Abstract
Semen from 5210 sperm bank donors was analyzed and trends in semen quality were evaluated at Shandong Human Sperm Bank between 2008 and 2014. After 2–7 days of abstinence, semen samples were collected. Measurements of semen volume, sperm concentration, sperm forward motility, and total sperm count were performed. There were significant declining trends in semen volume, sperm concentration, sperm forward motility, and total sperm count. Our results indicate that the quality of semen in this cohort of sperm donors had decreased during the study period.
Keywords: male infertility, semen quality, sperm donation
INTRODUCTION
Carlsen and coworkers (1992) reviewed 61 heterogeneous observational studies on semen quality published between 1938 and 1991.1 After that, numerous studies suggest a decline in semen quality in some parts of the world.2,3,4,5,6,7,8,9,10,11,12 In contrast, other studies have reported no significant change in semen quality.13,14,15,16,17,18,19,20,21 However, the debate regarding semen quality remains ongoing due to the possible effects of geographic differences. Of all the studies, some concern infertile couples, while others analyze normal sperm parameters. Until today, large studies on the semen quality of Chinese men have been rare. The objective of this study was to analyze the semen parameters of 5210 sperm donors from Shandong Human Sperm Bank of China, and look for changes that may have occurred during a period of 7 years (2008–2014). Shandong Human Sperm Bank opened in 2006, from then on, human sperm laboratory has specialized in semen analysis and has been focused on spermatozoa research. To this end, we have performed a very precise, body mass index (BMI, kg m−2) and age-adjusted study of donors’ semen parameters. These results were then computerized and are now presented, for the first time, in a detailed analysis of the semen parameters over a course of time.
MATERIALS AND METHODS
Subjects
This was a retrospective longitudinal cohort study analyzing the semen quality of 5210 qualified sperm bank donors recorded in the Shandong Human Sperm Bank from January 1, 2008 to December 31, 2014. All the participants were healthy sperm bank donors, living in Shandong province at the time of sperm donation; 98.17% were Han race.
The screening criteria of sperm donors in our study were conducted strictly in accordance to the standard published by the Chinese Ministry of Health in 2003. All donors in our study were qualified sperm bank donors who had passed the screening from January 1, 2008 to December 31, 2014. We used their first ejaculate sperm parameters for statistical analysis. Besides semen analysis, relevant demographic and clinical information was collected and analyzed. Demographic information included age. Clinical information included BMI, semen parameters, duration of abstinence, and the date of semen analysis. A total of 5210 donors’ semen samples were eligible and screened for entry into the data analysis.
During the entirety of the study, all the technicians working in the human sperm bank laboratory had received the same training. The methods of analysis did not change during the course of the 7 years. The medical director of the laboratory remained the same since 2006. The quality of semen analysis remained a constant. Quality control procedures were carried out for the whole process.
This study was approved by the Ethics Committee of the Hospital for Reproductive Medicine Affiliated to Shandong University. All volunteers signed informed consent forms during their first visit to the human sperm bank, agreeing that their semen samples and data could be used by the human sperm bank for scientific research.
Semen collection and semen analysis
All semen samples were obtained by masturbation in a separate room in the human sperm bank and ejaculated into a wide-mouthed sterile plastic container. Then, they were immediately delivered to the laboratory. The semen samples were incubated to liquefy in a water bath at 37°C analyzed within 1 h after collection.
Semen analysis was carried out following the recommendations of the World Health Organization (WHO) Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction.22 It is done manually. The semen quality parameters that were assessed included appearance, semen volume, viscosity, agglutination, liquefaction time, pH value, sperm concentration, sperm forward motility, and total motility. Semen volume was evaluated by graduated pipettes. The pH value was measured using pH paper and compared with the calibration strip to determine the pH value. For the assessment of sperm concentration and motility, 10 μl of well-mixed semen was placed in a clean Makler chamber (which had been held at 37°C) and covered gently with the cover glass, then examined at a total magnification of ×200. Ten of the 100 squares in the microscope field were randomly scanned and the sperm count was recorded by cytometer. The proportion in each of the four motility categories was assessed: fast progressive sperm (a), slow progressive sperm (b), nonprogressive sperm (c), and immotile sperm (d). During the analysis, specimens were not diluted before using the Makler chamber. To reduce variation in the assessment of sperm characteristics, each semen sample was analyzed twice every time. During the research, internal quality control was performed to ensure that there was no significant difference between the results of the technicians.
Statistical analysis
Descriptive statistical results are presented as nontransformed data. Because the distributions of the analyzed parameters were not normal, the percentiles, medians, and means were calculated. The data were summarized using medians, 25th, and 75th percentiles. The nonparametric test (Kruskal–Wallis method) was used for the comparison of the raw data. Linear regression analysis was used to examine trends in semen characteristics over time. Multiple linear regression analyses were performed controlling for appropriate covariates (age, BMI, duration of abstinence, and season) to look for a calendar-year effect on semen parameters over the study period (2008–2014). Season was re-evaluated as a dummy variable: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February of the following year), with winter as the reference value. When performing linear regression analyses, semen volume, sperm concentration, sperm forward motility, and total sperm count were log-transformed (base 10) prior to analysis because of a skewed distribution. Residual plot was used to test the homogeneity of variance. All statistical tests were two-sided, and statistical significance was defined as P < 0.05. All statistical analyses were performed with SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Subject characteristics
A total of 5210 eligible semen samples from 5210 donors were screened for inclusion in the final analysis. General characteristics of the 5210 donors are summarized in Table 1. The mean age, BMI, and duration of abstinence of the donors were 25.76 ± 5.87 years, 22.59 ± 2.71 kg m−2, and 4.56 ± 1.21 days, respectively. Over the time span of 2008–2014, the mean age of the donors increased from 22.48 ± 2.10 years to 27.80 ± 6.44 years (R2 = 0.877, P = 0.002), the mean BMI increased from 21.80 ± 2.21 kg m−2 to 23.35 ± 3.02 kg m−2 (R2 = 0.903, P = 0.001), and the mean duration of abstinence increased from 4.31 ± 1.12 days to 4.65 ± 1.19 days (R2 = 0.705, P = 0.018).
Table 1.
Descriptive characteristics of the participants
Trends of semen parameters
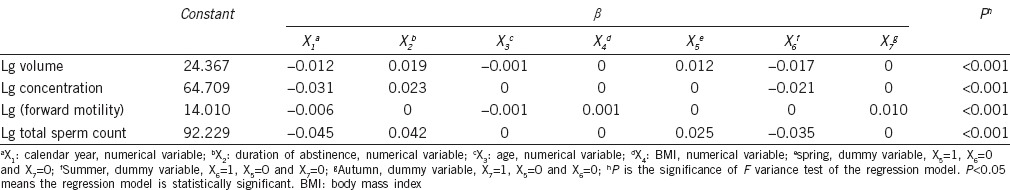
Table 2 shows the semen parameters of the sperm bank donors according to calendar year. Figure 1 shows the decreases in mean values for semen volume, sperm concentration, mean (a + b)% (sperm forward motility), and total sperm count (R2 = 0.563, P = 0.052, β = −0.012; R2 = 0.848, P = 0.003, β = −0.032; R2 = 0.829, P = 0.004, β = −0.008; and R2 = 0.796, P = 0.007, β = −0.045, respectively). Moreover, after adjusting for age, BMI, duration of abstinence and season, semen volume, sperm concentration, sperm forward motility, and total sperm count also showed a tendency to decrease with calendar year (β = −0.012, P < 0.001; β = −0.031, P < 0.001; β = −0.006, P < 0.001 and β = −0.045, P < 0.001, respectively) (Table 3).
Table 2.
Descriptive characteristics of participants’ semen parameters between 2008 and 2014
Figure 1.
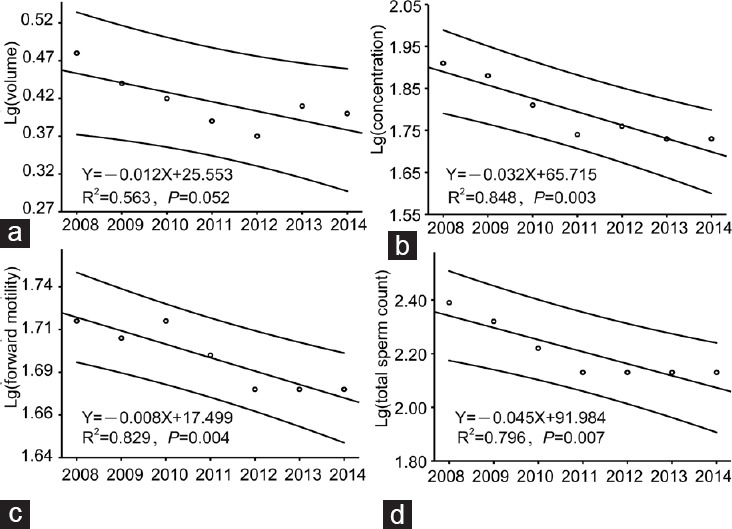
Linear regression lines of the means per year of the main sperm parameters. For each parameter, the graph shows the linear regression line with 95% confidence interval over the study period. Significant decreases in mean semen volume (a), mean sperm concentration (b), mean sperm forward motility (c), and mean total sperm count (d) were observed.
Table 3.
Beta of multiple linear regression equation of semen parameters
DISCUSSION
Semen analysis is one of the most valuable methods for evaluating male reproductive health, and it plays an important role in the field of andrology. During the past several decades, many reports have suggested that the semen quality in healthy men is declining,1,2,3,4,5,6,7,8,9,10,11,12 Swan et al.5 also corroborated a large annual decline in sperm concentration in European men (2.3%) and a smaller decline in the US men (0.8%). However, few studies have focused on the semen quality and temporal trends in the semen quality of Chinese men. In our study, 5210 eligible semen samples from Shandong Human Sperm Bank were screened and analyzed. The results indicated that semen volume, sperm concentration, sperm forward motility, and total sperm count of Shandong Sperm Bank donors may have declined in sperm bank donors between the years 2008 and 2014 in Shandong. To our knowledge, this is one of the largest studies focusing on the semen quality and temporal trends in the semen quality of Chinese sperm bank donors. Therefore, our study expands the current data on semen quality and temporal trends of semen quality among men with unknown fertility.
Our analysis of sperm samples from 5210 men who qualified to be sperm donors at Shandong Human Sperm Bank in China showed declining trends in semen volume, sperm concentration, sperm forward motility, and total sperm count between 2008 and 2014. These trends continued to be present even after adjusting for age, BMI, duration of abstinence, and season. Compared with other similar Chinese studies,23,24,25,26 most values for the semen parameters examined in our study (Table 2) were in the middle of the other reported results. The probable reasons for this include: (1) the different criteria used to select participants; (2) regional differences; and (3) the different study periods.
Abstinence time is strongly related to sperm concentration,5,27,28,29 and this relationship was further verified by the data shown in Table 3. Besides calendar year, abstinence time was a relatively more important predictor of sperm concentration. However, similar to a previous result,5 age was not retained in the final model for prediction of sperm concentration. Data in Table 3 show that the yearly rates of decline in semen volume, sperm concentration, sperm forward motility, and total sperm count were 2.73%, 6.89%, 1.37%, and 9.84%, respectively. Data in Table 2 and Figure 1 indicate that the declining trends in sperm parameters were obvious during the 4-year period of 2008–2011, however the declines seem to have stopped during the final 3 years of our study. A long-term observational study is needed to reach a true and real objective conclusion regarding changing trends in the parameters of semen quality.
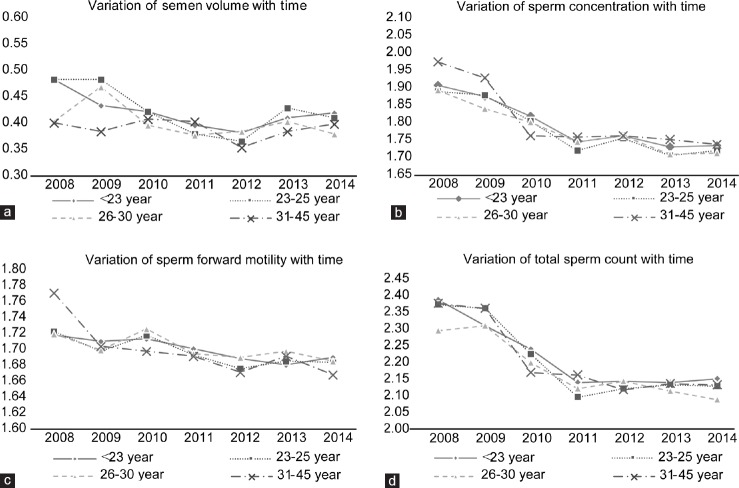
Since the mean age of participants in our study increased to 5.32 years during the study period, we re-evaluated the effect of age by examining four different categories (<23 years, 23–25 years, 26–30 years, and 31–45 years) to check for the changes in semen parameters with time in those different age groups (Figure 2a–2d). Our results showed that the values for almost all semen parameters in every age group showed a tendency to decline with time.
Figure 2.
Variation of semen parameter of different age groups with time. Semen volume (a), sperm concentration (b), sperm forward motility (c), and total sperm count (d) show tendencies to decline with time in every age group.
The declining trend in our study would be consistent with a decline in the magnitude reported by Carlsen et al.1 However, the yearly decline rates of sperm concentration and total sperm count calculated in our study were much higher than those reported in previous studies.8,9,10,11,12 In addition, our study found a slight decline in sperm forward motility over time, which was different from other reported results.10,11,30
The screening criteria used to screen sperm bank donors in China have not changed since 2003. Furthermore, it is noteworthy that the percentage of qualified donors who satisfied sperm bank criteria declined during the study period, suggesting that the semen quality of men residing in Shandong may have declined.
We cannot explain the larger decline in donor semen quality found in our 7-year study when compared to changes found in other studies. In the year 2000, Swan et al.5 corroborated a large annual decline in sperm concentration in European men (2.3%) and a smaller decline in the US men (0.8%). Geoffroy-Siraudin et al.10 found declining trends in sperm concentration and total sperm count of about 1.5%/year and 1.6%/year, respectively, among 10 392 males consulting for couple infertility in 2012. In addition, in France in 2012, Rolland et al.11 found a continuous decrease in sperm concentration of about 1.9%/year in a sample of 26 609 partners of totally infertile women. However, such studies have rarely been conducted in China. In 1999, Zhang et al.30 proposed that sperm quality in China had declined significantly faster than that in Western countries during the same time period. Recently, Rao et al.23 reported that in 1808 Wuhan University students, there was a decrease in sperm concentration during the 4-year observation (from 58.0 × 106 ml−1 in 2010 to 41.8 × 106 ml−1 in 2013). After adjusting for potential confounders (age, year, season, and duration of abstinence), sperm concentration and total sperm count also showed a tendency to decrease. It seems that the yearly decline rate in sperm concentration in their study was even higher than that in ours. It has been proposed that the semen quality can be influenced by geographic and ethnic factors.31,32 By now, we cannot resolve if the reasons for the downward trends might be related to environmental or occupational factors or differences in lifestyles of the individuals. However, we are certain that the decreases in sperm concentration and total sperm count do exist in Shandong sperm bank donors. In the year 2014, Jiang et al.33 reported that semen quality of adult men in Sichuan (China) declined. This indicates that the decline in semen quality found in our study was not an isolated event, which should be paid high attention to.
Le Moal et al.34 found that the highest decreases and lowest values of concentration and morphology were consistently observed in two proximate regions that are both highly agricultural and densely populated. Those investigators proposed that their results were consistent with the endocrine disruptor hypothesis, and most probably due to changes in environmental exposure or lifestyle that equally impacted all participants during their study period. Further studies are needed to determine the potential causes for the declines in semen quality found in our study.
Semen quality is known to be a well-recognized marker of fertility. Besides fertility outcomes, semen quality is a sentinel indicator of gamete deterioration and thus should be considered as a biomarker of the next development outcomes.35 And, Jensen et al.36 reported lower rates of mortality among men with good semen quality, and speculated that good semen quality may be a fundamental biomarker of overall male health. All these aspects strengthen the need to pay attention to semen quality.
Our study had some potential weaknesses that should be mentioned: first, this was a retrospective analysis, subjected to inherent biases, and the data analyzed were relatively crude. Although the data had been adjusted for several factors, they were not adjusted for some potential confounders and factors known to impact semen quality such as tobacco use, alcohol use, dietary patterns, occupational exposure, and lifestyle. Second, the data were obtained by analyzing semen from donors at a sperm bank, and thus the quality of semen may not be representative of that found in the general population, resulting in selection bias. Nonetheless, the relatively large sample size in this study, the use of a longitudinal cohort, the fact that few changes in the laboratory staff, and the unique and very precise sperm analyses conducted are the main strengths of the present study.
CONCLUSION
Among a sample of 5210 sperm bank donors from Shandong Human Sperm Bank in China, we found evidence suggesting there may have decreases in semen volume, sperm concentration, sperm forward motility, and total sperm count between 2008 and 2014, of about 2.73%, 6.89%, 1.38% and 9.84%/year, respectively. To our knowledge, this is the first study to focus on qualified sperm bank donors’ semen quality in China. These results indicate that there may be a severe and generalized decrease in semen quality among males in Shandong. The decline of semen quality among sperm bank donors in our study may be alarming, and should receive great attention. Long-term observation should be conducted to confirm the present results, and determine the potential causes of this decline in semen quality.
AUTHOR CONTRIBUTIONS
LW and XHS carried out the semen analysis. HBZ collected and collated the data, LW conceived the study, LW and LZ drafted the manuscript and performed the statistical analysis. CYX and ZJC supervised the project and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the Science research foundation item of no-earnings health vocation (201402004) and National Basic Research Program of China (973 Program) (2012CB944700).
REFERENCES
- 1.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 3.Adamopoulos DA, Pappa A, Nicopoulou S, Andreou E, Karamertzansi M, et al. Seminal volume and total sperm number trends in men attending subfertility clinics in the Greater Athens area during the period 1977-1993. Hum Reprod. 1996;11:1936–41. doi: 10.1093/oxfordjournals.humrep.a019520. [DOI] [PubMed] [Google Scholar]
- 4.Van Waeleghem K, de Clercq N, Vermeulen L, Schoonjans F, Comhaire F. Deterioration of sperm quality in young healthy Belgian men. Hum Reprod. 1996;11:325–9. doi: 10.1093/humrep/11.2.325. [DOI] [PubMed] [Google Scholar]
- 5.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lackner J, Schatzl G, Waldhor T, Resch K, Kratzik C, et al. Constant decline in sperm concentration in infertile males in an urban population: experience over 18 years. Fertil Steril. 2005;84:1657–61. doi: 10.1016/j.fertnstert.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 7.Spirada S, Fonseca S, Lee A, Harrild K, Giannaris D, et al. Trends in semen parameters in the Northeast of Scotland. J Androl. 2007;28:313–9. doi: 10.2164/jandrol.106.000729. [DOI] [PubMed] [Google Scholar]
- 8.Shine R, Peek J, Birdsall M. Declining sperm quality in New Zealand over 20 years. N Z Med J. 2008;121:50–6. [PubMed] [Google Scholar]
- 9.Mukhopadhyay D, Varghese A, Pal M, Banerjee S, Bhattacharyya A, et al. Semen quality and age-specific changes: a study between two decades on 3729 male partners of couples with normal sperm count and attending an andrology laboratory for infertility-related problems in an Indian city. Fertil Steril. 2010;93:2247–54. doi: 10.1016/j.fertnstert.2009.01.135. [DOI] [PubMed] [Google Scholar]
- 10.Geoffroy-Siraudin C, Loundou AD, Romain F, Achard V, Courbiere B, et al. Decline of semen quality among 10932 males consulting for couple infertility over a 20-year period in Marseille. Asian J Androl. 2012;14:584–90. doi: 10.1038/aja.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2012;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendiola J, Jorgensen N, Minguez-Alarcon L, Sarabia-Cos L, Lopez-Espin JJ, et al. Sperm counts may have declined in young university students in Southern Spain. Andrology. 2013;1:408–13. doi: 10.1111/j.2047-2927.2012.00058.x. [DOI] [PubMed] [Google Scholar]
- 13.Bujan L, Mansat A, Pontonnier F, Mieusset R. Time series analysis of sperm concentration in fertile men in Toulouse between 1977 and 1992. BMJ. 1996;312:471–2. doi: 10.1136/bmj.312.7029.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berling S, Wölner-Hanssen P. No evidence of deteriorating semen quality among men in infertile relationships during the last decade: a study of males from Southern Sweden. Hum Reprod. 1997;12:1002–5. doi: 10.1093/humrep/12.5.1002. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen PE, Erb K, Westergaard LG, Laursen SB. No evidence for decreasing semen quality in four birth cohorts of 1055 Danish men born between 1950 and 1970. Fertil Steril. 1997;68:1059–64. doi: 10.1016/s0015-0282(97)00377-4. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel E, Gobuloff E, Fisch H. MacLeod revisited: sperm counts distributions in 374 fertile men from 1971 to 1994. Urology. 1998;51:86–8. doi: 10.1016/s0090-4295(97)00462-7. [DOI] [PubMed] [Google Scholar]
- 17.Seo JT, Rha K, Park YS, Lee MS. Semen quality over a 10 year period in 22249 men in Korea. Int J Androl. 2000;3:194–8. doi: 10.1046/j.1365-2605.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 18.Itoh N, Kayama F, Tatsuki J, Tsukamoto T. Have sperm counts deteriorated over the past 20 years in healthy young Japanese men. Results from the Sapporo area? J Androl. 2001;22:40–4. [PubMed] [Google Scholar]
- 19.Costello MF, Sjoblom P, Haddad Y, Steigrad SJ, Bosch EG. No decline in semen quality among potential sperm donors in Sydney, Australia, between 1983 and 2001. J Assist Reprod Genet. 2002;19:284–90. doi: 10.1023/A:1015729314081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marimuthu P, Kapilashrami MC, Misro MM, Singh G. Evaluation of trend in semen analysis for 11 years in subjects attending a fertility clinic in India. Asian J Androl. 2003;5:221–5. [PubMed] [Google Scholar]
- 21.Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod. 2011;26:1012–6. doi: 10.1093/humrep/der045. [DOI] [PubMed] [Google Scholar]
- 22.WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 4th ed. New York: Cambridge University Press; 1999. [Google Scholar]
- 23.Rao M, Meng TQ, Hu SH, Guan HT, Wei QY, et al. Evaluation of semen quality in 1808 university students, from Wuhan, Central China. Asian J Androl. 2015;17:111–6. doi: 10.4103/1008-682X.135984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JQ, Yang QY, Tao JG, Yuan W, Bo LW, et al. Reference value of semen quality in Chinese young men. Contraception. 2002;65:365–8. doi: 10.1016/s0010-7824(02)00281-0. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Gao ES, Walker M, Yang Q, Wu JQ, et al. Reference values of semen parameters for healthy Chinese men. Urol Int. 2008;81:256–62. doi: 10.1159/000151400. [DOI] [PubMed] [Google Scholar]
- 26.Li YF, Lin H, Ma MF, Li LB, Cai M, et al. Semen quality of 1346 healthy men, results from the Chongqing area of Southwest China. Hum Reprod. 2009;24:459–69. doi: 10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- 27.Macleod J, Gold RZ. The male factor in fertility and infertility: spermatozoon counts in 1000 men of known fertility and in 1000 cases of infertile marriage. J Urol. 1951;66:436–49. doi: 10.1016/S0022-5347(17)74358-3. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz D, Laplanche A, Jouannet P, David G. Within-subject variability of human semen in regard to sperm count, volume, total number of spermatozoa and length of abstinence. J Reprod Fertil. 1979;57:391–5. doi: 10.1530/jrf.0.0570391. [DOI] [PubMed] [Google Scholar]
- 29.Magnus O, Tollefsrud A, Abyholm T, Purvis K. Effects of varying the abstinence period in the same individuals on sperm quality. Arch Androl. 1991;26:199–203. doi: 10.3109/01485019108987644. [DOI] [PubMed] [Google Scholar]
- 30.Zhang SC, Wang HY, Wang JD. Analysis of change in sperm quality of Chinese fertile men during 1981-1996. Reprod Contracept. 1999;10:33–9. [PubMed] [Google Scholar]
- 31.Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–20. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu JQ, Yang QY, Tao JG, Li WY, Gao ES, et al. [Epidemiological study on semen quality of 562 volunteers aged 22-30] Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:44–8. Article in Chinese. [PubMed] [Google Scholar]
- 33.Jiang M, Chen X, Yue H, Xu W, Lin L, et al. Semen quality evaluation in a cohort of 28213 adult males from Sichuan area of South-West China. Andrologia. 2014;46:842–7. doi: 10.1111/and.12168. [DOI] [PubMed] [Google Scholar]
- 34.Le Moal J, Rolland M, Goria S, Wagner V, De Crouy-Chanel P, et al. Semen quality trends in French regions are consistent with a global change in environmental exposure. Reproduction. 2014;147:567–74. [Google Scholar]
- 35.Joffe M. What has happened to human fertility? Hum Reprod. 2010;2:295–307. doi: 10.1093/humrep/dep390. [DOI] [PubMed] [Google Scholar]
- 36.Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol. 2009;5:559–65. doi: 10.1093/aje/kwp168. [DOI] [PubMed] [Google Scholar]