum. Evol. 51, 274–296. ( 10.1016/j.jhevol.2006.04.005) [DOI] [PubMed] [Google Scholar]
- 103.Simpson GG. 1961. Principles of animal taxonomy. New York, NY: Columbia University Press. [DOI] [PubMed] [Google Scholar]
- 104.Howell FC. 1999. Paleo-demes, species, clades and extinctions in the Pleistocene hominid record. J. Anthropol. Res. 55, 191–243. ( 10.1086/jar.55.2.3631209) [DOI] [Google Scholar]
- 105.Lorenzo C, Carretero JM, Arsuaga JL, Gracia A, Martínez I. 1998. Intrapopulational body size variation and cranial capacity variation in middle pleistocene humans: the Sima de los Huesos sample (Sierra de Atapuerca, Spain). Am. J. Phys. Anthrop. 106, 19–33. ( 10.1002/(SICI)1096-8644(199805)106:1%3C19::AID-AJPA2%3E3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 106.Antón SC, Taboada HG, Middleton ER, Rainwater CW, Taylor AB, Turner TR, Turnquist JE, Weinstein KJ, Williams SA. 2016. Morphological variation in Homo erectus and the origins of developmental plasticity. Phil. Trans. R. Soc. B 371, 20150236 ( 10.1098/rstb.2015.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Howell FC. 1951. The place of Neanderthal man in human evolution. Am. J. Phys. Anthropol. 9, 379–416. ( 10.1002/ajpa.1330090402) [DOI] [PubMed] [Google Scholar]
- 108.Howell FC. 1952. Pleistocene glacial ecology and the evolution of ‘classic Neandertal’ man. Southwestern J. Anthropol. 8, 377–410. ( 10.1086/soutjanth.8.4.3628480) [DOI] [Google Scholar]
- 109.Antón SC, Snodgrass JJ. 2012. Origins and evolution of the genus Homo . Curr. Anthropol. 53(Suppl. 6), S479–S496. ( 10.1086/667692) [DOI] [Google Scholar]
- 110.Plavcan JM. 2012. Body size, size variation, and sexual size dimorphism in early Homo. Curr. Anthropol. 53(Suppl. 6), S409–S423. ( 10.1086/667605) [DOI] [Google Scholar]
- 111.Rightmire GP. 1981. Patterns of evolution in Homo erectus. Paleobiology 7, 241–246. ( 10.1017/S0094837300004012) [DOI] [Google Scholar]
- 112.Wolpoff MH. 1984. Evolution in Homo erectus: the question of stasis. Paleobiology 10, 389–406. ( 10.1017/S0094837300008411) [DOI] [Google Scholar]
- 113.Lahr MM. 1996. The evolution of modern human diversity: a study of cranial variation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 114.Leigh SR. 1992. Cranial capacity evolution in Homo erectus and early Homo sapiens. Am. J. Phys. Anthropol. 87, 1–13. ( 10.1002/ajpa.1330870102) [DOI] [PubMed] [Google Scholar]
- 115.Wood B, Wood C, Konigsberg L. 1994. Paranthropus boisei: an example of evolutionary stasis? Am. J. Phys. Anthropol. 95, 117–136. ( 10.1002/ajpa.1330950202) [DOI] [PubMed] [Google Scholar]
- 116.Porto A, de Oliveira FB, Shirai LT, de Conto V, Marroig G. 2009. The evolution of modularity in the mammalian skull I: morphological integration patterns and magnitudes. Evol. Biol. 36, 118–135. ( 10.1007/s11692-008-9038-3) [DOI] [Google Scholar]
- 117.Marroig G, Shirai LT, Porto A, de Oliveira FB, de Conto V. 2009. The evolution of modularity in the mammalian skull II: evolutionary consequences. Evol. Biol. 36, 136–148. ( 10.1007/s11692-009-9051-1) [DOI] [Google Scholar]
- 118.Leakey MG, Spoor F, Dean MC, Feibel CS, Antón SC, Kiarie C, Leakey LN. 2012. New fossils from Koobi Fora in northern Kenya confirm taxonomic diversity in early Homo. Nature 488, 201–204. ( 10.1038/nature11322) [DOI] [PubMed] [Google Scholar]
- 119.Grabowski M, Hatala KG, Jungers WL, Richmond BG. 2015. Body mass estimates of hominin fossils and the evolution of human body size. J. Hum. Evol. 85, 75–93. ( 10.1016/j.jhevol.2015.05.005) [DOI] [PubMed] [Google Scholar]
- 120.Antón SC. 2012. Early Homo: who, when, and where . Curr. Anthro 53(Suppl. 6), S278–S298. ( 10.1086/667695) [DOI] [Google Scholar]
- 121.Arriaza MC, Dominguez-Rodrigo M, Yravedra J, Baquedano E. 2016. Lions as bone accumulators? Paleontological and ecological implications of a modern bone assemblage from Olduvai Gorge. PLoS ONE 11, e0153797 ( 10.1371/journal.pone.0153797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hardy BL, Moncel M-H. 2011. Neanderthal use of fish, mammals, birds, starchy plants and wood 125–250,000 years ago. PLoS ONE 6, e23768 ( 10.1371/journal.pone.0023768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Archer W, Braun DR. 2013. Investigating the signature of aquatic resource use within Pleistocene hominin dietary adaptations. PLoS ONE 8, e69899 ( 10.1371/journal.pone.0069899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Henry AG, Ungar PS, Passey BH, Sponheimer M, Rossouw L, Bamford M, Sandberg P, de Ruiter DJ, Berger L. 2012. The diet of Australopithecus sediba. Nature 487, 90–93. ( 10.1038/nature11185) [DOI] [PubMed] [Google Scholar]
- 125.Garcia N, Feranec RS, Passey BH, Cerling T, Arsuaga JL. 2015. Exploring the potential of laser ablation carbon isotope analysis for examining ecology during the ontogeny of Middle Pleistocene hominins from Sima de los Huesos (Northern Spain). PLoS ONE 10, e142895 ( 10.1371/journal.pone.0142895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaatari ES, Grine FE, Ungar PS, Hublin J-J. 2016. Neandertal versus modern human dietary responses to climatic fluctuations. PLoS ONE 11, e153277 ( 10.1371/journal.pone.0153277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.King T, Humphrey LT, Hillson S. 2005. Linear enamel hypoplasias as indicators of systemic physiological stress: evidence from two known age-at-death and sex populations from postmedieval London. Am. J. Phys. Anthrop. 128, 547–559. ( 10.1002/ajpa.20232) [DOI] [PubMed] [Google Scholar]
- 128.Alfonso-Durruty MP. 2011. Experimental assessment of nutrition and bone growth's velocity effects on Harris lines formation. Am. J. Phys. Anthrop. 145, 169–180. ( 10.1002/ajpa.21480) [DOI] [PubMed] [Google Scholar]
- 129.Bromage TG, Juwayeyi YM, Katris JA, Gomez S, Ovsiy O, Goldstein J, Janal MN, Hu B, Schrenk F. 2016. The scaling of human osteocyte lacuna density with body size and metabolism. C. R. Palevol. 15, 32–39. ( 10.1016/j.crpv.2015.09.001) [DOI] [Google Scholar]
- 130.Bromage TG, Juwayeyi YM, Smolyar I, Hu B, Gomez S, Chisi J. 2011. Enamel calibrated lamellar bone reveals long period growth rate variability in humans. Cells Tissues Organs 194, 124–130. ( 10.1159/000324216) [DOI] [PubMed] [Google Scholar]
- 131.Sulloway FJ. 1982. The Beagle collections of Darwin's finches (Geospizinae). Brit. Mus. 43, 49–94. [Google Scholar]
- 132.Turner TR, Anapol F, Jolly CJ. 1997. Growth, development and sexual dimorphism in free ranging vervet monkeys in Kenya. Am. J. Phys. Anthropol. 103, 19–35. ( 10.1002/(SICI)1096-8644(199705)103:1%3C19::AID-AJPA3%3E3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 133.Fernandez-Duque E. 2011. Rensch's rule, Bergman's effect and adult sexual dimorphism in wild monogamous owl monkeys of Argentina. Am. J. Phys. Anthrop. 146, 38–48. ( 10.1002/ajpa.21541) [DOI] [PubMed] [Google Scholar]
- 134.Spoor F, Gunz P, Neubauer S, Stelzer S, Scott N, Kwekason A, Dean MC. 2015. Reconstructed Homo habilis type OH 7 suggests deep-rooted species diversity in early Homo. Nature 519, 83–86. ( 10.1038/nature14224) [DOI] [PubMed] [Google Scholar]
- 135.Spoor F, Leakey MG, Gathogo PN, Brown FH, Antón SC, McDougall I, Kiarie C, Manthi FK, Leakey LN. 2007. Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya. Nature 448, 688–691. ( 10.1038/nature05986) [DOI] [PubMed] [Google Scholar]
- 136.Antón SC, Potts R, Aiello LC. 2014. Evolution of early Homo: an integrated biological perspective. Science 345, 1236828 ( 10.1126/science.1236828) [DOI] [PubMed] [Google Scholar]
- 137.Holliday T. 2012. Body size, body shape, and the circumscription of the genus Homo. Curr. Anthropol. 53(Suppl. 6), S330–S345. ( 10.1086/667360) [DOI] [Google Scholar]
- 138.Leonard WR, Robertson ML. 1995. Energetic efficiency of human bipedality. Am. J. Phys. Anthropol. 97, 335–338. ( 10.1002/ajpa.1330970308) [DOI] [PubMed] [Google Scholar]
- 139.Shipman P, Walker A. 1989. The costs of becoming a predator. J. Hum. Evol. 18, 373–392. ( 10.1016/0047-2484(89)90037-7) [DOI] [Google Scholar]
- 140.Vekua A, et al. 2002. New skull of early Homo from Dmanisi, Georgia. Science 297, 85–89. ( 10.1126/science.1072953) [DOI] [PubMed] [Google Scholar]
- 141.Lordkipanidze D, de León MS, Margvelashvili A, Rak Y, Rightmire GP, Vekua A, Zollikofer CP. 2013. A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo. Science 342, 326–331. ( 10.1126/science.1238484) [DOI] [PubMed] [Google Scholar]
- 142.Jungers WL, Grabowski M, Hatala KG, Richmond BG. 2016. The evolution of body size and shape in the human career. Phil. Trans. R. Soc. B 371, 20150247 ( 10.1098/rstb.2015.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lieberman DE. 2007. Palaeoanthropology: homing in on early Homo. Nature. 449, 291–292. ( 10.1038/449291a) [DOI] [PubMed] [Google Scholar]
- 144.Schwartz JH, Tattersall I, Chi Z. 2014. Comment on ‘A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo’. Science 344, 360 ( 10.1126/science.1250056) [DOI] [PubMed] [Google Scholar]
- 145.Zollikofer CP, de Leon MP, Margvelashvili A, Rightmire GP, Lordkipanidze D. 2014. Response to Comment on ‘A complete skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo’. Science 360, 360 ( 10.1126/science.1250081) [DOI] [PubMed] [Google Scholar]
- 146.Baab KL. 2008. The taxonomic implications of cranial shape variation in Homo erectus. J. Hum. Evol. 54, 827–847. ( 10.1016/j.jhevol.2007.11.003) [DOI] [PubMed] [Google Scholar]
- 147.Grabowski M, Voje KL, Hansen TF. 2016. Evolutionary modeling and correcting for observation error support a 3/5 brain–body allometry for primates. J. Hum. Evol. 94, 106–116. ( 10.1016/j.jhevol.2016.03.001) [DOI] [PubMed] [Google Scholar]
- 148.Grabowski M. 2016. Bigger brains led to bigger bodies? The correlated evolution of human brain and body size. Curr. Anthropol. 57, 174–185. ( 10.1086/685655) [DOI] [Google Scholar]
- 149.DeSilva J. 2016. Comment on Grabowski, M. 2016. Bigger brains led to bigger bodies? The correlated evolution of human brain and body size. Curr. Anthro. 57, 187–188. [Google Scholar]
- 150.Gould SJ. 1977. Ontogeny and phylogeny. Cambridge, UK: Harvard University Press. [Google Scholar]
- 151.Antón SC, Leigh SR. 2003. Growth and life history in Homo erectus. In Patterns of growth and development in the genus Homo (eds Thompson J, Krovits G, Nelson A), pp. 219–245. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 152.Kuzawa CW, et al. 2014. Metabolic costs and evolutionary implications of human brain development. Proc. Natl Acad. Sci. USA 111, 13 010–13 015. ( 10.1073/pnas.1323099111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kruska DC. 2005. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication, and feralization. Brain Behav. Evol. 65, 73–108. ( 10.1159/000082979) [DOI] [PubMed] [Google Scholar]
- 154.Trut L, Dzerzhinsky FJ, Nikolsky VS. 1991. Intracranial allometry and morphological changes in silver foxes (Vulpes vulpes) under domestication. Genetika 27, 1605–1611. (In Russian.) [PubMed] [Google Scholar]
- 155.Ruff CB, Walker A. 1993. Body size and body shape. In The Nariokotome Homo erectus skeleton, pp. 234–265. Cambridge, MA: Harvard University Press. [Google Scholar]
- 156.McHenry HM. 1992. Body size and proportions in early hominids. Am. J. Phys. Anthropol. 87, 407–431. ( 10.1002/ajpa.1330870404) [DOI] [PubMed] [Google Scholar]
- 157.Brown P. Research Resources. See http://www.peterbrown-palaeoanthropology.net/resource.html .
- 158.Potts R.2017. Climate effects on human evolution figure. See http://humanorigins.si.edu/research/climate-and-human-evolution/climate-effects-human-evolution .
- 159.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]