Abstract
Context:
Cyavanaprāśa (CP) is an Ayurvedic immune booster formulation that confers vigor and vitality while delaying the ageing process. Benefits of CP have been studied widely in adult population.
Objectives:
Current study assessed beneficial effects of CP on health and immunity related parameters in healthy children.
Methods:
This study was a 6 month long two armed, randomized, open labeled, prospective clinical study. School going healthy children between ages of 5-12 years were randomized to receive orally daily either CP (approx. 6 g) followed by a cup of milk (100 – 200 ml) or cup of milk only twice a day while continuing with their normal/routine diet. Results were analyzed based on number of episodes, severity, duration of illness (infections and allergies) and number of absent days due to illness during the study duration and changes in levels of energy, physical fitness, strength, stamina and quality of life in children which were recorded in subject diary by their parents/Legally Acceptable Representative (LAR).
Results:
702 participants were randomized, out of which 627 completed the study (CP n = 313; Control n = 314). Results of immunity (episodes of infections or allergy related conditions) showed more than 2 times protection from immunity related illness in CP Group as compared to the control. CP also showed better percentage improvement in energy levels, physical fitness, strength, stamina and quality of life assessed through KIDSCREEN QOL-27 questionnaires in children.
Conclusion:
Regular consumption of CP for a period of six months could significantly improve immunity, energy levels, physical fitness, strength, stamina and quality of life in school going healthy children.
Study Registration:
Clinical Trail Registry of India vide CTRI/2015/02/005574, Dated 24 February 2015.
Keywords: Allergy, children, chyawanprash, immunity, infection, KIDSCREEN-27 questionnaire, strength, stamina
Introduction
Background
Children suffer from infections more than adults. On an average, a child suffers from four to eight respiratory infections per year.[1] This number varies depending on the presence or absence of risk factors that predispose to an increase in infective or allergic agents exposure; such as day-care attendance, school-aged siblings, and second-hand smoke etc.[2,3] Children with atopic disease seem more likely to develop recurrent and persistent upper respiratory infections probably due to enhanced adherence of micro-organisms to inflamed respiratory epithelium.[4] It is estimated that consumers purchase about 95 million packages of OTC medication for cough and cold for use in such situations in children each year.[5]
Cyavanaprāśa (CP) is an Ayurvedic health product that helps in boosting immunity. CP has been found to be effective as an immunity booster, vitalizer and a preventer of day to day infections and allergies such as common cold and cough etc.[6]
In recent times, CP has gained immense popularity all over the world as a time tested immunity booster. CP can be consumed in all seasons as it contains weather friendly ingredients which nullify unpleasant effects due to extreme environmental and climatic conditions.[7] Beneficial effects of CP have been observed in nasal allergies and viral infections and seasonal influences.[7,8,9] There exists enough evidence suggesting its multifaceted biological activity in favor of positive health. Till now CP has been studied in diverse population and there is scarcity of documented data regarding its use in children, specially, school going children.[9]
Objective
The current study was planned to study comprehensively the beneficial effects of CP in school going children for various immunity related health parameters.
Methods
Study design
Current study was a 6 month long two arm, randomized, open labeled, prospective, clinical study conducted between Mar 2015 to Feb 2016 at two school sites in Maharashtra, India viz. Sane Guruji Vidya Mandir, Hadapsar and Shishuvihar pre-primary and primary school, Akrudi that were attached to Sumatibhai Shah Ayurvedic Hospital, Hadapsar and PDEA's College of Ayurveda and Research Center, Akrudi, respectively. The study was conducted in compliance with applicable ethical guidelines. Ethics committee approvals were obtained before study initiation. The Study was registered with the Clinical Trail Registry of India vide CTRI/2015/02/005574, Dated 24 Feb 2015. The CONSORT statement guidelines have been followed in reporting the outcomes of the study.[10]
Study participants
School going children of either sex were screened for eligibility (Day 3) and were recruited if they fulfilled the inclusion and exclusion criteria. Written informed consent was taken from parents/Legally Acceptable Representatives (LAR) along with participant assent where applicable.
Inclusion criteria
Healthy children of either sex aged between 5-12 years, having no history of allergic conditions to CP like products or any ingredient of CP and who were not averse to consuming CP for the study duration were included.
Exclusion criteria
(i) Children with presence of any clinically significant medical or surgical condition requiring long-term treatment; (ii) subjects participating/having participated in another clinical study within 3 months before recruitment; (iii) evidence of significant uncontrolled concomitant disease which in the investigator's opinion would preclude patient participation and (iv) immunologically compromised children were excluded.
Study interventions
Cyavanaprāśa is a classical Ayurvedic formulation comprising ingredients such as Pāṭalā (Stereospermum suaveolens, St. Bk.), Agnimantha (Clerodendrum phlomidis, St. Bk.), Gambhārī (Gmelina arborea, St. Bk.), Bilva (Aegle marmelos, St. Bk.), Syonāka (Oroxylum indicum, St. Bk.), Gokṣura (Tribulus terrestris, Fr.), Śālaparṇī (Desmodium gangeticum, Pl.), Prśṇiparṇī (Uraria picta, Pl.), Bṛhatī (Solanum indicum, Pl.), Kaṇṭakārī (Solanum surattense, Pl.), Pippalī (Piper longum, Fr.), Karkaṭaśṛṅgī (Pistacia integerrima, Gl.), Drākṣā (Vitis vinifera, Fr.), Guḍūcī (Tinospora cordifolia, St.), Harītakī (Terminalia chebula, P.), Balā (Sida cordifolia, Rt.), Tāmalakī (Phyllanthus niruri, Pl.), Vasā (Adhatoda vasica, Lf.), Jīvantī (Leptadenia reticulata, Pl.), Shaṭī (Heydichum spicatum, Rz.), Mustā (Cyperus rotundus, Rz.), Puṣkara (Inula racemosa, Rt.), Kākanāsikā (Martynia annua, Ft.), Mudgaparṇī (Phaseolus trilobus, Pl.), Māṣaparṇī (Teramnus labialis, Pl.), Punarnavā (Boerhavia diffusa, Rt.), Utpala (Nymphaea stellata, Fl.), Sūkṣmaila (Elettaria cardamomum, Sd., Ft. Tvak (Cinnamomum zeylanicum, Bk.), Candana Śveta (Santalum album, Ht. wd.), Vārāhī (Dioscorea bulbifera, Rt. Tr.), Śatāvarī (Asparagus racemosus, Rt.), Aśvagandhā (Withania somnifera, Rt.), Vidārī (Pueraria tuberosa, Rt. Tr.), Āmalakī (Emblica officinalis, Fr.), Ghṛta (Clarified butter), Tila taila (Sesamum indicum, oil), Śarkara (Crystal sugar), Madhu (Honey), Pippalī (Piper longum, Fr.), Vamśa (Bambusa bambos, S.C.), Tvakpatra (Cinnamomum tamala, lf.), Nāgakesara (Mesua ferrea, Stmn.), Abhraka Bhasma (Calcined mica), Muktā Piṣṭi (Anhydrous paste of pearl), Akarakarabha (Anacyclus pyrethrum, Rt.), Lavaṅga (Syzygium aromaticum, Fl.Bd.), Kuṅkuma (Crocus sativus, sty./stg.). In the current study, Cyavanaprāśa (Mfd: Dabur India Ltd.) manufactured following traditional methods[11] was used. The proximate analysis of CP showed Moisture content- 18.0—23.0% w/w, Total Fat- 1.5—2.5% w/w, Protein- Not detected, Dietary Fibre- 2.0—3.0% w/w, Total Ash- 3.0—5.0% w/w, Total Carbohydrate- 70.0—80.0% w/w.
Study procedure
At screening/baseline visit, a parent- teacher meeting was conducted to brief parents/LAR about study requirements and their written informed consent was obtained after one to one session with them. Assent for participation in the study was also obtained from participant, if applicable. Participants’ physical and systemic examinations were done and vitals were recorded. Parents/LAR were handed over a subject diary and were trained to record episodes of illness (number of episodes, severity and duration of infection/allergies for assessment of immunity) and the KIDSCREEN QOL-27 Health Questionnaire in it.
Recruited children were assigned to either CP group or Milk Group as per computer generated randomization list in 1:1 ratio. Children in CP Group were provided 500 gm jar of CP and parents/LAR were asked to give their child half a teaspoon (approx. 6 gm) of CP orally two times followed by a cup of milk daily. Parents in Milk group were asked to give their child a cup of milk only twice daily. The cup of milk was considered to be between 100-200 ml. Both the interventions were prescribed for a period for 6 months; during which, participants in both the groups were advised to continue with their normal/routine diet. A record of the children who were absent during the treatment period was observed and the reason for the same was recorded.
Follow up and end of study assessment
Participants were followed up for 6 months from baseline on Day 30, 60, 90, 120, 150 and 180 (±7 d). Last participant follow up was observed in the month of Feb 2016. At each follow-up visit, filled in questionnaires/scales were collected, study drug compliance was checked and CP supply was replenished as applicable. If 80% study medication was consumed over 80% time, the participant was considered compliant. Parents/LAR were enquired for any adverse event (s). At the end of the study, Parents/LAR were asked to stop giving CP and take the advice of investigator for further treatment, if any.
Outcomes
The primary outcome of the study was the assessment of immunity through episodes, severity and duration of immunity related illness (infections or allergies) in both study groups based on the subject diary.
The secondary outcomes of the study were assessment of immunity through number of children having episode of immunity related illness; number of absent days and number of children absent from school due infection or allergy; children's Quality of life was evaluated using KIDSCREEN QOL-27 Health Questionnaires for Children. Their energy levels, physical fitness, strength and stamina; physician's global evaluation for overall improvement on CGI-I Scale; incidence of adverse events, serious adverse events and vitals during the study period; overall safety and tolerability by the physician and participant/parent was evaluated at the end of the study. Prakṛti evaluation was also conducted at the screening/baseline visit.
Levels of Energy, physical strength, stamina and, the physical fitness were measured on a 7-point scale ranging from +3 to -3 [-3 (very dissatisfied), -2 (somewhat dissatisfied), -1 (a little dissatisfied), 0 (neutral), +1 (a little satisfied), +2 (somewhat satisfied), and +3 (very satisfied)].
KIDSCREEN QOL-27 Health Questionnaires were scored based on the scale ranging from 1 to 5 (1 - Excellent, 2 - Very Good, 3 - Good, 4 -Fair, 5- Poor; 1 - Not at all, 2 - Slightly, 3 - Moderately, 4 - Very, 5 - Extremely) depending on the response of the questionnaire).
Statistical analysis
The primary population for this study was Per-Protocol population. With more than 300 participants in each group, the sample size was considered sufficient for analysis. The primary efficacy endpoints were analyzed using two proportion test. A 95% confidence interval was constructed for the proportion. All secondary outcomes were analyzed by applying appropriate statistical methods such as proportion test, t-test etc.
Results and Observations
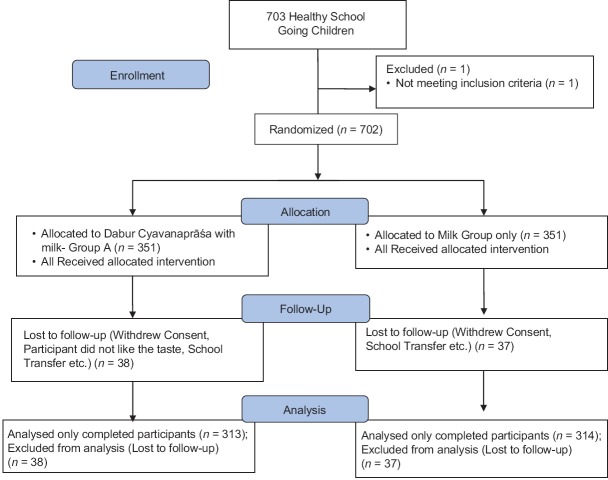
A total of 703 school going children were screened in the study of which one was a screen failure and 702 were randomized. Of the 702 participants, there were 75 dropouts while 627 participants completed the study. Drop out reasons included (i) withdrawal of consent, (ii) Investigational Product (IP) taste dislike, (iii) lost to follow-up, (iv) change of school due to shifting of home to a different place or job transfer of parent to a far away place from current location etc., None of the drop-outs was due to adverse event.
Out of the participants who completed the study, 313 participants were in CP Group and 314 were in Control Group. There were a total of 325 males (51.83%) and 302 females (48.16%). Of the 325 males, 161 (51.43%) were in CP group while 164 (52.22%) were in the control group while out of the 302 females, 152 (48.56%) were in the CP group while 150 (47.77%) were in the control group. Only those participants who completed the total study duration were included in the analysis. The average age of participants in the CP group was 7.31 ± 1.75 while in the control group it was 7.36 ± 1.80. The participant flow chart for the study is shown in Figure 1.
Figure 1.
Patient enrollment flow chart: CONSORT 2010 flow diagram
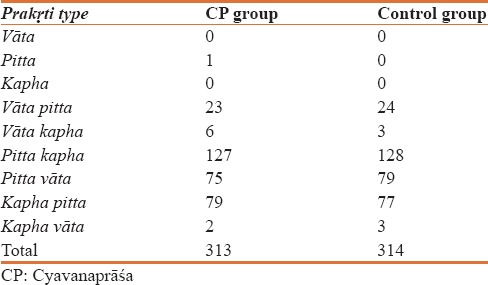
The Prakṛti wise distribution of participants is shown in Table 1. It was observed that a majority of participants in both the groups were in the category of pitta-kapha, pitta-vāta and kapha-pitta prakṛti. There were 281 participants (89.77%) belonging to these phenotypes in CP group while in the control group; there were 284 participants (90.44%) of these phenotypes.
Table 1.
Prakṛti wise distribution of participants in the two groups
Efficacy parameters
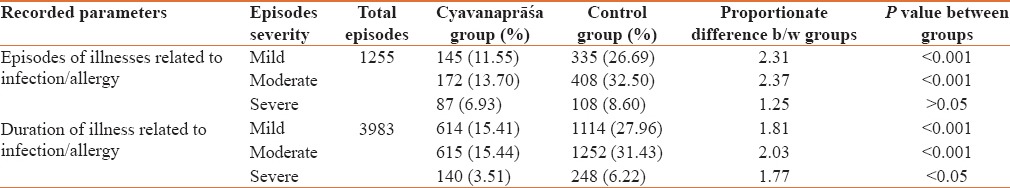
Primary and secondary outcomes of current study were observed in term of episodes of infection or allergy which were further divided into various categories [Table 2].
Table 2.
Infection/allergy related disorders observed in the study in the two groups

Assessment of immunity related parameters
Assessment of episodes of infection or allergy
A total of 1255 episodes related to infection or allergy were recorded in the study. Of these, 404 episodes (32.19%) were in CP group while 851 (67.80%) were in control group, which was assessed to be statistically significant (P < 0.001) and the proportionate difference was more than 2 times (2.10) when compared between the groups [Figure 2].
Figure 2.
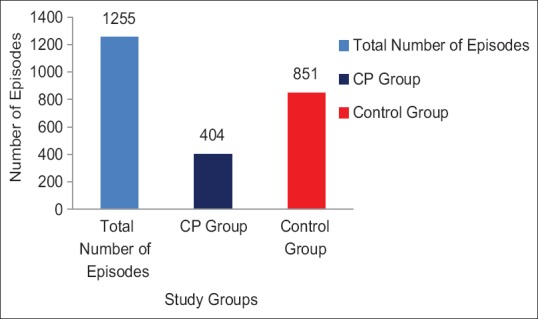
Number of episodes of illness related to infection/allergy
Evaluation of total duration of and severity of illnesses
Evaluation of total duration of illnesses related to infection and allergy showed that of the total duration of 3983 days of illness, there were 1369 (34.37%) illness days in CP group whereas it was 2614 (65.62%) days in the control group. This difference was observed statistically significant (P < 0.01) and the proportionate difference was about 2 times (1.91) [Figure 3]. A proportionate difference of 1.25 to 2.37 fold were observed between CP and control group while assessing episodes and duration of illness related to infection or allergy in terms of severity [Table 3].
Figure 3.
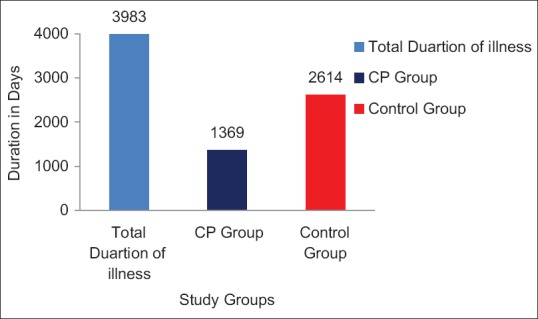
Duration of illness related to infection/allergy
Table 3.
Assessment of episodes and duration of illness related to infection/allergy based on severity
Assessment of number of participants having episodes of infection/allergy
A total of 540 participants had episodes of infection/allergy related to immunity. Of these, 254 participants (47.03%) participants were in CP group while 286 (52.96%) were in the control group. The difference observed was statistically not significant (P > 0.05). 87 participants did not have any episode related to infection/allergy. Of these, 60 participants (68.96%) were in CP group while 27 (31.03%) participants were control group. The difference was statistically significant (P < 0.01).
Absenteeism due to infection or allergy
Numbers of absent days: There were a total of 702 absent days in 627 participants. Of these, 215 days (30.62%) were in CP group while 487 (69.37%) were in the control group. This difference was observed to be statistically significant (P < 0.001) [Figure 4]. There were a total of 281 participants who were absent due to infection/allergy/immunity related illnesses. Of these, 93 participants (33.09%) were in CP group while 188 (66.90%) were in the control group. This difference was found to be statistically significant (P < 0.001) [Figure 5].
Figure 4.

Assessment of number of absent days due to infection or allergy
Figure 5.
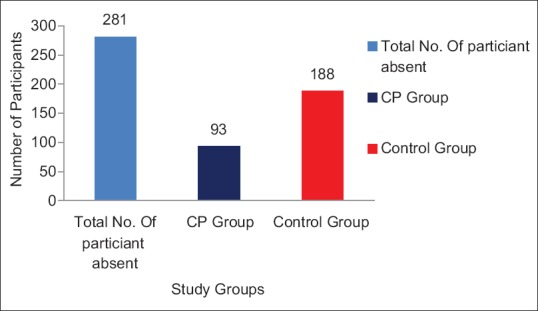
Number of participants absent due to infection/allergy related illnesses
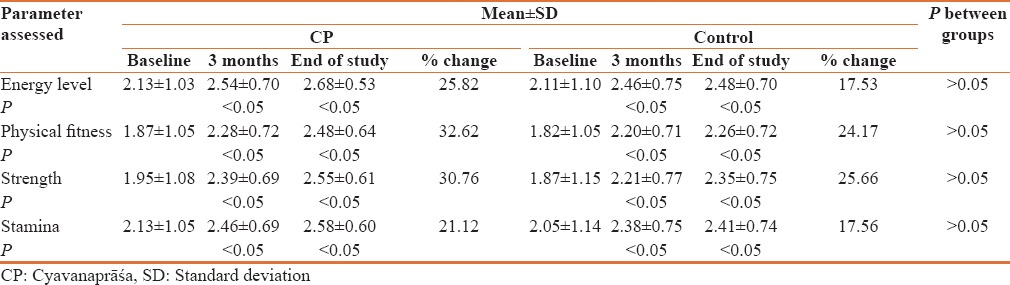
Assessment of energy, physical fitness, strength and stamina
Though no significant difference was observed between the groups at 3 and 6 months follow up, CP group showed a better improvement in energy levels, physical fitness, strength and stamina as compared to control group [Table 4].
Table 4.
Assessment of energy, physical fitness, strength and stamina
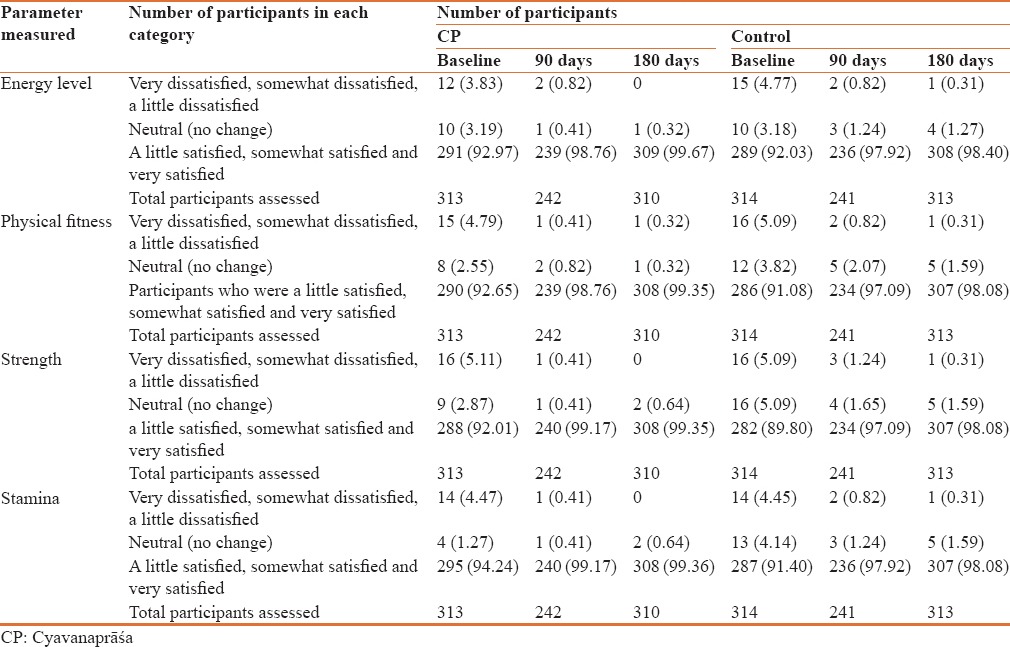
In CP group, there were 12 participants who were dissatisfied (Very dissatisfied, somewhat dissatisfied, A little dissatisfied) with their energy level at the baseline visit, which was reduced to two participants at the end of three months. At the end of six months, there was no participant who was dissatisfied with energy levels in CP Group. In the control group, however, there was one participant left dissatisfied out of 15 at baseline at the end of six months.
15 participants were dissatisfied with their physical fitness at the baseline visit in CP group; this was reduced to one participant at the end of three and six months. However, in control group one participant out of 16 at baseline was dissatisfied with his physical fitness participants at the end of 6 months.
In CP group, 16 participants were dissatisfied with their strength at the baseline visit, which was reduced to one participant at the end of 3 months and none at the end of 6 months. In control group, however, one participant was left in dissatisfied out of 16 participants at baseline at the end of 6 months in control group.
There were 14 participants who were dissatisfied with their stamina at the baseline visit in CP group, which was reduced to one participant at the end of 3 months and none at the end of 6 months. In control group, there was no dissatisfied participant out of 14 at baseline at the end of 6 months [Table 5].
Table 5.
Assessment of number of participants showing change in energy level, physical fitness, strength and stamina
Assessment of quality of life through KIDSCREEN-27 Questionnaire
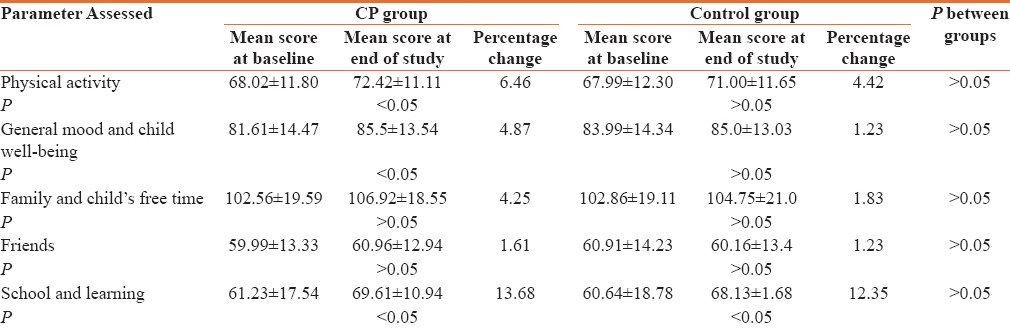
Assessment of Quality of life through KIDSCREEN-27 Questionnaire over a period of 6 months showed an improvement in the domain score of “Physical Activity and Health” and “General Mood and Child's Feeling” in both the groups at the end of 6 months of the study [Table 6]. As per one previously conducted survey on children, KIDSCREEN QOL-27 health questionnaire has been found to be a valid tool for accessing child's quality of life.[12]
Table 6.
Assessment of change in quality of life through KIDSCREEN-27 questionnaire
Assessment of participants on CGI-I scale
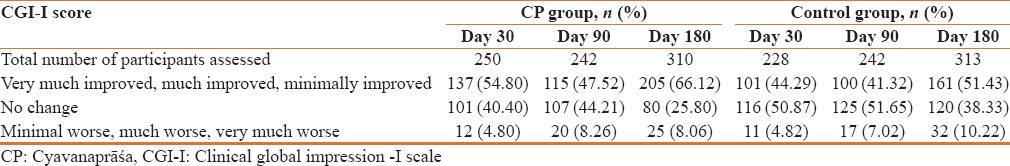
Assessment of participants on CGI-I scale showed that a higher percentage of participants (66.12%) showed improvement on this scale in CP Group as compared to control group (51.43%) [Table 7].
Table 7.
Physician's global evaluation for overall improvement on CGI-I scale
Assessment of Safety
Assessment of overall safety and tolerability as per physician
Assessment of overall safety by the physician showed that of the total 313 participants who were in the CP group, 92.45% showed excellent safety while the remaining 7.55% participants showed good or fair overall safety [Table 8].
Table 8.
Assessment of overall safety and tolerability of cyavanaprāśa as per physician

Assessment of overall safety through adverse events monitoring
Adverse events which were not related to infection or allergy were recorded separately as AE in both the groups. A total of 248 participants were reported to have AE of which 133 were in CP group and 115 in the Control group. A total of 599 AE were recorded in the study, 316 in CP group and 283 in Control Group. These AE were, however, not found to be related to the study drugs i.e. there were no Adverse Drug Reaction in any participant. Fourteen events were assessed and reported as SAE, of which 6 were in CP group and 8 were from Control Group. None of the SAE's was related to the study drug or procedure. Assessment of vitals such as pulse, respiration and body temperature did not show any significant difference either within or between the groups. The study drug was well tolerated by the participant.
Discussion
In recent times, CP has gained immense popularity all over the world as a time-tested immunity booster. Various pre-clinical[13,14,15,16,17] and clinical studies have been conducted to evaluate its safety and efficacy. Efficacy of CP in term of anti-allergic property, activity of Natural Killer (NK) cells and phagocytic activity, level of Tumor Necrosis Factor- alpha (TNF-a), IL- 1-b and MIP-1- a secreted by Dendritic Cells (DC,[14] improvement of quality of life in patient with recurrent cough and cold and health benefits under seasonal variations has been established.[8,9,18,19,20]
The benefits of CP have been validated in adults. In the current study, recruited children were randomized into two groups in which male and females were almost equally divided.
Demography parameters analysis showed that age and gender were equally represented in both groups. The Prakṛti classification of the majority of participants enrolled in the study were Pitta-Kapha, Pitta-Vāta and Kapha-Pitta which were almost equally distributed in CP and control group.
In the current study, CP significantly improved the immunity of school going children. The study was conducted from the month of Mar 2015 to Feb 2016 which covered one summer, one rainy and one winter season. It showed that the children were protected from various illnesses related to infection or allergy under the different seasonal variations though out the year. These findings are also in consensus with earlier published literature which demonstrated that CP provides protection from various illnesses irrespective of seasonal influence.[18,19,20]
Out of a total of 1255 episodes related to immunity related infections or allergies, control group had 2.10 times more number of episodes in comparison to CP group which was statistically significant (P < 0.01). Out of the total duration 3983 days of all episodes, the duration of such illness days was 1.91 times lesser in the CP group as compared to control group and was found to be statistically significant (P < 0.01). 540 participants were found to be affected by various illnesses related to infection or allergy and 87 participants did not have any such episode. The number of children having no immunity related illnesses was 2.22 times higher in the CP group as compared to control group which was found to be statistically significant (P < 0.01). Out of the total absent days from school, 2.27 times higher number of days were observed in the control group in comparison to CP group and found to be statistically significant (P < 0.001) as well. Out of the total participants who were absent from school due to such illness, 2.02 times more were observed in the control group and was found to be statistically significant (P < 0.001).
These above mentioned effects are most likely related to the immune boosting potential of CP. The mechanism of these benefits of CP can be explained with the help of previously published literature and studies conducted on CP. CP has demonstrated the in vitro immune boosting potential by various mechanisms. In the concentration range of 20 μg/ml – 500 μg/ml, CP was found to increase the activity of NK cells by 1.8 to 10.3 fold, levels of TNF-α, IL- 1-β and MIP-1- α secreted by DCs were found to be increased by 1.3 to 3.2 fold and phagocytic activity was found to be increased by 28.9% to 65.2%.[13,14] In two separate in-vivo studies, the level of IgE and plasma histamine were observed to be almost equal to the vehicle when challenged with allergens in animals (mice and rats respectively) pretreated with CP.[21] These two studies are suggestive of suppression of allergic response in response to allergens implying protection against various infections.
Studies on CP have indicated that it contains a number of phenolics which may account for its therapeutic activity.[22] CP also contains honey which works as ‘a carrier of herbs’, or Yogavāhī, thereby potentiates absorption of various herbs deep into the tissues and imparts youth, charm, vigour and longevity.[7] In the current study as well, the subject diaries were evaluated to assess the change in energy, physical fitness, strength and stamina. Under all of these parameters, fair amounts of improvement were observed at end of three months that continued until the end of the study in both groups. The improvement was higher in CP group in comparison to control. However, comparison within and between the groups was not statistically significant. Assessment of above mentioned parameters in terms of number of children revealed that in CP group all participants except one, moved from the category of dissatisfied to satisfied at the end of the study in comparison to control where 4 participants were still in category of dissatisfied at the end of study.
Assessment of Quality of life through KIDSCREEN-27 Questionnaires showed improvement in the domain score of “Physical Activity and Health” and “General Mood and Child's Feeling” in both the groups at the end of 6 months of the study. The domain score of “Physical Activity and Health” in CP as well as in control group improved from baseline to the end of the study and was found to be significant. The improvement in this domain was however found to be higher in CP group however; there was no significant difference between the two groups. Similarly the “general mood and child's feeling” showed a better improvement in CP group as compared to control group over 6 months period. The domain score of ‘School & learning’ showed a significant improvement in both the groups from one month further to 3 months and 6 months. The other domains of ‘Family and child's free time and Friends’ did not show any significant change both within the group and between the two groups.
Assessment of participants on CGI-I scale showed that a higher percentage of participants showed improvement on this scale in CP group as compared to control group. Assessment of overall safety by the physician showed that of the total 313 participants in CP group, 92.45% of the participants showed excellent safety while the remaining 7.55% participants showed good or fair overall safety. All reported AEs and SAEs were found to be not related to the study drugs. Assessment of vitals did not show any significant finding.
As per earlier published literature as well, CP was found to be safe in the recommended dosage for the defined period of time in toxicity studies. In all the toxicity studies i.e. acute dose oral toxicity, 28 days repeated dose oral toxicity studies and in the 90 days repeated dose oral toxicity studies, CP did not cause any signs of toxicity, body weight loss with all animals surviving throughout the study periods. In acute oral dose toxicity, the LD50 value of CP in both sexes of rats and mice was found to be greater than 2000 mg/kg of the body weight. The no observed adverse effect level (NOAEL) of CP was found to be 1000 mg/Kg body weight in both 28 day and 90 day toxicity studies.[13]
The results of the above study on immunity related benefits of CP and safety parameters appear to be in line with the earlier studies and published data on the same.
Conclusion
The present study concluded that regular consumption of CP for a period of three to six months helps to improve immunity, energy levels, physical fitness, strength, stamina and quality of life of children as assessed by various parameters. The primary parameter of immunity (episodes of infections or allergy related conditions) showed more than 2 times protection from illness in CP group as compared to the control group. The duration of illness related to infection or allergy was also observed to be significantly lesser in CP group as compared to control group. Other parameters of assessment of immunity such as number of absent days and number of absent participants in school due to these illnesses, also confirmed that CP showed more than twice the protection from immunity related illness as compared to the control group. There was a significant change in energy levels, physical fitness levels, strength and stamina in both the groups, however, CP showed better percentage improvement on these parameters as compared to the control group. Assessment of quality of life by KIDSCREEN-27 Questionnaire showed better improvement in CP group as compared to control group on domains of “Physical activity and Health”, “General Mood and Child's Feelings” and “School and learning.” Assessment of overall improvement by the physician (CGI-I) also showed that over a period of 6 months more number of participants improved in CP group as compared to control group. CP showed excellent to good safety in all the participants in the study. No Adverse events/Serious Adverse events reported was assessed to be related to study product or procedure.
Financial support and sponsorship
The study was sponsored by Dabur Research and Development Centre, Dabur India Limited, Sahibabad (Ghaziabad) Uttar Pradesh, India.
Conflicts of interest
Some of the Authors (Arun Gupta, Sunil Kumar and V. Sasibhushan) are currently employed with Dabur India Ltd.
References
- 1.Grüber C, Keil T, Kulig M, Roll S, Wahn U, Wahn V MAS-Study Group. History of respiratory infections in the first 12 yr among children from a birth cohort. Pediatr Allergy Immunol. 2008;19:505–12. doi: 10.1111/j.1399-3038.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 2.Alkhater SA. Approach to the child with recurrent infections. J Family Community Med. 2009;16:77–82. [PMC free article] [PubMed] [Google Scholar]
- 3.The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Centers for Disease Control and Prevention (US) 2006. [Last accessed on 2016 Dec 23]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44318/ [PubMed]
- 4.Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: Effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156:986–91. doi: 10.1001/archpedi.156.10.986. [DOI] [PubMed] [Google Scholar]
- 5.Smith SM, Henman M, Schroeder K, Fahey T. Over-the-counter cough medicines in children: Neither safe or efficacious? Br J Gen Pract. 2008;58:757–8. doi: 10.3399/bjgp08X342642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sastry K, Chaturvedi G, editors. Charak Samhita of Angnivesa. Chikitsasthana: Divitiya Rasayana Prakran Adhyay. Ch. 2, Ver. 70-74. Vol. 2. Varanasi, India: Chaukhambha Bharati Academy; Reprint 2011. pp. 16–7. [Google Scholar]
- 7.Parle M, Bansal N. Traditional medicinal formulation, Chyawanprash – A review. Indian J Tradit Knowl. 2006;5:484–8. [Google Scholar]
- 8.Bode M. Assembling cyavanaprash, Ayurveda's best-selling medicine. Anthropol Med. 2015;22:23–33. doi: 10.1080/13648470.2015.1005285. [DOI] [PubMed] [Google Scholar]
- 9.Narayana DB, Durg S, Manohar PR, Mahapatra A, Aramya AR. Chyawanprash: A review of therapeutic benefits as in authoritative texts and documented clinical literature. J Ethnopharmacol. 2017;197:52–60. doi: 10.1016/j.jep.2016.07.078. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Schulz KF, Altman DG. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized studys. Ann Intern Med. 2001;134:657–62. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 11.Rasa Tantra Sara Siddha Prayog Sangraha. Pratham Khanda. Krishan-Gopal Ayurveda Bhawan. 9th ed. Ajmer: Kaleda-Krishangopal; 1961. pp. 779–83. [Google Scholar]
- 12.Ravens-Sieberer U, Herdman M, Devine J, Otto C, Bullinger M, Rose M, et al. The European KIDSCREEN approach to measure quality of life and well-being in children: Development, current application, and future advances. Qual Life Res. 2014;23:791–803. doi: 10.1007/s11136-013-0428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sastry JLN, Gupta A, Brindavanam NB, Kanjilal S, Kumar S, et al. Quantification of immunity status of Dabur Chyawanprash – A review part-1 (Experimental Studies) Indian J Appl Res. 2014;4:20–4. [Google Scholar]
- 14.Madaan A, Kanjilal S, Gupta A, Sastry JL, Verma R, Singh AT, et al. Evaluation of immunostimulatory activity of Chyawanprash using in vitro assays. Indian J Exp Biol. 2015;53:158–63. [PubMed] [Google Scholar]
- 15.Parle M, Bansal N. Antiamnesic activity of an ayurvedic formulation chyawanprash in mice. Evid Based Complement Alternat Med 2011. 2011:898593. doi: 10.1093/ecam/neq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bansal N, Parle M. Beneficial effect of chyawanprash on cognitive function in aged mice. Pharm Biol. 2011;49:2–8. doi: 10.3109/13880209.2010.489904. [DOI] [PubMed] [Google Scholar]
- 17.Manjunatha S, Jaryal AK, Bijlani RL, Sachdeva U, Gupta SK. Effect of Chyawanprash and Vitamin C on glucose tolerance and lipoprotein profile. Indian J Physiol Pharmacol. 2001;45:71–9. [PubMed] [Google Scholar]
- 18.Sastry JL, Gupta A, Brindavanam B, et al. Quantification of immunity status of Dabur Chyawanprash – A review part-2 (Clinical Studies) Indian J Appl Res. 2014;4:205–11. [Google Scholar]
- 19.Uma A, Pajanivel R, Raj S, Lokeshmaran Smoking-induced satellite associations in a rural population of south India: An in vitro study. Int J Appl Basic Med Res. 2011;1:75–9. doi: 10.4103/2229-516X.91148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar SS, Archana R, Mishra S, Symphoria, Mukkadan JK. Effect of Chyawanprash on cognitive, autonomic and respiratory parameters in college students. Int J Res Ayurveda Pharm. 2014;5:435–8. [Google Scholar]
- 21.Singh P, Bhat TA, Madaan A. New Delhi: Althea Lifesciences Limited; 2010. Determination of anti-allergic potential of DRDC/AY/8044 (Dabur Chyawanprash) by estimation of levels of histamine in plasma of Wistar rats. Data on files; pp. 1–27. [Google Scholar]
- 22.Govindarajan R, Singh DP, Rawat AK. High-performance liquid chromatographic method for the quantification of phenolics in 'Chyavanprash'a potent Ayurvedic drug. J Pharm Biomed Anal. 2007;43:527–32. doi: 10.1016/j.jpba.2006.08.005. [DOI] [PubMed] [Google Scholar]