Abstract
Androgen deficiency is strongly associated with erectile dysfunction (ED). Inadequate penile arterial blood flow is one of the major causes of ED. The blood flow to the corpus cavernosum is mainly derived from the internal pudendal arteries (IPAs); however, no study has evaluated the effects of androgen deprivation on IPA's function. We hypothesized that castration impairs IPAs reactivity and structure, contributing to ED. In our study, Wistar male rats, 8-week-old, were castrated and studied 30 days after orchiectomy. Functional and structural properties of rat IPAs were determined using wire and pressure myograph systems, respectively. Protein expression was determined by Western blot and immunohistochemistry. Plasma testosterone levels were determined using the IMMULITE 1000 Immunoassay System. Castrated rats exhibited impaired erectile function, represented by decreased intracavernosal pressure/mean arterial pressure ratio. IPAs from castrated rats exhibited decreased phenylephrine- and electrical field stimulation (EFS)-induced contraction and decreased acetylcholine- and EFS-induced vasodilatation. IPAs from castrated rats exhibited decreased internal diameter, external diameter, thickness of the arterial wall, and cross-sectional area. Castration decreased nNOS and α-actin expression and increased collagen expression, p38 (Thr180/Tyr182) phosphorylation, as well as caspase 3 cleavage. In conclusion, androgen deficiency is associated with impairment of IPA reactivity and structure and increased apoptosis signaling markers. Our findings suggest that androgen deficiency-induced vascular dysfunction is an event involving hypotrophic vascular remodeling of IPAs.
Keywords: androgen, castration, internal pudendal artery
INTRODUCTION
Erectile function is a complex neurovascular phenomenon that encompasses the interplay among neural, hormonal, psychological factors as well as the integrity of the penile vasculature.1,2 It is well established that erectile responses involve both: (1) vasodilation of penile arteries to produce an adequate level of blood inflow into the cavernous sinuses and (2) smooth muscle relaxation of the cavernosal tissue. Therefore, insufficient arterial blood inflow to the penis is widely accepted as one of the biggest causes of erectile dysfunction (ED).3 However, the exact mechanism of the insufficient arterial blood inflow is often difficult to elucidate and may involve functional as well as structural vascular abnormalities. In humans and many experimental animals, the arterial blood flow to the corpora cavernosa is normally derived from bilateral cavernous arteries, which branch off the internal pudendal arteries (IPAs).4,5
More than a decade ago, studies on penile vasculature focused solely on intrapenile control mechanisms of erection (helical arteries, cavernous arteries, and the cavernous smooth muscle)4,5 rather than the resistance properties of the prepenile vasculature. However, Manabe and co-workers demonstrated that around 70% of the blood flow resistance to the penis is located in IPAs, and only the remaining 30% of the resistance lies in intrapenile tissues.6 These findings not only emphasize the predominant role of prepenile vascular resistance, but also drop the penocentric concept of erection control.
Clinical and experimental evidence have demonstrated a crucial role of IPAs on erectile function. Aboseif and co-workers3 have shown that acute bilateral occlusion of IPAs in dogs decreases around 60% of the intracavernosal pressure during erection following neurostimulation. Angiographic studies performed in men with ED demonstrated that vascular narrowing caused by atherosclerotic disease in IPAs, iliac arteries, and cavernous arteries is one of the mechanisms contributing to ED.7,8,9 The important role that IPAs play in erection is further demonstrated in patients who start suffering of ED after pelvic trauma as a consequence of IPAs rupture or stenosis.9 Likewise, it was demonstrated that unilateral occlusion of IPAs in rabbits caused various functional changes in the corpus cavernosum, which may additionally contribute to the onset of ED.10
ED is defined as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance.11 The etiology of ED is complex and involves vascular, neurogenic, psychological, and hormonal components.12 Androgens are involved in male sexual development and they also play a crucial role in the maintenance of sexual health along the entire life of a male individual. Testosterone (TESTO) and dihydrotestosterone (DHT) are the main androgens playing key regulatory roles in determining and later supporting the male phenotype.13 Experimental studies have massively demonstrated that androgen deprivation induces ED in several animal species (cats,14 dogs,15 rats,16,17 rabbits,18 and mice19). In humans, numerous reports also indicate that androgens play a crucial role in erectile function.20,21,22 Moreover, clinical studies have largely demonstrated that surgical or pharmacological castration induces loss of libido and ED.23,24,25,26,27,28
Clinical and experimental studies suggest that there are many mechanisms responsible for androgen deficiency (AD)-associated ED, such as: (1) reduced trabecular smooth muscle content and increased deposition of extracellular connective tissue,15,17,18,19 a tissue architecture change that may contribute to veno-occlusive dysfunction; (2) functional and structural impairment of pelvic ganglia,29,30 cavernosal, and dorsal nerves;31,32 (3) endothelial damage;33,34 and (4) hypercontractile state of the corpus cavernosum.35
To date, despite the growing number of evidence demonstrating the important role that IPAs play on blood flow resistance and erectile function, no study has evaluated the effects of androgen deprivation on IPAs function and structure. Therefore, the present study tested the hypothesis that androgen deprivation induces IPAs dysfunction, which in turn, may contribute to ED development.
MATERIALS AND METHODS
Animals
Male Wistar rats were housed individually with free access to water and standard chow and maintained on 12 h light-dark cycle. The Ethics Committee in Animal Research of Ribeirao Preto Medical School approved the experimental protocols used in this study (protocol number 002/2015-1).
Orchiectomy
Male, 8-week-old Wistar rats were anesthetized, the efferent duct of each testicle was ligated, and the testicles were removed (20 animals). The sham animals (20 animals) were submitted to the same surgical procedures without efferent duct ligation. All experimental protocols were carried out 4 weeks after the orchiectomy surgery.
Functional studies in internal pudendal arteries
IPAs were cut into 2 mm ring segments and mounted on a wire myograph (Danish Myo Technology, Aarhus, Denmark) filled with 5 ml of Krebs–Henseleit modified solution ([in mmol l−1] 130 NaCl, 14.9 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4 ∙ 7H2O, 5.5 glucose, 1.56 CaCl2 ∙ 2H2O, and 0.026 EDTA) gassed with 5% CO2/95% O2 to maintain a pH of 7.4. Vessel viability was checked by KCl 120 mmol l−1. To investigate the contractile response, cumulative concentration–response curves to phenylephrine (Phe, 10−9 mol l−1 to 3 × 10−5 mol l−1) and electrical field stimulation (EFS) were performed (20 V, 1-ms pulse width and trains of stimuli lasting 10 s at varying frequencies −0.2–12 Hz). Relaxation response curves to acetylcholine (ACh, 10−9 mol l−1 to 10−4 mol l−1) and sodium nitroprusside (SNP, 10−10 mol l−1 to 10−4 mol l−1) were determined in vessels precontracted with U46619 (10−7 mol l−1). To evaluate relaxation induced by nonadrenergic-noncholinergic (NANC) nerves stimulation, vessels were incubated for 30 min with guanethidine (3 × 10−5 mol l−1) plus atropine (10−6 mol l−1) and EFS was performed (0.2–12 Hz).
Mechanical studies in internal pudendal arteries
Mechanical and structural properties of IPA segments were studied in pressure myograph system. IPAs segments (2–3 mm) were placed between two glass microcannulae. Intraluminal pressure was adjusted to 3 mmHg and incrementally increased (3–140 mmHg) to determine pressure-dependent changes in lumen diameter under passive conditions (calcium-free Krebs). Values of internal and external diameters were used to calculate wall thickness and cross-sectional area (CSA).
Internal pudendal artery histomorphometry and immunohistochemistry
IPAs segments were isolated and immersed in 4% paraformaldehyde for 24 h. After this period, the segments were washed in tap water and kept in 70% alcohol. Thereafter, arterial segments were cut on a microtome (5-μm thick) and mounted on glass slides. To determine collagen content, IPAs sections were incubated with picrosirius red (0.1% Sirius red F3B in saturated aqueous picric acid) for 30 min. Tunica media, lumen, and outer border were traced to calculate internal and external diameters and cross-sectional area.
To identify neuronal nitric oxide synthase (nNOS) and α-actin immunoreactivity, IPAs sections were incubated with the primary antibody anti-NOS1 (Santa Cruz Biotechnology) and then incubated with the biotinylated secondary antibody (Vector). The diaminobenzidine reaction produced a brown color, which was representative of nNOS immunoreactivity.
Plasma testosterone measurement
Plasma testosterone levels were determined using the IMMULITE 1000 Immunoassay System (Enzo Life Sciences). The sample and the reagent containing the enzyme alkaline phosphatase conjugated with testosterone were dispensed on the bead, serving as a container for incubation, washing and signal development. After 60 min of incubation, the bead was washed to eliminate any remaining unbound fraction. The bound fraction was then quantified using the dioxetane chemiluminescent substrate.
In vivo measurements of ICP/MAP
Animals were anaesthetized with 4% isoflurane in 10% oxygen (O2). To monitor and calculate mean arterial pressure (MAP) and intracavernosal pressure (ICP), the left femoral artery and the right crura were cannulated. The right major pelvic ganglion was assessed via a midline incision and stimulated with bipolar silver electrode. ICP changes were then monitored in response to 0.2–20 Hz. The erectile response was calculated using the maximum ICP response normalized to MAP at the time of maximum ICP. The area under the curve (AUC) ratio of the ICP/MAP was recorded performing stimulation at 12 Hz during 40 s. Right after the in vivo experiment, the penis was removed and dried at 60°C for 24 h. Penis dry weight was determined in an analytical scale and normalized by tibia length.
Western blot
A total of 20 μg of IPAs protein were separated on a Hoefer Mini VE system (GE) (Thermofisher), using a precast 8%–25% PhastGel. After, SDS/PAGE proteins were transferred to a nitrocellulose filter (Amersham). Membranes were then probed with rabbit anti-phospho-p38 (Thr180/Tyr182) (Cell Signaling); rabbit anti-p38 (Cell Signaling), rabbit anti-Procaspase 3 (Santa Cruz) and rabbit anti-caspase 3 (Santa Cruz); rabbit anti-nNOS (Cell Signaling); and rabbit anti-α/β-tubulin (Cell Signaling). Immunostaining was detected using horseradish peroxidase-conjugated anti-rabbit IgG (GE). Immunoblots were revealed by the ECL prime (Amersham) and quantitated by densitometry using ImageJ software (NIH).
Statistical analysis
Contractile responses to Phe and EFS are expressed as percentage of KCl-induced response. The ACh and NANC relaxation curves are expressed as percentage change from U46619-induced contraction. Phe and ACh concentration–response curves were fitted by nonlinear regression analysis. pD2 (negative logarithm of the EC50 values) and maximal response (Emax) were compared by Student's t-test, as well as the remaining experiments. The results are shown as mean ± s.e.m., with “n” representing the number of animals used. P < 0.05 was considered significant.
RESULTS
In vivo erectile function
Electric neurostimulation of the major pelvic ganglion increased ICP/MAP ratio in both castrated and sham rats (Figure 1a). However, castrated rats displayed a significant reduction in erectile response (Figure 1b). This impairment was characterized by a decrease not only in maximal ICP/MAP ratio, but also in the AUC ratio of the ICP/MAP (stimulation at 12 Hz) was reduced in castrated rats compared to the sham group (sham: 0.47 ± 0.04 vs castrated: 0.17 ± 0.01; P < 0.05). Orchiectomy induced a dramatic reduction in total serum TESTO levels (Figure 1c). In addition, the effectiveness of androgen deprivation was confirmed by changes in the external genitalia. Castrated rats displayed a decreased penis weight when compared to the sham group (sham: 32 ± 1.5 vs 25 ± 1.8; g/tibia length ×100).
Figure 1.
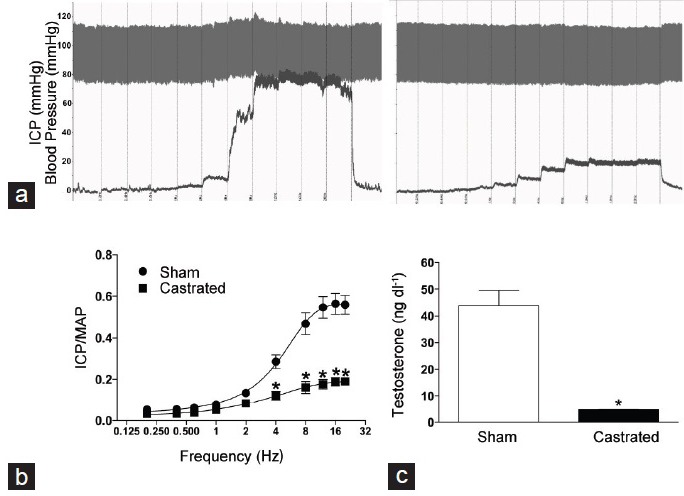
Orchiectomy reduces total serum testosterone levels and decreases erectile function. (a) Representative tracings showing changes in intracavernosal pressure (ICP, mmHg) and blood pressure (mmHg) in response to electrical stimulation of the major pelvic ganglion (0.2–20 Hz; 1 ms pulses at 6 V stimulations for 45 s every 5 min) in sham (left) and castrated (right) rats. (b) The erectile response was calculated using the peak of ICP response normalized to mean arterial pressure (MAP) at the respective peak of ICP. The points represent the mean ± standard error of the mean. The average of three measurements was calculated for each animal (n = 5–6). (c) Serum testosterone levels (ng dl−1) were determined by enzyme immunoassay in sham (open bars) and castrated (filled bars) rats. *P < 0.05 versus sham.
Functional studies in internal pudendal arteries
The contractile response induced by KCl was not different between the groups ([mN], sham: 10.34 ± 1.4 vs castrated: 10.35 ± 1.4). IPAs from orchidectomized rats displayed decreased maximal contractile response to phenylephrine (Figure 2a) when compared to IPAs from sham rats (Emax [% of KCl-induced contraction], sham: 176.6 ± 7.6 vs castrated: 138.4 ± 7.9), but no changes were observed in pD2 values between the sham and castrated groups (pD2 : sham: 6.98 ± 0.1 vs castrated: 7.26 ± 0.2). EFS induced frequency-dependent contractile responses in IPAs from both groups (Figure 2b). However, the contractile response in IPAs from castrated rats was decreased when compared to the sham group (Figure 2b). Addition of L-NAME (10−4 mol l−1) did not abolish the difference between the groups (data not shown).
Figure 2.
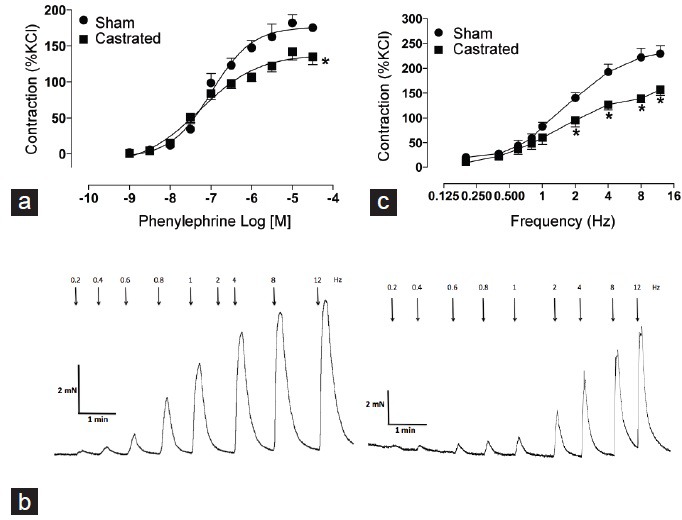
Orchiectomy decreases contractile responses of internal pudendal arteries (IPAs). (a) Concentration-response curves to the α1-adrenergic receptor agonist phenylephrine (Phe) on IPAs from sham (•) and castrated (▪) rats. (b) Representative tracings showing changes in tension in response to electric field stimulation (adrenergic nerves-mediated responses) (at 20 V, 1-ms pulse width, and trains of stimuli lasting 10 s at varying frequencies [0.2–12 Hz]) in sham (left) and castrated (right) rats. Graph shows contractile responses induced by frequency-response curves in IPAs from sham (•) and castrated (▪) rats. (c) Electric field stimulation-induced contraction on IPAs from sham and castrated rats. The points represent the mean ± standard error of the mean (n = 5–9). *P < 0.05 versus sham.
The endothelial function was determined by evaluating acetylcholine-induced relaxation. IPAs from castrated animals showed decreased relaxation response to ACh when compared to IPAs from sham rats (Figure 3a). However, differences in sodium nitroprusside-induced relaxation were not observed (Figure 3b). EFS induced a frequency-dependent relaxation in IPAs from both groups (Figure 3c). Nevertheless, IPAs from castrated animals displayed a decreased relaxation response to NANC stimulation when compared to IPAs from sham rats (Figure 3c).
Figure 3.
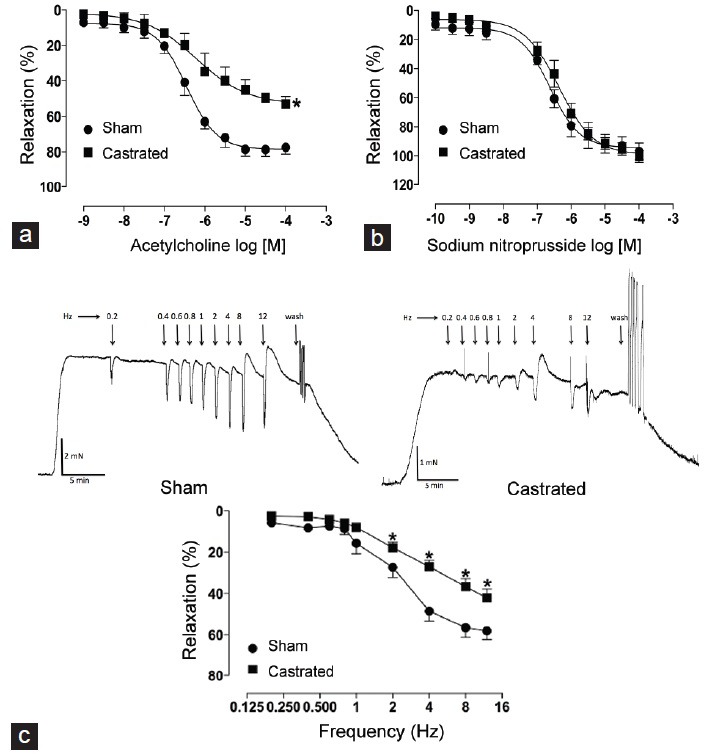
Orchiectomy decreases relaxation responses of internal pudendal arteries (IPAs). (a) Concentration-response curves to acetylcholine (ACh), (b) sodium nitroprusside (SNP) and frequency-response curves (nonadrenergic-noncholinergic [NANC]-induced relaxation) (c) in IPAs from sham (•) and castrated (▪) rats. Representative tracings show NANC-induced relaxation in response to electric field stimulation (at 20 V, 1-ms pulse width, and trains of stimuli lasting 10 s at varying frequencies [0.2–12 Hz] in the presence of atropine and guanethidine) in IPAs from sham (left) and castrated (right) rats. The points represent the mean ± standard error of the mean (n = 5–6). *P < 0.05 versus sham.
Structural studies in internal pudendal arteries
Considering the decreased contractile responses to an adrenergic agonist and also to neurogenic stimulation in IPAs from castrated animals, studies were carried out to test the hypothesis that androgen deprivation induces changes in the structural properties of IPAs due to remodeling. Experiments were performed with pressurized IPAs under passive conditions (absence of Ca2+). IPAs from castrated animals displayed decreased internal and external diameters compared to sham rats (Figure 4a and 4b, respectively). Moreover, androgen deprivation reduced IPAs wall thickness and cross-sectional area (Figure 4c and 4d, respectively).
Figure 4.
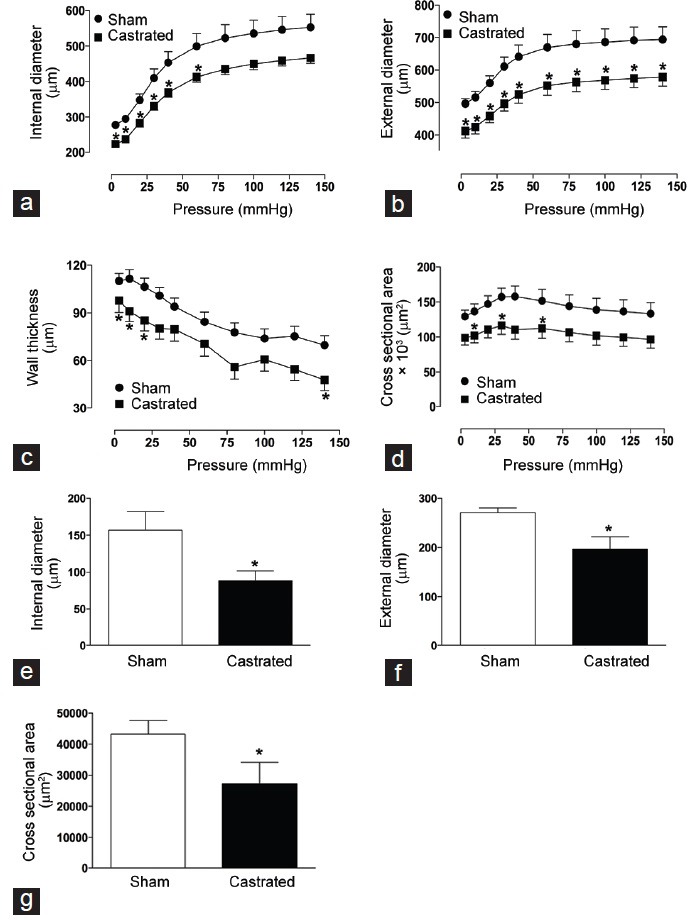
Orchiectomy changes internal pudendal arteries (IPAs) structural properties. (a) Internal and (b) external diameters, (c) vascular wall thickness and (d) cross-sectional area of IPAs from sham (•) and castrated (▪) rats. Values were obtained in arteries gradually pressurized in passive conditions (Ca2+-free Krebs–Henseleit buffer). Graphs show histological measurement of (e) internal and (f) external diameters and (g) cross-sectional area of IPAs, stained with picrosirius red, from sham (open bars) and castrated (filled bars) rats. Data are expressed as mean ± standard error of the mean (n = 5–7). *P < 0.05 versus sham.
Internal pudendal artery histomorphometry
Histomorphometric studies were performed in IPAs from sham and castrated animals to determine changes in the vascular structure and collagen expression. Similarly to what was observed in the functional studies with pressurized vessels, IPAs from castrated animals displayed decreased internal diameter (Figure 4e), external diameter (Figure 4f), and cross-sectional area (Figure 4g). Collagen expression was increased in IPAs from castrated animals as represented in Figure 5a.
Figure 5.
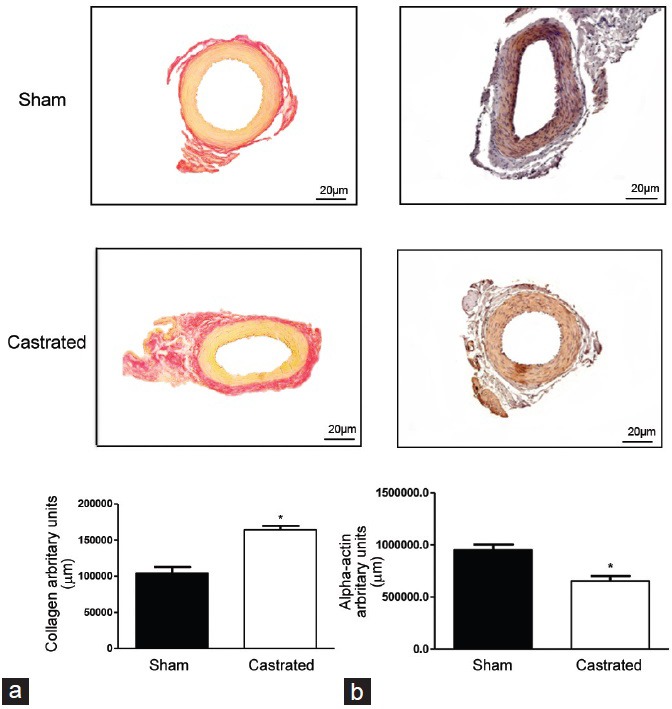
Orchiectomy increases internal pudendal arteries (IPAs) collagen and decreases α-actin expression. Representative images depict (a) collagen deposition (top) and (b) α-actin expression (bottom) in IPAs from sham (left) and castrated (right) rats. Light microscopy of IPAs stained with picrosirius red shows collagen in red color. α-actin expression, indicated by the brown color, was determined by immunohistochemistry. Error bars represent mean ± standard error of the mean, n = 5. *P < 0.05 versus sham.
Immunohistochemistry and protein expression
Vascular remodeling is a complex set of vascular changes, ranging from changes in the phenotype and function of vascular smooth muscle cells (VSMC) to changes in the extracellular matrix structure and composition, which lead to altered vessel wall-to-lumen ratio. To determine whether IPAs from castrated rats underwent vascular changes in composition, the expression of α-actin was evaluated. IPAs from castrated animals presented a decrease in α-actin expression when compared to sham rats as represented in Figure 5b.
Considering the decreased NANC relaxation in IPAs from castrated animals, immunohistochemical experiments were performed in sham and castrated animals to test the hypothesis that androgen deprivation induces a decrease in nNOS protein expression. nNOS expression was mainly located in the intersection between adventitia and media layer on vascular wall (Figure 6a). In addition, nNOS expression was decreased in IPAs from castrated animals (Figure 6b). These results were further confirmed by evaluation of total nNOS protein expression.
Figure 6.
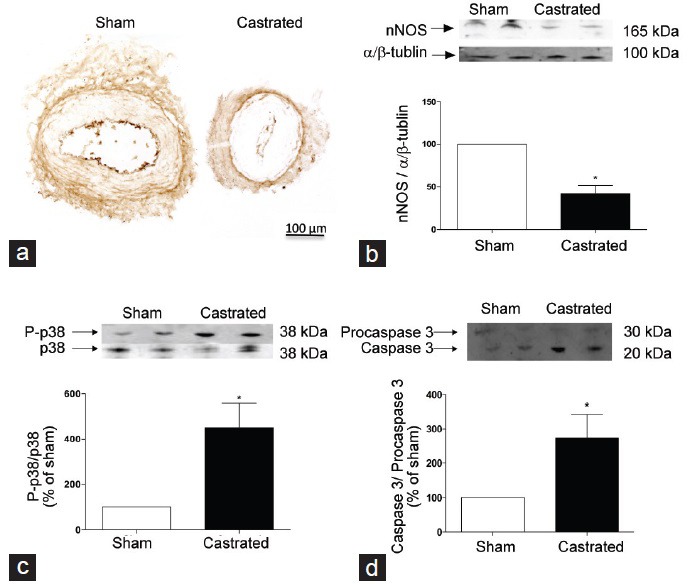
Orchiectomy induces changes in neuronal nitric oxide synthase (nNOS), phosphorylated p38 MAP-kinase and caspase 3 in internal pudendal arteries (IPAs). (a) Representative immunohistochemistry photomicrographies depict nNOS expression in IPAs from sham (left) and castrated (right) rats. (b) Western blot representative images (on top) and densitometric analysis (bar graphs) of nNOS, (c) phosphorylated p38 MAP-kinase and (d) caspase 3 protein expression in IPAs from sham (open bars) and castrated (filled bars) rats. Data are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus sham.
Orchiectomy increased the phosphorylation levels of the p38 mitogen-activated protein kinase (MAPK) in IPAs when compared to sham animals (Figure 6c). Moreover, castrated animals displayed increased caspase 3/pro-caspase 3 expression ratio (Figure 6d).
DISCUSSION
Hypogonadism is widely accepted as the major endocrinopathy leading to ED.36 The present study shows evidence that androgen deprivation induces functional and structural changes in IPAs, which could be a new target in the treatment of hypogonadism-associated ED.
Orchiectomy caused a marked decrease in the circulating plasma levels of testosterone, which was followed by ED, characterized by decreased ICP/MAP ratio. The contractile responses induced by Phe and EFS were reduced in castrated rats, as well as vasodilatation induced by ACh and NANC stimulation, when compared to IPAs from sham rats. IPAs from castrated animals displayed increased collagen expression and vascular remodeling, characterized by decreased internal diameter as well as decreased external diameter and cross-sectional area. Although nNOS and α-actin protein expression was decreased, p38 MAPK phosphorylation and caspase 3 cleavage were increased in IPAs isolated from castrated animals.
Although testosterone effects on IPAs function are scarcely known, many studies have addressed the effects of androgen on erectile function. Cavernosal strips obtained from castrated rabbits present decreased phenylephrine-induced contractions and adenosine-induced vasodilation, which are restored by in vivo testosterone replacement.37 In young men, treatment with gonadotropin-releasing hormone antagonist for 6 weeks decreased the frequency of sexual desire, sexual fantasies, spontaneous erections, and remarkably decreased the frequency of masturbation, which was reversed by 3 weeks posttreatment.38 Testosterone replacement also reversed ED, or at least improved erectile function, in patients with hypogonadism.37,39
Adrenergic receptors have an important role in erectile function. In clinical studies, intracavernous injection of prazosin, an α1-adrenergic receptor antagonist, increases intracavernosal pressure,40,41 an effect also observed by injection of α1, 2-adrenergic receptors antagonist.42 On the other hand, administration of an α2 receptor antagonist is not able to increase the ICP at the same magnitude.43 One of the mechanisms by which androgens maintain erectile function is via regulation of α1-adrenergic responses,44 an effect that could be impaired by hypogonadism. Castration reduces the number of functional α1-adrenergic receptors in corpus cavernosum, an effect restored by testosterone replacement.18 Androgen deprivation also decreases α1-adrenergic receptors-mediated contractility of rat prostate strips, which was associated to down-regulation of α1 adrenoceptors population.45 It is possible that, as in cavernosal tissue and prostate strips, α1 receptors are also downregulated by hypogonadism in IPAs. This as well as the hypotrophic vascular remodeling may explain how decreased androgen levels lead to decreased IPA constriction. The impairment of vasoconstrictor and vasodilatator responses in IPAs from castrated animals, plus the changes in the IPA's mechanical properties due to remodeling, provide strong evidence that hypogonadism-induced ED is a vasculogenic event, defined as impaired perfusion of the corpora cavernosa.46,47
Erectile function involves an increase in arterial blood inflow to the corpus cavernosum, relaxation of its smooth muscle, where nitric oxide (NO) is important, and control of venous outflow.48 It is possible that NO has an important role in castration-induced IPAs dysfunction. Endothelial- and NANC-mediated smooth muscle relaxation is evident in IPAs from sham and castrated animals, but both are sharply impaired in orchidectomized animals. The studies evaluating NANC relaxation in pudendal arteries are limited, but in cavernosal tissue, orchiectomy decreases the density of nonadrenergic noncholinergic nerve fibers, effect proportional to the time postorchiectomy, and partially reversed by androgen treatment,49 it is possible that the same event occurs in pudendal artery after castration, which would lead to a decrease in NANC-induced vasorelaxation. Decreased IPAs relaxation in castrated animals may be due to decreased NO bioavailability, since nNOS expression decreased in arteries from those animals and vasorelaxation is not impaired in response to a NO donor, SNP. Castration modulates NOS not only in IPAs, Park et al. showed that hypogonadism caused a marked decrease in NOS activity, eNOS expression,50 and nNOS mRNA expression in cavernosal tissue, which was partially fixed by testosterone replacement and restored by DHT.51 Interestingly, androgens can have opposite outcomes in other tissues, since castration resulted in a 4-fold rise in pituitary nNOS protein expression.52
Vascular remodeling or alterations in the vascular structure as a result of cell growth and rearrangement of cellular components and extracellular matrix proteins, contributes to vascular resistance.53 Vascular remodeling can be either eutrophic, a process of changing the vessel wall without changes in the amount or characteristics of the VSMC; hypertrophic, a situation involving an increase in the amount of cells; or hypotrophic, which involves a reduction in the amount of VSMC content.53,54 Our data show that IPAs from castrated animals present inward hypotrophic vascular remodeling, which denotes a reduction in vessel size. This structural change may decrease blood flow to corpora cavernosa and may impact erectile function.
Whereas the number of VSMC was decreased, there was increased collagen deposition in IPAs from castrated animals, suggesting the replacement of VSMC by collagen. Collagen deposition contributes to the remodeling process and changes in vascular stiffness, which may be related to the decreased Phe-induced vasoconstriction, since changes in the amount of VSMC impair vascular function. Hypogonadism effect in collagen is not exclusive to IPAs, castration increases collagen staining, thickness, and epithelial atrophy in prostate lobes of Mongolian gerbil rodent.55
At least four processes are involved in vascular remodeling: cell death, cell growth, cell migration, and degradation or synthesis of extracellular matrix.53 It is not clear if apoptosis is a growth-related compensatory mechanism or a primary process in vascular remodeling and its modulator are numerous, such as reactive oxygen species, NO, angiotensin-II, and the endothelin-1 system.56 Mitogen-activated protein kinases such as p38 kinase, play an important regulatory role in apoptosis, regulating caspase 3 activation.57,58 IPAs from castrated animals present decrease in α-actin expression, which indicates a decrease in the amount of VSMC, increase in phosphor p38 and cleaved caspase 3, events that regulate apoptosis signaling and therefore, vascular remodeling. In a rabbit model, castration also reduces trabecular smooth muscle content in corpus cavernosum when compared to control, event restored by testosterone replacement.18
This study is not the first one associating androgen to apoptosis signaling pathway. In human prostatic epithelium, castration gradually increases the level of epithelial apoptosis from 0.026% on day 0 to 1.54% on postcastration day 3.59 In rat ventral prostate, 6–12 h after castration is enough to cause an increase in apoptosis.60,61 In mice, apoptosis is observed 2–4 days after castration in prostate cells.62 In terms of muscle cell (MC), 7 days of castration leads to decrease in the volume of MC from rat ventral prostate.63 In addition, castration increased cardiomyocyte apoptosis and fibrosis in Wistar rats, which was followed by decreased anti-apoptosis Bcl-2 protein expression.64 Hypogonadism is also related to tissue degeneration and trabecular smooth muscle apoptosis in corpora cavernosa.65,66
Androgen receptors are distributed in several cells/tissues, including endothelial cells and vascular smooth muscle cells, but it is unclear the relationship between testosterone levels and its effects, ranging from protective to deleterious ones.67,68,69 Whereas high doses of testosterone have been associated with liver disease, sudden cardiac death, and ROS generation, low levels are associated with the progression of atherosclerosis, production of pro-inflammatory cytokines and low-density lipoprotein, increased levels of glucose, total cholesterol, and increased arterial thickness.70,71,72,73,74
Testosterone is the major circulating androgen and is converted by 5α-reductase to DHT,75 which is mentioned as much more potent than testosterone.75,76 An important limitation of our study is the absence of evidence showing which androgen is involved in hypogonadism-induced IPAs vascular dysfunction, such as testosterone, DHT, or androstenedione, and if castration modulates enzymes important for androgen generation, such as 5α-reductase. Finally, whether androgen replacement is able to revert or prevent IPAs dysfunction warrants further investigation.
CONCLUSIONS
We demonstrated that androgen deprivation contributes to functional and structural dysfunction of IPAs, which is followed by decrease in α-actin expression and increase in collagen and proteins that regulates apoptosis pathway. Our data support the idea that hypogonadism-induced vascular dysfunction is an event involving hypotrophic vascular remodeling of IPAs, which may compromise blood inflow to the cavernous tissue. This new approach presents IPAs as a novel therapeutic target in hypogonadism-induced erectile dysfunction.
AUTHOR CONTRIBUTIONS
RAML, RCT, and FSC prepared the study conception and designed the study. RAML, KBN, MABS, VCO, SGR, LNZR, and FSC acquired the data. RAML, KBN, VCO, SGR, LNZR, RCT, and FSC analyzed and interpreted the data. RAML and FSC drafted the manuscript. JAR, SGR, LNZR, RCT, and FSC performed critical revision.
COMPETING INTERESTS
None of the authors declare competing financial interests.
ACKNOWLEDGMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-2010-17362-4 and 2011-23060-3), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
REFERENCES
- 1.Leite R, Giachini FR, Carneiro FS, Nunes KP, Tostes RC, et al. Targets for the treatment of erectile dysfunction: is NO/cGMP still the answer? Recent Pat Cardiovasc Drug Discov. 2007;2:119–32. doi: 10.2174/157489007780832579. [DOI] [PubMed] [Google Scholar]
- 2.Priviero FB, Leite R, Webb RC, Teixeira CE. Neurophysiological basis of penile erection. Acta Pharmacol Sin. 2007;28:751–5. doi: 10.1111/j.1745-7254.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 3.Aboseif SR, Breza J, Orvis BR, Lue TF, Tanagho EA. Erectile response to acute and chronic occlusion of the internal pudendal and penile arteries. J Urol. 1989;141:398–402. doi: 10.1016/s0022-5347(17)40782-8. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- 5.Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 6.Manabe K, Heaton JP, Morales A, Kumon H, Adams MA. Pre-penile arteries are dominant in the regulation of penile vascular resistance in the rat. Int J Impot Res. 2000;12:183–9. doi: 10.1038/sj.ijir.3900526. [DOI] [PubMed] [Google Scholar]
- 7.Bookstein JJ, Valji K, Parsons L, Kessler W. Pharmacoarteriography in the evaluation of impotence. J Urol. 1987;137:333–7. doi: 10.1016/s0022-5347(17)44017-1. [DOI] [PubMed] [Google Scholar]
- 8.Mueller SC, von Wallenberg-Pachaly H, Voges GE, Schild HH. Comparison of selective internal iliac pharmaco-angiography, penile brachial index and duplex sonography with pulsed Doppler analysis for the evaluation of vasculogenic (arteriogenic) impotence. J Urol. 1990;143:928–32. doi: 10.1016/s0022-5347(17)40140-6. [DOI] [PubMed] [Google Scholar]
- 9.Rosen MP, Greenfield AJ, Walker TG, Grant P, Guben JK, et al. Arteriogenic impotence: findings in 195 impotent men examined with selective internal pudendal angiography. Young Investigator's Award. Radiology. 1990;174:1043–8. doi: 10.1148/radiology.174.3.174-3-1043. [DOI] [PubMed] [Google Scholar]
- 10.Utkan T, Sarioglu Y, Utkan NZ, Kurnaz F, Yildirim S. Effects of chronic unilateral internal pudendal arterial occlusion on reactivity of isolated corpus cavernosum strips from rabbits. Eur J Pharmacol. 1999;367:73–9. doi: 10.1016/s0014-2999(98)00930-3. [DOI] [PubMed] [Google Scholar]
- 11.NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 12.Celik O, Yucel S. Testosterone replacement therapy: should it be performed in erectile dysfunction? Nephrourol Mon. 2013;5:858–61. doi: 10.5812/numonthly.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales A. Androgens are fundamental in the maintenance of male sexual health. Curr Urol Rep. 2011;12:453–60. doi: 10.1007/s11934-011-0202-4. [DOI] [PubMed] [Google Scholar]
- 14.Simpson SM, Marshal FHA. On the effect of stimulating the nervi erigentes in castrated animals. Exp Physiol. 1908;1:257–9. [Google Scholar]
- 15.Muller SC, Hsieh JT, Lue TF, Tanagho EA. Castration and erection. An animal study. Eur Urol. 1988;15:118–24. doi: 10.1159/000473410. [DOI] [PubMed] [Google Scholar]
- 16.Gray GD, Smith ER, Davidson JM. Hormonal regulation of penile erection in castrated male rats. Physiol Behav. 1980;24:463–8. doi: 10.1016/0031-9384(80)90237-1. [DOI] [PubMed] [Google Scholar]
- 17.Mills TM, Stopper VS, Wiedmeier VT. Effects of castration and androgen replacement on the hemodynamics of penile erection in the rat. Biol Reprod. 1994;51:234–8. doi: 10.1095/biolreprod51.2.234. [DOI] [PubMed] [Google Scholar]
- 18.Traish AM, Park K, Dhir V, Kim NN, Moreland RB, et al. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999;140:1861–8. doi: 10.1210/endo.140.4.6655. [DOI] [PubMed] [Google Scholar]
- 19.Palese MA, Crone JK, Burnett AL. A castrated mouse model of erectile dysfunction. J Androl. 2003;24:699–703. doi: 10.1002/j.1939-4640.2003.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 20.Morales A, Johnston B, Heaton JP, Lundie M. Testosterone supplementation for hypogonadal impotence: assessment of biochemical measures and therapeutic outcomes. J Urol. 1997;157:849–54. doi: 10.1016/s0022-5347(01)65062-6. [DOI] [PubMed] [Google Scholar]
- 21.Saad F, Gooren LJ, Haider A, Yassin A. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl. 2008;29:102–5. doi: 10.2164/jandrol.107.002774. [DOI] [PubMed] [Google Scholar]
- 22.Hwang TI, Lin YC. The relationship between hypogonadism and erectile dysfunction. Int J Impot Res. 2008;20:231–5. doi: 10.1038/sj.ijir.3901633. [DOI] [PubMed] [Google Scholar]
- 23.Peters CA, Walsh PC. The effect of nafarelin acetate, a luteinizing-hormone-releasing hormone agonist, on benign prostatic hyperplasia. N Engl J Med. 1987;317:599–604. doi: 10.1056/NEJM198709033171004. [DOI] [PubMed] [Google Scholar]
- 24.Rousseau L, Dupont A, Labrie F, Couture M. Sexuality changes in prostate cancer patients receiving antihormonal therapy combining the antiandrogen flutamide with medical (LHRH agonist) or surgical castration. Arch Sex Behav. 1988;17:87–98. doi: 10.1007/BF01542054. [DOI] [PubMed] [Google Scholar]
- 25.Marumo K, Baba S, Murai M. Erectile function and nocturnal penile tumescence in patients with prostate cancer undergoing luteinizing hormone-releasing hormone agonist therapy. Int J Urol. 1999;6:19–23. doi: 10.1046/j.1442-2042.1999.06128.x. [DOI] [PubMed] [Google Scholar]
- 26.Greenstein A, Plymate SR, Katz PG. Visually stimulated erection in castrated men. J Urol. 1995;153:650–2. doi: 10.1097/00005392-199503000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Eri LM, Tveter KJ. Safety, side effects and patient acceptance of the luteinizing hormone releasing hormone agonist leuprolide in treatment of benign prostatic hyperplasia. J Urol. 1994;152:448–52. doi: 10.1016/s0022-5347(17)32760-x. [DOI] [PubMed] [Google Scholar]
- 28.Hirshkowitz M, Moore CA, O’Connor S, Bellamy M, Cunningham GR. Androgen and sleep-related erections. J Psychosom Res. 1997;42:541–6. doi: 10.1016/s0022-3999(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 29.Meusburger SM, Keast JR. Testosterone and nerve growth factor have distinct but interacting effects on structure and neurotransmitter expression of adult pelvic ganglion cells in vitro. Neuroscience. 2001;108:331–40. doi: 10.1016/s0306-4522(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 30.Keast JR, Gleeson RJ, Shulkes A, Morris MJ. Maturational and maintenance effects of testosterone on terminal axon density and neuropeptide expression in the rat vas deferens. Neuroscience. 2002;112:391–8. doi: 10.1016/s0306-4522(02)00077-5. [DOI] [PubMed] [Google Scholar]
- 31.Armagan A, Hatsushi K, Toselli P. The effects of testosterone deficiency on the structural integrity of the penile dorsal nerve in the rat. Int J Impot Res. 2008;20:73–8. doi: 10.1038/sj.ijir.3901614. [DOI] [PubMed] [Google Scholar]
- 32.Baba K, Yajima M, Carrier S, Akkus E, Reman J, et al. Effect of testosterone on the number of NADPH diaphorase-stained nerve fibers in the rat corpus cavernosum and dorsal nerve. Urology. 2000;56:533–8. doi: 10.1016/s0090-4295(00)00667-1. [DOI] [PubMed] [Google Scholar]
- 33.Lu YL, Kuang L, Zhu H, Wu H, Wang XF, et al. Changes in aortic endothelium ultrastructure in male rats following castration, replacement with testosterone and administration of 5 alpha-reductase inhibitor. Asian J Androl. 2007;9:843–7. doi: 10.1111/j.1745-7262.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 34.Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029–34. doi: 10.1291/hypres.30.1029. [DOI] [PubMed] [Google Scholar]
- 35.Zhang XH, Melman A, Disanto ME. Update on corpus cavernosum smooth muscle contractile pathways in erectile function: a role for testosterone? J Sex Med. 2011;8:1865–79. doi: 10.1111/j.1743-6109.2011.02218.x. [DOI] [PubMed] [Google Scholar]
- 36.Rajfer J. Relationship between testosterone and erectile dysfunction. Rev Urol. 2000;2:122–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Yildirim MK, Yildirim S, Utkan T, Sarioglu Y, Yalman Y. Effects of castration on adrenergic, cholinergic and nonadrenergic, noncholinergic responses of isolated corpus cavernosum from rabbit. Br J Urol. 1997;79:964–70. doi: 10.1046/j.1464-410x.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- 38.Bagatell CJ, Heiman JR, Rivier JE, Bremner WJ. Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. J Clin Endocrinol Metab. 1994;78:711–6. doi: 10.1210/jcem.78.3.8126146. [DOI] [PubMed] [Google Scholar]
- 39.O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146–51. doi: 10.1192/bjp.145.2.146. [DOI] [PubMed] [Google Scholar]
- 40.Blum MD, Bahnson RR, Porter TN, Carter MF. Effect of local alpha-adrenergic blockade on human penile erection. J Urol. 1985;134:479–81. doi: 10.1016/s0022-5347(17)47248-x. [DOI] [PubMed] [Google Scholar]
- 41.Holmquist F, Hedlund H, Andersson KE. Effects of the alpha 1-adrenoceptor antagonist R-(-)-YM12617 on isolated human penile erectile tissue and vas deferens. Eur J Pharmacol. 1990;186:87–93. doi: 10.1016/0014-2999(90)94063-4. [DOI] [PubMed] [Google Scholar]
- 42.Diederichs W, Lue TF. Reduction of sympathetic influences on penile erection by phentolamine. Urol Int. 1991;46:64–6. doi: 10.1159/000281778. [DOI] [PubMed] [Google Scholar]
- 43.Argiolas A, Melis MR. Neuromodulation of penile erection: an overview of the role of neurotransmitters and neuropeptides. Prog Neurobiol. 1995;47:235–55. [PubMed] [Google Scholar]
- 44.Reilly CM, Stopper VS, Mills TM. Androgens modulate the alpha-adrenergic responsiveness of vascular smooth muscle in the corpus cavernosum. J Androl. 1997;18:26–31. [PubMed] [Google Scholar]
- 45.Homma Y, Hamada K, Nakayama Y, Tsujimoto G, Kawabe K. Effects of castration on contraction and alpha(1)-adrenoceptor expression in rat prostate. Br J Pharmacol. 2000;131:1454–60. doi: 10.1038/sj.bjp.0703706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hale TM, Hannan JL, Carrier S, deBlois D, Adams MA. Targeting vascular structure for the treatment of sexual dysfunction. J Sex Med. 2009;6(Suppl 3):210–20. doi: 10.1111/j.1743-6109.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- 47.Saenz de Tejada I, Angulo J, Cellek S, Gonzalez-Cadavid N, Heaton J, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005;2:26–39. doi: 10.1111/j.1743-6109.2005.20103.x. [DOI] [PubMed] [Google Scholar]
- 48.Nangle MR, Cotter MA, Cameron NE. An in vitro investigation of aorta and corpus cavernosum from eNOS and nNOS gene-deficient mice. Pflugers Arch. 2004;448:139–45. doi: 10.1007/s00424-003-1232-7. [DOI] [PubMed] [Google Scholar]
- 49.Zvara P, Sioufi R, Schipper HM, Begin LR, Brock GB. Nitric oxide mediated erectile activity is a testosterone dependent event: a rat erection model. Int J Impot Res. 1995;7:209–19. [PubMed] [Google Scholar]
- 50.Park KH, Kim SW, Kim KD, Paick JS. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int. 1999;83:327–33. doi: 10.1046/j.1464-410x.1999.00913.x. [DOI] [PubMed] [Google Scholar]
- 51.Park KH, Kim SW, Kim KD, Paick JS. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int. 1999;83:327–33. doi: 10.1046/j.1464-410x.1999.00913.x. [DOI] [PubMed] [Google Scholar]
- 52.Shi Q, LaPaglia N, Emanuele NV, Emanuele MA. Castration differentially regulates nitric oxide synthase in the hypothalamus and pituitary. Endocr Res. 1998;24:29–54. doi: 10.3109/07435809809031867. [DOI] [PubMed] [Google Scholar]
- 53.Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. 2013;2013:808353. doi: 10.1155/2013/808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, et al. Vascular remodeling. Hypertension. 1996;28:505–6. [PubMed] [Google Scholar]
- 55.Goes RM, Zanetoni C, Tomiosso TC, Ribeiro DL, Taboga SR. Surgical and chemical castration induce differential histological response in prostate lobes of Mongolian gerbil. Micron. 2007;38:231–6. doi: 10.1016/j.micron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 56.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–7. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Sun L, Xia C, Ye L, Wang B. P38MAPK regulates caspase-3 by binding to caspase-3 in nucleus of human hepatoma Bel-7402 cells during anti-Fas antibody- and actinomycin D-induced apoptosis. Biomed Pharmacother. 2009;63:343–50. doi: 10.1016/j.biopha.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Sarker KP, Nakata M, Kitajima I, Nakajima T, Maruyama I. Inhibition of caspase-3 activation by SB 203580, p38 mitogen-activated protein kinase inhibitor in nitric oxide-induced apoptosis of PC-12 cells. J Mol Neurosci. 2000;15:243–50. doi: 10.1385/JMN:15:3:243. [DOI] [PubMed] [Google Scholar]
- 59.Staack A, Kassis AP, Olshen A, Wang Y, Wu D, et al. Quantitation of apoptotic activity following castration in human prostatic tissue in vivo. Prostate. 2003;54:212–9. doi: 10.1002/pros.10179. [DOI] [PubMed] [Google Scholar]
- 60.Nickerson T, Pollak M, Huynh H. Castration-induced apoptosis in the rat ventral prostate is associated with increased expression of genes encoding insulin-like growth factor binding proteins 2,3,4 and 5. Endocrinology. 1998;139:807–10. doi: 10.1210/endo.139.2.5912. [DOI] [PubMed] [Google Scholar]
- 61.Shabisgh A, Tanji N, D’Agati V, Burchardt M, Rubin M, et al. Early effects of castration on the vascular system of the rat ventral prostate gland. Endocrinology. 1999;140:1920–6. doi: 10.1210/endo.140.4.6644. [DOI] [PubMed] [Google Scholar]
- 62.Feng Z, Joos HJ, Vallan C, Muhlbauer R, Altermatt HJ, et al. Apoptosis during castration-induced regression of the prostate is fos dependent. Oncogene. 1998;17:2593–600. doi: 10.1038/sj.onc.1202195. [DOI] [PubMed] [Google Scholar]
- 63.Antonioli E, Della-Colleta HH, Carvalho HF. Smooth muscle cell behavior in the ventral prostate of castrated rats. J Androl. 2004;25:50–6. doi: 10.1002/j.1939-4640.2004.tb02758.x. [DOI] [PubMed] [Google Scholar]
- 64.Kang NN, Fu L, Xu J, Han Y, Cao JX, et al. Testosterone improves cardiac function and alters angiotensin II receptors in isoproterenol-induced heart failure. Arch Cardiovasc Dis. 2012;105:68–76. doi: 10.1016/j.acvd.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Shabsigh R, Raymond JF, Olsson CA, O’Toole K, Buttyan R. Androgen induction of DNA synthesis in the rat penis. Urology. 1998;52:723–8. doi: 10.1016/s0090-4295(98)00233-7. [DOI] [PubMed] [Google Scholar]
- 66.Traish AM, Toselli P, Jeong SJ, Kim NN. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiency. J Androl. 2005;26:242–8. doi: 10.1002/j.1939-4640.2005.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 67.Reckelhoff JF. Sex steroids, cardiovascular disease, and hypertension: unanswered questions and some speculations. Hypertension. 2005;45:170–4. doi: 10.1161/01.HYP.0000151825.36598.36. [DOI] [PubMed] [Google Scholar]
- 68.Lopes RA, Neves KB, Carneiro FS, Tostes RC. Testosterone and vascular function in aging. Front Physiol. 2012;3:89. doi: 10.3389/fphys.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab. 1999;84:3459–62. doi: 10.1210/jcem.84.10.6122. [DOI] [PubMed] [Google Scholar]
- 70.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–9. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 71.Miller VM, Tindall DJ, Liu PY. Of mice, men, and hormones. Arterioscler Thromb Vasc Biol. 2004;24:995–7. doi: 10.1161/01.ATV.0000130660.53541.a4. [DOI] [PubMed] [Google Scholar]
- 72.Francomano D, Bruzziches R, Natali M, Aversa A, Spera G. Cardiovascular effect of testosterone replacement therapy in aging male. Acta Biomed. 2010;81(Suppl 1):101–6. [PubMed] [Google Scholar]
- 73.Bagatell CJ, Bremner WJ. Androgens in men – Uses and abuses. N Engl J Med. 1996;334:707–14. doi: 10.1056/NEJM199603143341107. [DOI] [PubMed] [Google Scholar]
- 74.Lopes RA, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, et al. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am J Physiol Heart Circ Physiol. 2014;306:H1485–94. doi: 10.1152/ajpheart.00809.2013. [DOI] [PubMed] [Google Scholar]
- 75.Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88:15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 76.Wright AS, Thomas LN, Douglas RC, Lazier CB, Rittmaster RS. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest. 1996;98:2558–63. doi: 10.1172/JCI119074. [DOI] [PMC free article] [PubMed] [Google Scholar]


