Abstract
Background:
Helleborus niger L (Ranunculaceae) is used Ayurvedic and Unani systems and other herbal medicine systems. The roots of H. niger have a good medicinal value.
Aims:
To conduct a pharmacognostical and phytochemical study of H. niger.
Materials and Methods:
The pharmacognostical studies on roots including parameters such as taxonomical, macroscopic, microscopic characters, physico-chemical, ultra-violet analysis and phytochemical studies are established.
Results:
Macroscopically, the roots are brownish-black in colour, cylindrical in shape, feeble odour, slightly acrid taste with irregularly branched. Microscopically the root showed the presence of epidermis, air-chambers, fissure periderm, periderm, inner cortex, pith, phloem, xylem, vessels and xylem vessels. Microscopic examination of the powder showed the presence of parenchyma cells, parenchyma mass, periderm, cell inclusion, laticifer, lateral wall pith, perforation, xylem bundle and xylem elements. Ultra-violet and ordinary light analyses with different reagents were conducted to identify the drug in powder form. Physico-chemical evaluation established, Ash values - Total, acid insoluble, water soluble and sulphated ash values were 7.3%, 4.1%, 3.7% and 5.2%, respectively. Extractive values - Alcohol soluble, water soluble and ether soluble extractive values were 22.8%, 7.4% and 5.6%, respectively. Loss on drying was 3.3%. Preliminary phytochemical screening showed the presence of carbohydrate, glycoside, saponins, flavonoid, phytosterols, tannins and phenolic compounds.
Conclusions:
The results of the study can serve as a valuable resource of pharmacognostic and phytochemical information. This will serve as appropriate, standards for discovery of this plant material in future investigations and applications and also contribute towards establishing pharmacopoeial standards.
Keywords: Helleborus niger, macroscopy, microscopy, physicochemical, phytochemical, ultra-violet analysis
Introduction
Hellebores niger L (Ranunculaceae) commonly known as Kadagaruganie in Tamil. It is a small, perennial herb about 30 cm in height.[1] The plant is native to Central and Southern Europe, not reaching British or North Germany, but extending eastward to South Poland, and Westward to Dauphiny and Provence.[2] Its dried roots and rhizomes are used in diverse ailments in traditional and folklore remedies. The Black hellebore contains three crystalline cardiac glycoside (Hellborin, hellebrin and hellebrin). These substances have an action similar to that of the glycosides found in common Foxglove (Digitalis purpurea). The drug occasionally employed in veterinary practice, but is obsolete in ordinary medicine. The plants also contains aconitic acid and kaempferol glycosides.[3,4] The roots and rhizomes contain hellebrin, desgluco-hellebrin, hellebrigenin, bufatetraeno-lide, beta-ecdysterone and 5-beta-hy-droxyecdysterone.[5]
Traditionally, the H. niger root is used as hydragogue cathartic, anthelmintic; in epilepsy, dropsy, hypochondriosis, mania melancholia, chronic skin affections and worms. The powdered roots are given in dyspepsia and as a purgative.[6,7] An Unani drug quantity sufficient (QS), containing H. niger and Anacyclus pyrethrum De Candolle (3:1), reduces the cholesterol level and the dose of insulin in diabetic patients. The plant extract, an anti-inflammatory agent, induces inhibition of the enzyme in the androsterone oxidation and 5- andro-stane -17 β-ol-3-one reduction reaction in rat liver in vitro.[1] Literature survey did not provide sufficient information about pharmacognostical and phytochemical studies of this plant. The current work aims to contribute in solving the problems of controversial drugs prevalent in Ayurveda beside helping in laying down pharmacopoeial standards. Therefore, the present investigation was conducted with the objective of evaluating various parameters such as macroscopic, microscopic characters and phytochemical evaluation of the plant.
Materials and Methods
Collection and authentication
H. niger (HN) plant was obtained from a local traditional healer in Erode, Tamil Nadu (India). The plant was identified by Prof. P. Jayaraman and a voucher specimen (Ref. No: PARC/2012/2177) was deposited in the pharmacognosy department herbarium, JKKMMRF's - Annai JKK Sampoorani Ammal College of Pharmacy, - B. Komarapalayam, Tamil Nadu. Taxonomic description, regional language names, habit and habitat of the plant and botanical descriptions were noted from the available literatures.
Macroscopic studies
Organoleptic characters
In organoleptic evaluation, appropriate parameters like taste, odor, size, shape and color of the roots were studied.[1]
Morphological characters
Morphological investigations of the plant roots were conducted.[1,2]
Microscopic studies
Treatment of roots
Care was taken to select healthy plant and normal parts. The required samples of different parts were cut and removed from the plant and fixed in FAA (Formalin-5 ml + Acetic acid-5 ml + 70% Ethyl alcohol-90 ml). After 24 hrs of fixing, the specimens were dehydrated with graded series of tertiary-butyl alcohol (TBA) as per the schedule.[8] Infiltration of the specimens was carried by gradual addition of paraffin wax (melting point 58-60°C) until TBA solution attained super saturation. The specimens were cast into paraffin blocks.
Sectioning
The paraffin embedded specimens were sectioned with the help of rotary microtome. The thickness of the sections were 10-12 μm. dewaxing of the sections was done by customary procedure.[9] The sections were stained with toluidine blue as per the methods.[10] Since toluidine blue is a polychromatic stain. The staining results were remarkably good; and some cytochemical reactions were also obtained. The dye rendered pink colour to the cellulose walls, blue to the lignified cells, dark green to suberin, violet to the mucilage, blue to the protein bodies, etc., Wherever necessary, sections were also stained with safranin and Fast-green and IKI (for starch). Glycerin mounted temporary preparations were made from macerated/cleared materials. Powdered materials of different parts were cleared with NaOH and mounted in glycerine medium after staining. Different cell components were studied and measured.[11]
Photomicrograph
Microscopic descriptions of tissue are supplemented with micrographs wherever necessary. Photographs of different magnifications were taken with Nikon Labphoto 2 microscopic units. For normal observation, bright field was used. For the study of crystals, starch grains and lignified cells, polarized light was employed. Since these structures have birefringent property, under polarized light they appear bright against dark background. Magnifications of the figures are indicated by the scale-bars. Descriptive terms of the anatomical features are as given in the standard anatomy book.[12,13]
Ultra-violet analysis
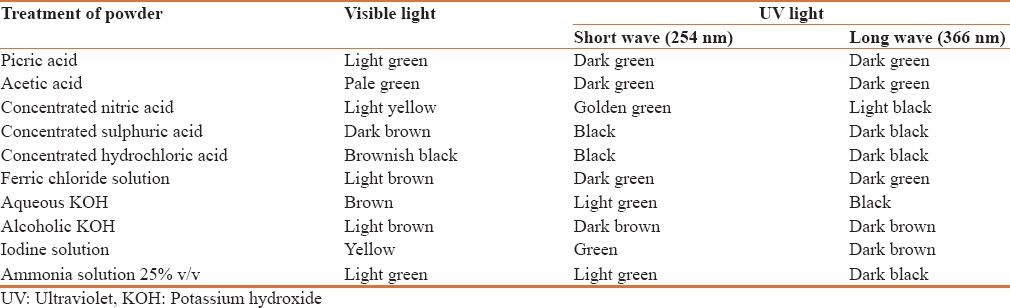
Ultra-violet analysis of the powdered drugs with different chemicals were observed in day light and ultra-violet light. The powdered roots were treated with various solvents such as picric acid, acetic acid, concentrated nitric acid, concentrated sulphuric acid, concentrated hydrochloric acid, ferric chloride, aqueous KOH, Alcoholic KOH, Iodine solution, Ammonia solution 25% v/v and observed under day light and also U.V. 254 nm, U.V. 366 nm.[14,15]
Physico-chemical standards
In the physico-chemical evaluation, ash values, viz. total ash, acid insoluble ash, water soluble ash and sulphated ash and extractive values, viz. alcohol soluble extractive value, water soluble extractive value and loss on drying were determined as per standard procedures. The ash values represent the inorganic salts present in the drug. Extracts obtained by exhausting crude drugs are indicative of approximate measures of certain chemical compounds they contain, their diversity in chemical compounds, diversity in chemical natures and properties of the contents of drug. The percentage w/w values were calculated with reference to the air-dried drug.[16]
Phytochemical studies
Phytochemical analyses were performed by preliminary phytochemical screening to identify various secondary metabolites present in the plant roots using the standard procedures described by Khandelwal.[16]
Results
Scientific classification
Kingdom : Plantae,
Division : Spermatophyta,
Sub division : Magnoliophyta,
Class : Magnoliopsida,
Subclass : Magnoliidae,
Order : Ranunculales,
Family : Ranunculaceae,
Tribes : Helleboreae,
Genus : Helleborus,
Species : Niger,[17]
Botanical name: Helleborus niger L,[1,4]
Synonyms : Helleborus grandiflorus salisb, Helleborus niger legitimus, Helleborus niger verus, Helleborus niger flore roseo.[4,18,19]
Regional language names
Arabic : Khurbuc usivud, Khartu, Kuerbeck;[6,19]
Bengali : Kala kutki;[1]
English : Black hellebore, Christmas rose, Hellebore;[4,5,6,7]
French : L’Hellebore a fleurs roses, Hellebore noir, Rose de Noel;[19]
Gujarati : Koddu;[19]
Hindi : Khorasani-kutki, Kalikatuki;[1,6,7]
Malayalam : Kadagaruganie, Katukarohini;[1,6,7]
Persian : Kharabekahindi, Kharakahindi;[6]
Sanskrit : Kaṭuracīni, Kaṭurohinī, Kaṭukī, Vakrāgra, Krṣṇabhedī;[1,6,7]
Sinhalese : Calurana;[6]
Habit and habitat
A small, perennial herb, about 30 cm in height. Rhizomes black, joined; leaves pale green, ovate-cun-eate, slightly toothed towards apex; flowers white or pink, usually borne singly on red-spotted peduncles.[1] The roots are more or less detached from rhizomes, and mixed with it. The roots are numerous, unbranched, cylindrical in shape. Both rhizomes and roots have a brownish-black colour; a feeble odour, which has been compared to that of senega root; and a bitterish, slightly acrid taste.[2]
Botanical description
A perennial herb with a cylindrical, brownish-black, knotted, brittle, fleshly, subterranean, bracteated, definite rhizome, with its numerous branches much interlaced, and giving off many stout, fibrous, straight, brown roots. Leaves from the extremities of the rhizome with red petioles, pedate, the lateral divisions deeply divided into 2-4 nearly separate lobes successively smaller towards the petiole, coriaceous, nearly evergreen, smooth, shining, dark-green above, paler and reticulated beneath, lobes coarsely serrate above. Flower-stalks terminating the rhizome, surrounded at the base with a loose entire bract, shorter than the leaves, cylindrical, smooth, tapering, mottled with pink below, 1-(rarely 2-) flowered, with 2 or 3 large, ovate, acute, concave bracts a little below the flower. Fruits follicular, sessile; pericarp leathery, dehiscing along the ventral suture. Seeds several, in two rows, oval, black, shining; embryo small, at the base of plentiful horny endosperm.[2]
Macroscopic studies
Organoleptic characters
In organoleptic evaluation, appropriate parameters such as taste, odour, size, shape, and colour of the roots were studied. They are brownish black in colour, cylindrical in shape with slight and feeble odour and pungent with slightly acrid taste.
Morphological character
A perennial herb with a cylindrical, irregularly branched, brownish black roots. The roots are usually 3-6 cm in length and 5-8 mm in diameter. When broken across they present a central undivided, or very slightly stellate, woody axis or meditullium, of a whitish or yellowish-white colour. The roots produce a tingling sensation on the tongue [Figure 1].
Figure 1.

Helleborus niger L root
Microscopic studies
Transverse section of root
Microscopically the root is circular in cross sectional outline, measuring 3.6 mm in thickness. The root consists of uniformly thick, continuous periderm, all along the circumference. The periderm in 250 μm thick. There are shallow wide, V-shaped fissures at several places of the periderm [Figure 1]. The periderm consists of several layers of thin walled, homogenous quadrangular phellem cells and about three layers of palladium cells [Figure 2b and c]. Cortical zone is wide and it measures 900 μm of radial width. The outer part of the cortex includes two or three layers of tangentially stretched air-chambers [Figure 2a]. The remaining inner zone comprises angular, thin walled compact parenchyma cells. Many of the parenchyma cells contain tannins.
Figure 2.
(a) Transverse section of Helleborus niger – ground plan. AC: Air-chamber, Fi: Fissue in the periderm, IC: Inner cortex, Pe: Periderm, Pi: Pith, Ph: Pholeum, X: Xyleum. (b) Transverse section of Helleborus niger – A sector enlarged. AC: Air-chamber, Fi: Fissue in the periderm, IC: Inner cortex, Pe: Periderm, Ph: Pholeum, X: Xyleum. (c) Transverse section of Helleborus niger – periderm zone and xylem cylinder. Ep: Epidermis, Fi: Fissue, Pe: Periderm, Ve: Vessels, XFi: Xylem fibers
The vascular cylinder is hollow and encloses wide pith. The vascular cylinder includes, thick spindle shaped segments with constructions in between the segment. The segments have collateral phloem and xylem elements [Figure 2a and b].
The phloem forms a thick cylinder outside the xylem cylinder. The phloem elements are small, compaer and and are darkly stained [Figure 2c] the xylem is densely distributed, compaer and diffuse. Xylem elements, are circular angular in outline. They are solitary and intermixed with xylem fibres. The fibres and fairly wide, thick walled and liquefied [Figure 2c] the vessels are 10-20 μm wide. Vessel frequency is 280/mm2.
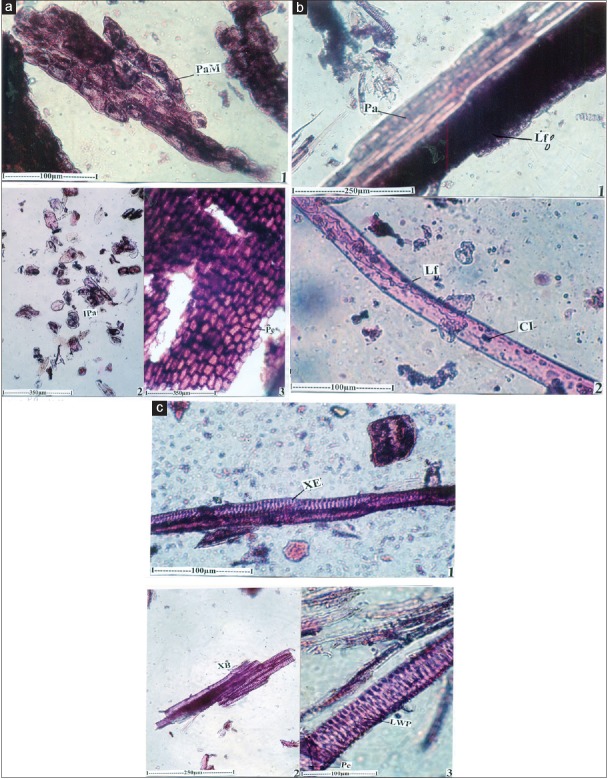
Powder microscopy
The powder preparation of the root was studied under the microscope and the following inclusions were observed
-
(i)
Parenchyma cells
Thick masses or solitary parenchyma cells are abundant in the powder. The cells vary in shape and size. They have dense, darkly stained amorphous inclusions [Figure 3a].
-
(ii)
Periderm cells
Large flakes of periderm cells (phellem cells) are occasionally found in the powder. The cells are tabular in shape, and are thick walled. They are homogenous with similar type of cells and are in compaer rows. The cells are 50-80 × 30-50 μm in size [Figure 3a].
-
(iii)
Laticifers
Laticifers are latex secreting and conducting canals distributed in the ground tissue. In the powder, laticifers are seen either associated with the lendle sheath cells or in an isolated condition. The laticifers are wide, thin walled, non separate and unbranched. They contain solid bodies of starch grains suspended in the latex [Figure 3b].
-
(iv)
Xylem elements
Xylem elements are common in the powder. They are seen either in the form of continuous canal of vessels or broken individual vessel elements. The vessel elements are long, narrow and cylindrical. They have sealariform lateral walls pits which are wide and dense. The end wall perforation in simple, circular or elliptical and oblique. The vessel elements are 70-100 μm long [Figure 3c].
Figure 3.
(a) Powder microscopy of Helleborus niger – thick masses of parenchyma cells with periderm. IPa: Isolated parenchyma cells, PaM: Paranchyma mass, Pe: Periderm. (b) Powder microscopy of Helleborus niger – A laticifier enlarged with parenchyma. CI: Cell inclusion, Lf: Laticifer, Pa: Parenchyma. (c) Powder microscopy of Helleborus niger – xylem elements of vessles. LWP: Lateral wall pits, Pe: Perforation, XB: Xylem bundle, XE: Xylem elements
Ultra-violet analysis
Powdered drug under ultra-violet and ordinary light when treated with different reagent emitted various colour radiations which helps in identifying the drug in powder form [Table 1].
Table 1.
Ultra-violet analysis of the powdered Helleborus niger L root
Physico-chemical standards
The physico-chemical evaluation, ash values, viz. total ash, acid insoluble ash, water soluble ash and sulphated ash and extractive values, viz. alcohol soluble, water soluble and ether soluble extractive values and loss on drying were calculated and recorded. The ash values, viz. total ash, acid insoluble ash, water soluble ash and sulphated ash values were 7.3%, 4.1%, 3.7% and 5.2%, respectively. Extractive values, viz. alcohol soluble, water soluble and ether soluble extractive values were 22.8%, 7.4% and 5.6%, respectively. The moisture content (Loss on drying) was 3.3%.
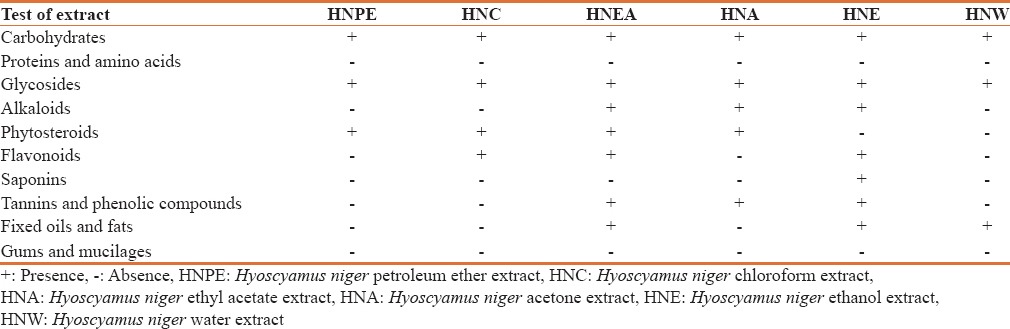
Phytochemical studies
The results of phytochemical screening of the various extracts of H. niger roots revealed the presence of carbohydrate, glycoside, saponins, flavonoid, phytosterols, tannins and phenolic compounds shown in [Table 2]. These secondary metabolites are known to possess various pharmacological effects and may be responsible for various actions of H. niger.
Table 2.
Preliminary phytochemical screening of Helleborus niger L root extracts
Discussion
To establish the identity, purity, safety and quality of herbal drugs, standardization is an vital tool. In order to standardize a drug, various macroscopic, microscopic, fluorescence analyses are done. Microscopic analysis is one of the inexpensive and the simplest methods to begin with establishing the accurate identification of the plant material.[20] According to the world health organization (WHO) the macroscopic and microscopic description of a plant is the first step to estabilish the identity and the degree of purity of such materials and should be carried out before any tests are undertaken.[21]
Thus, the present work was taken up with an objective to lay down detail pharmacognostical and phytochemical standards that will contribute significantly to the quality control of H. niger. This is the first report for the macroscopy and microscopy of root of H. niger. Macroscopic evaluation is a qualitative evaluation based on the study of morphological and organoleptic characters of drugs and establishes diagnostic parameters.
The microscopic studies of the transverse sections showed presence of air-chamber, fissures in periderm and inner cortex. Powder microscopy also plays an important role in pharmacognostical evaluation and sometimes may be an identifying parameter of herbal drugs. Powder microscopy revealed the characters such as cell inclusion, laticifer and lateral wall pits as the specified identification characters.
Ash values of drug give an idea of earthly matter or the inorganic composition and other impurities present along with drugs. It is used as a reliable aid to detect adulteration. The total ash is particularly important in the evaluation of purity of drugs, i.e. the presence or absence of foreign inorganic matter such as metallic salts and/or silica.[22] Percentage ash analysis was carried out and results showed the total ash value of the root to be higher than other ash values.
Extractive values are preliminarily helpful for the determination of exhausted and adulterated drugs. They are also helpful to evaluate the chemical composition of the crude drug and also help in the estimation of specific constituents soluble in particular solvents.[23,24] Among extracts alcohol soluble extractive value of root was higher than water soluble and ether soluble extractive values.
Determination of the moisture content of the drugs used in Ayurvedic system of medicine is very important. A higher or lower percentage shows that the drug was resorted in humid, wet or dry climate. Excessive moisture may favour the growth of fungi or may cause other micro-organic contamination that may result in the deterioration of the drug. Percentage of loss of weight on drying indicates the loss of volatile substance along with water, which is determined by subtracting the moisture content of the powder drug from the loss of weight on drying. Thus the loss on drying percentage was also determined. Lesser values of moisture content could prevent bacteria, fungal or yeast growth.[21,25]
Fluorescence is a vital phenomeno exhibited by various chemical constituents present in plant materials. If the substances themselves are not fluorescent, they may often be converted into fluorescent derivatives by applying different reagents. Hence some crude drugs are often assessed qualitatively in this way and it is an important parameter of pharmacognostical evaluation.[26,27] The micro-chemical tests of the drug were carried out with different concentrated mineral acids, the colours produced by these reagents represent the presence of active constituents. The analysis of powdered drug under ultraviolet light establishes the colour of the drug, as such and after treatment with different reagents.
Preliminary phytochemical screening showed the presence of carbohydrate, glycoside, saponins, flavonoid, phytosterols, tannins and phenolic compounds in various extracts of roots. Phytochemical screening suggests that, the root extracts of H. niger probably contain active agent(s) and this provides the basis for their use as a cure in folklore.
Conclusion
The present study concludes for the first time, the complete pharmacognostical parameters of the root of H. niger. This will provide useful information for identification and to determine the quality and purity of the plant materials. It also provides data for identification of biologically active phytoconstituents, and pharmacopoeia standards for easy identification of H. niger and hence differentiating it from closely related species. These observations could be helpful in setting up of the diagnostic characters for the identification and preparation of a monograph of the H. niger and also contribute towards establishing pharmacopoeial standards.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are thankful to Prof. P. Jayaraman, Director, National Institute of Herbal Science, Chennai, for authentication of the plant for our study.
References
- 1.Anonymous. The Wealth of India: Raw Materials Series. Vol. 3. New Delhi: NISCARI, CSIR; 2005. pp. 251–2. [Google Scholar]
- 2.Bentley R, Trimen H. Medical Plants. Vol. 1. New Delhi: Ajay Book Services; 2006. pp. 5–7. [Google Scholar]
- 3.Sammbamurthy AVSS. Dictionary of Medicinal Plants. 1st ed. New Delhi: CBS Publishers and Distributors; 2006. p. 153. [Google Scholar]
- 4.Prajapati ND, Purohit SS, Sharma AK, Kumar T. A Hand Book of Medicinal Plants: A Complete Source Book. Jodhpur: Agrobios (India); 2007. pp. 266–7. [Google Scholar]
- 5.Khare CP. Indian Medicinal Plants: An Illustrated Dictionary. New Delhi: Springer Publications; 2007. p. 306. [Google Scholar]
- 6.Board N. Compendium of Medicinal Plants. Delhi: National Institute of Industrial Research; 2005. pp. 205–7. [Google Scholar]
- 7.Nadkarni KM. Indian Plants and Drugs. New Delhi: Ajay Book Services; 2010. pp. 158–9. [Google Scholar]
- 8.Sass JE. Elements of Botanical Micro Technique. 1st ed. New York: McGraw Hill Book and Co; 1940. p. 222. [Google Scholar]
- 9.Johanson DA. Plant Micro Technique. 1st ed. New York: McGraw Hill Book Co; 1940. pp. 183–203, 523. [Google Scholar]
- 10.O’Brien TP, Feder N, Mc Cull ME. Polychromatic staining of plant cell walls by toluidin blue-O. Protoplasma. 1964;59:364–73. [Google Scholar]
- 11.Evans WC. Trease and Evans: Pharmacognosy. 15th ed. London: Harcourt Publisher Limited; 2002. p. 313, 513-25, 538-47. [Google Scholar]
- 12.Easu K. Plant Anatomy. 3rd ed. New York: John Wiley and Sons; 1964. p. 767. [Google Scholar]
- 13.Easu K. Anatomy of Seed Plants. 4th ed. New York: John Wiley and Sons; 1979. p. 550. [Google Scholar]
- 14.Kokoski CJ, Kokoski RJ, Slama FJ. Fluorescence of powdered vegetable drugs under ultraviolet radiation. J Am Pharm Assoc Am Pharm Assoc. 1958;47:715–7. doi: 10.1002/jps.3030471010. [DOI] [PubMed] [Google Scholar]
- 15.Chase CR, Jr, Pratt R. Fluorescence of powdered vegetable drugs with particular reference to development of a system of identification. J Am Pharm Assoc Am Pharm Assoc. 1949;38:324–31. doi: 10.1002/jps.3030380612. [DOI] [PubMed] [Google Scholar]
- 16.Khandelwal KR. Practical Pharmacognosy. 19th ed. Pune: Nirali Prakashan; 2008. pp. 149–56, 157-9. [Google Scholar]
- 17.ITIS. Helleborus Niger. [Last accessed on 2017 July 14]. Available from: http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=195028 .
- 18.Stephenson J, Churchill JM. Medical Botany. Vol. 1. London: John Churchill; 1831. p. XI. [Google Scholar]
- 19.Hamilton E. The Flora Homoeopathica. London: Homoeopathic Trust; 1852. pp. 285–91. [Google Scholar]
- 20.Singh S, Manchawal L, Chauhan MG. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. with special emphasis on microscopic technique. J Pharmacogn Phytother. 2010;2:71–5. [Google Scholar]
- 21.Anonymous. World Health Organisation (WHO): Quality Control Methods for Medicinal Plant Materials. Geneva: WHO; 1998. [Google Scholar]
- 22.Nayak BS, Patel KN. Pharmacognostic study of Jatropha curcas leaves. Int J Pharm Tech Res. 2010;2:140–3. [Google Scholar]
- 23.Thomas S, Patil DA, Patil AG, Chandra N. Pharmacognostic evaluation and physicochemical analysis of Averrhoa carambola L fruit. J Herb Med Toxicol. 2008;2:51–4. [Google Scholar]
- 24.Kumar S, Kumar V, Prakash O. Pharmacognostic study and anti-inflammatory activity of Callistemon lanceolatus leaf. Asian Pac J Trop Biomed. 2011;1:177–81. doi: 10.1016/S2221-1691(11)60022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokate CK, Purohit AP, Gokhale SB. Practical Pharmacognosy. 4th ed. New Delhi: Vallabh Prakashan; 2006. p. 107. [Google Scholar]
- 26.Kumar D, Kumar K, Kumar S, Kumar T, Kumar A, Prakash O. Pharmacognostic evaluation of leaf and root bark of Holoptelea integrifolia Roxb. Asian Pac J Trop Biomed. 2012;2:169–75. doi: 10.1016/S2221-1691(12)60036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansari SH. Essentials of Pharmacognosy. 1st ed. New Delhi: Birla Publications Pvt., Ltd; 2006. [Google Scholar]