Abstract
Spermatogenesis is an androgen-regulated process that depends on the action of androgen receptor (AR). Sperm production may be affected in men treated for testicular cancer (TC), and it is important to identify the factors influencing the timing of spermatogenesis recovery following cancer treatment. It is known that the CAG and GGN repeat numbers affect the activity of the AR; therefore, the aim of this study is to investigate if the CAG and GGN polymorphisms in the AR gene predict recovery of sperm production after TC treatment. TC patients (n = 130) delivered ejaculates at the following time points: postorchiectomy and at 6, 12, 24, 36, and 60 months posttherapy (T0, T6, T12, T24, T36, and T60). The CAG lengths were categorized into three groups, <22 CAG, 22–23 CAG, and >23 CAG, and the GGN tracts were also categorized into three groups, <23 GGN, 23 GGN, and >23 GGN. At T12, men with 22–23 CAG presented with a statistically significantly (P = 0.045) lower sperm concentration than those with other CAG numbers (8.4 × 106 ml−1 vs 16 × 106 ml−1; 95% CI: 1.01–2.65). This association was robust to omitting adjustment for treatment type and sperm concentration at T0 (P = 0.021; 3.7 × 106 ml−1 vs 10 × 106 ml−1; 95% CI: 1.13–4.90). The same trends were observed for total sperm number. The least active AR variant seems to be associated with a more rapid recovery of spermatogenesis. This finding adds to our understanding of the biology of postcancer therapy recovery of fertility in males and has clinical implications.
Keywords: androgen receptor, CAG and GGN repeat, sperm number, testicular cancer
INTRODUCTION
The incidence of testicular germ cell cancer (TC) is constantly increasing in western countries. TC is a highly curable disease with an overall survival rate approaching 98%.1,2,3,4 One of the clinical challenges is the long-term sequelae of TC and its treatment. The available data show that total sperm number is decreased and fertility is impaired in this group of young men even before initiation of treatment. It has been discussed whether testis cancer, along with undescended testis and hypospadias, is part of the testicular dysgenesis syndrome and has common etiological factors. Spermatogenesis and fertility can be additionally hampered by the therapy given postorchiectomy.5,6,7 The time course is crucial in reproductive issues, and TC patients need information not only about semen preservation but also about the chance of recovery of spermatogenesis and fertility. Therefore, defining the prognostic factors of posttreatment spermatogenesis recovery is important in predicting fertility and in family planning.8
Spermatogenesis is a highly androgen-dependent process. Testosterone and 5α-dihydrotestosterone mediate their effects through the AR, acting as the transcription factor. The androgen action is empowered by the effect of available testosterone and 5α-dihydrotestosterone and the AR response in Sertoli cells.9,10,11,12 The activity of the AR has shown to be regulated by two repetitive sequences in exon I of the AR encoding gene, the CAG and GGN repeats.13,14,15 In the general population, CAG length is typically distributed between 10 and 30 repeats with an average length of 22–23 in most populations.13,15,16 Previous in vitro and in vivo studies have suggested a more active AR at lengths corresponding to average lengths17,18,19 in both animals and humans.20 For GGN, 23 is the most common variant in Caucasians, found in 50% of cases, and is also responsible for the highest AR activity.21,22,23,24
The available data indicate that there is considerable interindividual variation in the way certain TC treatments affect sperm production and also in the timing of spermatogenesis recovery.5,6,25,26,27,28,29,30 Earlier studies have indicated that pretreatment levels of follicle-stimulating hormone, sperm concentration, and sperm chromatin structure predict recovery of sperm concentration after treatment.5,6,30,31,32 The predictive values available are still scarce,5,6,30,31,32 and we have limited information for young patients in a clinical setting. Because the AR polymorphism affects the AR activity and some studies indicate that long CAG repeats are associated with male infertility,33 it is plausible that the genetic variant has an impact on sperm production recovery after TC.
A possible genetic association was first indicated in a previous pilot study in which we reported an inverse correlation between the AR CAG repeat length and sperm concentration 1–2 years after 3–4 cycles of cisplatin-based chemotherapy for TC.7 However, the analysis was based on only 9 patients. With this study, we sought to investigate this possible genetic association between variation in the lengths of the AR CAG and GGN tracts and sperm production recovery following TC treatment in a larger cohort of TC patients.
PATIENTS AND METHODS
Patients
The patients in the current study were selected from a larger cohort of TC patients participating in a study on reproductive function in men treated for TC in Lund or Stockholm between 2001 and 2011. All participants were of Caucasian origin. The prerequisite for inclusion in the study was that radiotherapy (RT) or chemotherapy (CT) was given postorchiectomy.
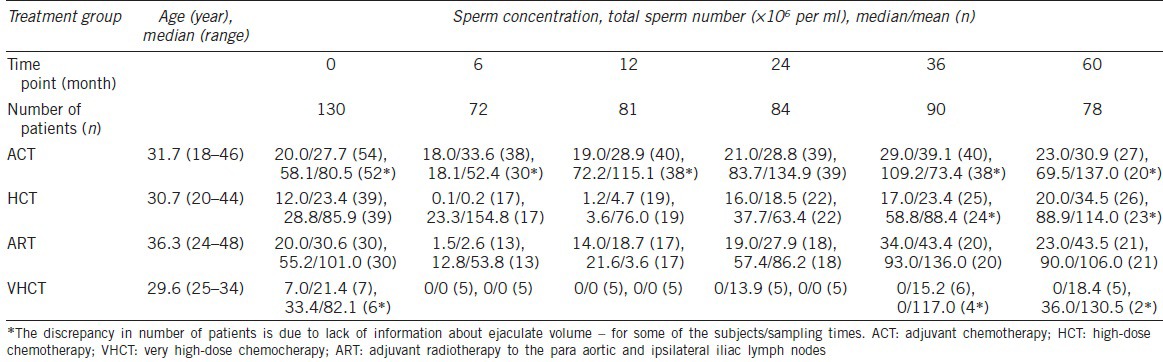
Six time points for delivery of ejaculates were defined as follows: after orchiectomy but before further treatment (T0) and 6 (T6), 12 (T12), 24 (T24), 36 (T36), and 60 (T60) months after completion of RT or CT. The follow-up was terminated at T60 or at the end of the study, whichever occurred first. After entering the study, the patients were asked to provide semen and blood samples at the remaining time points. Because we needed to adjust our findings for sperm number at T0 (see Statistical analysis) from this group, only those men who entered the study at T0 or for whom an ejaculate was delivered for cryopreservation before CT or RT was available were included in the final calculation. In the total cohort, 151 patients were eligible for the study and, as DNA was collected from 130 individuals, the final number included 130 participants, giving a participation rate of 86%. There was no difference in age or stage of disease between the included and excluded patients. The number of posttreatment ejaculates varied, being 72 at T6, 81 at T12, 84 at T24, 90 at T36, and finally, 78 at T60. Characteristics of the patients included in this study, sperm concentration and sperm count for the separate treatment groups at the different time points are given in Table 1.
Table 1.
Characteristics of 130 patients included in this study, and sperm concentration and sperm count for the separate treatment groups at the different time points
Cancer treatment
Cancer treatment was categorized into four groups: adjuvant CT given to patients without clinically obvious metastasis (clinical stage I, CSI) (ACT); standard CT given to patients with metastatic disease (HCT); adjuvant RT used only for seminoma patients with CSI disease (ART); and high-dose therapy given to patients with relapsing disease (VHCT). Depending on the histological type of TC and the disease stage, the patients were given treatment after orchiectomy according to the Swedish and Norwegian Testicular Cancer group (SWENOTECA)34 cancer care program. ACT was given to patients with nonseminomatous TC (NSGCT), and treated with 1–2 cycles of cisplatin-based CT (bleomycin, etoposide, and cisplatin [BEP], or cisplatin, vincristin, and bleomycin [CVB]); and, in patients with more advanced NSGCT disease, 3–4 cycles of cisplatin-based CT were given with HCT.
Patients with CSI seminomatous germ cell tumors (SGCT) received either ACT or ART. ART was administered in 14 fractions to a total target dose of 25 Gy; 2 Gy were delivered to the para-aortic and ipsilateral iliac lymph nodes of 29 patients. The dose of scattered radiation in the remaining lead-shielded testicle was retrospectively estimated in seven patients to be a maximum of 0.43 Gy.
The standard ACT treatment for SGCT patients was one or two cycles of carboplatin. Patients with metastatic seminoma were treated with four cycles of cisplatin-based CT (EP).
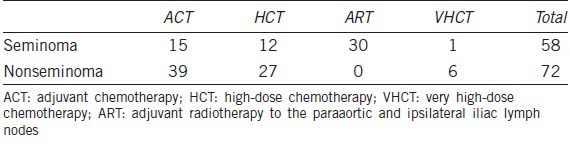
Patients with relapsing disease received VHCT ≥4 cycles of CT or CT combined with RT. CT used for relapse was also cisplatin based, but with the addition of an alkylating drug, ifosfamide (PEI). Information regarding the distribution of patients in the different treatment groups is given in Table 2.
Table 2.
Distribution of study participants according to the type of treatment given
The study was approved by the Ethics Committee at Lund University and at Karolinska Institute, and all patients provided written informed consent.
Biological samples
Semen analysis
All men were asked to maintain 2–7 days of ejaculation abstinence before delivering semen samples at home or in the laboratory. Ejaculates were analyzed according to the 1999 World Health Organization (WHO) recommendations.35 The sperm concentration was determined using positive displacement pipettes for diluting the samples and with an improved Neubauer chamber for counting. The total sperm number was calculated by multiplying the concentration and the semen volume. The latter was determined by weighing the ejaculate.
All but 29 T0 ejaculates were analyzed at the Reproductive Medicine Centre (RMC), Skåne University Hospital, Malmo, Sweden, or at the Andrology Unit, Karolinska Hospital, Stockholm, Sweden. The laboratories involved in the studies performed semen analysis according to the WHO guidelines and participated in the European Society of Human Reproduction and Embryology-Nordic Association for Andrology (ESHRE-NAFA) external quality control program.36 Among the remaining samples, 28 were analyzed at the Fertility Laboratory, Lund University Hospital, Lund, Sweden and one was analyzed at Linköping University Hospital, Linköping, Sweden.
DNA analysis
DNA was extracted from peripheral leukocytes. The AR CAG and GGN stretches were amplified by PCR, purified and directly sequenced in a Beckman Coulter CEQ 2000 XL (Beckman Coulter, Bromma, Sweden) sequencing gear as previously described.22
Statistical analysis
A unilateral regression model was used to analyze the association between CAG and GGN numbers and sperm concentration at all six time points (T0, T6, T12, T24, T36, and T60). For sperm concentrations and total sperm numbers in ejaculates delivered at T6 or later, the calculations were adjusted for age, sperm concentration at T0, and for the type of treatment given. If a statistically significant association between genotype and sperm concentration or total number was found, a corresponding nonadjusted analysis was performed.
The CAG and the GGN lengths were primarily trichotomized (<22 CAG, 22–23 CAG, and >23 CAG; <23 GGN, 23 GGN, and >23 GGN) according to the results of previous functional studies. Subsequently, to increase the statistical power, the two length intervals associated with the less active receptor were pooled together and compared with the most active receptor variant for each polymorphism (CAG: 22–23 set as reference and compared with <22 CAG or >23 CAG; GGN: 23 set as reference and compared with <23 GGN or >23 GGN).
To obtain a normal distribution of residuals, sperm concentration values and total sperm numbers were transformed using the natural logarithm (Ln). Because of some null values (azoospermic cases), 1 was added to all sperm concentrations/total numbers before the Ln transformation. Group characteristics were expressed as means and standard error (s.e.). Furthermore, back-transformed 95% confidence intervals of mean differences (corresponding to ratios) are also given.
All statistical analyses were performed with SPSS software version 21.0 (IBM, Chicago, Illinois, USA). P < 0.05 was considered statistically significant.
RESULTS
The number of CAG and GGN repeats in the AR is in accordance with the normal distribution for Caucasian men (Figure 1 and 2).
Figure 1.
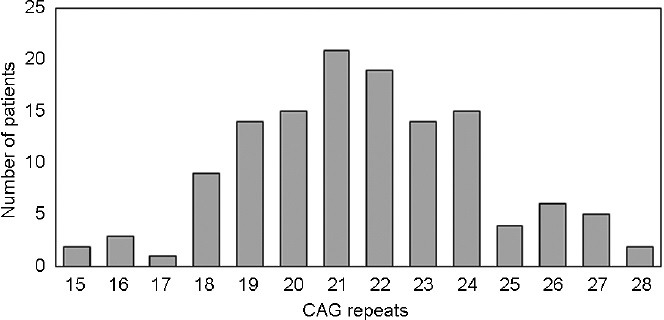
The graph bar shows the number and distribution of TC patients with different CAG repeats ranging from 15 to 28, which is in accordance with the normal distribution of CAG repeats in Caucasians. TC: testicular cancer.
Figure 2.
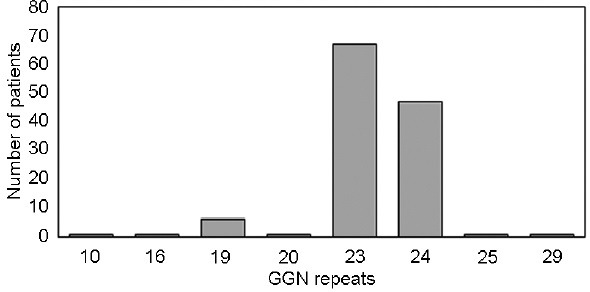
The graph bar shows the number and distribution of TC patients with different GGN repeats in this study. As for a normal population of Caucasians, the most common GGN repeat numbers are 23 and 24. TC: testicular cancer.
At T0, no statistically significant difference in the sperm concentration or total sperm number between the CAG groups was found (P = 0.16 and P = 0.14, respectively).
At T12 and T36, men with 22–23 CAG exhibited lower sperm concentrations than those with other CAG numbers; this difference was only statistically significant at T12 (95% CI for ratio: 1.01–2.65; P = 0.045). This association was robust to omitting adjustment for age, treatment type, and sperm concentration at T0 (95% CI for ratio: 1.13–4.9; P = 0.02). For trichotomized CAG numbers, both CAG <22 and CAG >23 exhibited higher sperm concentrations than CAG 22–23, and the difference was statistically significant (P = 0.04) for CAG <22 but not for CAG >23 (P = 0.18) (Figure 3). The same trends were observed for total sperm number (Table 3).
Figure 3.
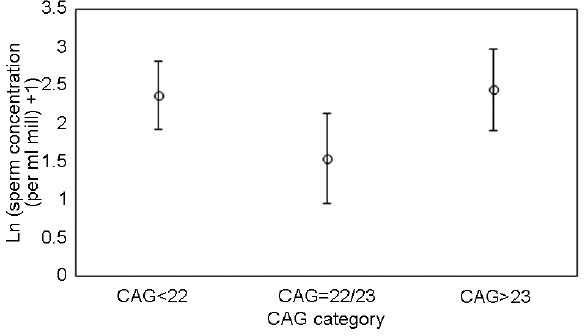
The error bar shows the androgen receptor CAG repeat number dependent 12 months posttreatment sperm concentration in testicular cancer patients given chemotherapy and/or irradiation. The sperm numbers are natural logarithm-transformed after adding 1 to all concentrations. The CAG numbers are categorized into three groups: CAG <22, CAG 22/23, CAG >23. Ln: logarithm.
Table 3.
The effect of CAG repeat number on total sperm number in relation to follow–up time
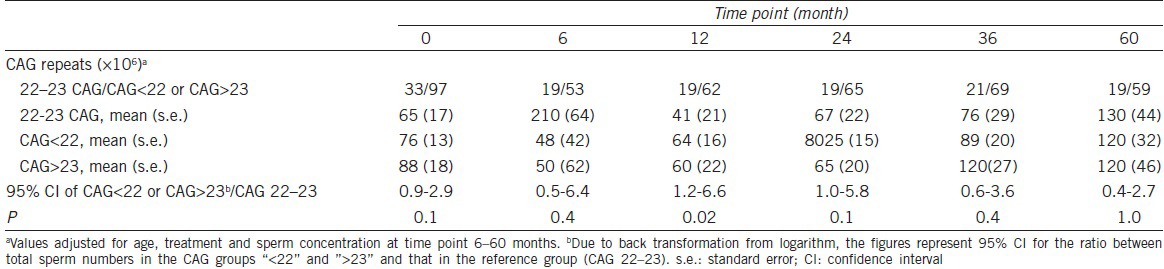
The sperm number in the GGN 23 group did not statistically significantly differ from that in the merged group GGN <23/GGN >23 at any of the included time points (for all comparisons: 0.55 < P < 1.0).
DISCUSSION
The main finding of this study is that 1 year after completion of therapy, a lower sperm concentration and total sperm number was observed in men having 22 or 23 CAG repeats in the AR gene compared to those with other lengths. This difference was primarily due to higher sperm numbers in men with short CAG tracts. The same trend, although not statistically significant, was seen in those with more than 23 CAG repeats.
From a clinical point of view, it is important to find reliable markers of postcancer treatment spermatogenesis recovery. Because the human reproductive time window is highly related to female age, the long lag time to spermatogenesis recovery may be an argument for applying methods of assisted reproduction, either with fresh ejaculated or cryopreserved spermatozoa. Previously, we reported on single-nucleotide polymorphisms (SNP) in the estrogen receptor alpha gene.37 However, using the CAG number or ER1 SNP for prediction of fertility recovery in cancer survivors had limited clinical value. However, our results represent a “proof of concept” regarding the impact of genetic polymorphisms on the dynamics of sperm production recovery following cancer treatment and may encourage researchers to look for other, more clinically useful markers.
From a biological perspective, our findings seem to fit with previous animal data showing that spermatogenesis recovery following testicular irradiation is enhanced by the use of gonadotropin-releasing hormone agonists or antagonists, lowering intratesticular testosterone levels.38,39 The authors of those studies suggested that suppression of intratesticular androgenic activity is the biological foundation of a more efficient recovery processes, a hypothesis fitting well with our clinical observation of a higher sperm concentration 12 months after completed cancer treatment in men with a less efficient AR variant.40
This report may seem to be contradictory to our previous report on the inverse association between CAG number and posttreatment sperm numbers. However, our earlier report was based on only 9 patients, and in that study, in agreement with the current report, those with <22 CAG exhibited higher sperm counts than the 22–23 group, but those with more than 23 CAG repeats had even lower sperm concentrations; thus, the association had a negative linear and not a U-shaped pattern. The data show that the >23 group included two observations only, one with a total sperm number higher than that in the 22–23 group and one with a lower sperm number. However, even those data might be compatible with the fact that the 22–23 CAG group presents with the lowest sperm number.7
A lack of association between GGN number and posttreatment sperm production recovery fits with the earlier observations that this polymorphism has a less pronounced impact on phenotype than does the variation in CAG.18
Our study has some weaknesses and some advantages to be discussed. Although all men were instructed to abstain for 2–7 days, we do not have information about the real length of the abstinence period. Although this can contribute to the level of noise and thereby reduce the power of the study, it can hardly explain the association found between CAG number and sperm concentration at T12. The same is true considering that we have access to only one ejaculate for each of the study participants at each time point, as there is significant intraindividual variation in sperm number.7
On the other hand, the study is based on a fairly high number of participants and, despite the cross-sectional design, semen samples at several time points after completion of therapy were included. The two laboratories performing the vast majority of sperm number assessments followed the same WHO guidelines for semen analysis, and assessment of sperm concentration seems robust to interlaboratory variation when this same protocol is applied.41,42
CONCLUSION
We found that in TC survivors, the AR CAG repeat number is associated with sperm concentration and total sperm number 12 months after completion of CT or RT. The least active receptor variant seems to indicate a more rapid spermatogenesis recovery. This adds to our understanding of the biology of postcancer therapy recovery of fertility in males. Further studies are needed for validation, but this new association may have clinical implications for predicting fertility in the future.
AUTHOR CONTRIBUTIONS
KB performed the analysis, calculations and wrote the manuscript in cooperation with AG. JE and OS planned the study, met the patients with GCC, and participated in revising and writing the manuscript together with YLG, GCC, ECS, and SA. SA was responsible for sperm analysis in Stockholm. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by grants from the Swedish Cancer Society (CAN 2012/661), Swedish Childhood Cancer Society (PROJ12/049), Malmö University Hospital Cancer Fund, Skane University Hospital Fund, Swedish governmental funding for clinical research, Region Scania Research Fund.
REFERENCES
- 1.Nur U, Rachet B, Mitry E, Cooper N, Coleman MP. Survival from testicular cancer in England and Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S80–2. doi: 10.1038/sj.bjc.6604597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dearnaley D, Huddart R, Horwich A. Regular review: managing testicular cancer. BMJ. 2001;322:1583–8. doi: 10.1136/bmj.322.7302.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, et al. NORDCAN - A nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49:725–36. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 4.Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol. 2014;65:1095–106. doi: 10.1016/j.eururo.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Lampe H, Horwich A, Norman A, Nicholls J, Dearnaley DP. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol. 1997;15:239–45. doi: 10.1200/JCO.1997.15.1.239. [DOI] [PubMed] [Google Scholar]
- 6.Aass N, Fossa SD, Theodorsen L, Norman N. Prediction of long-term gonadal toxicity after standard treatment for testicular cancer. Eur J Cancer. 1991;27:1087–91. doi: 10.1016/0277-5379(91)90298-r. [DOI] [PubMed] [Google Scholar]
- 7.Eberhard J, Stahl O, Giwercman Y, Cwikiel M, Cavallin-Stahl E, et al. Impact of therapy and androgen receptor polymorphism on sperm concentration in men treated for testicular germ cell cancer: a longitudinal study. Hum Reprod. 2004;19:1418–25. doi: 10.1093/humrep/deh231. [DOI] [PubMed] [Google Scholar]
- 8.Grassetti D, Giannandrea F, Paoli D, Masciandaro P, Figura V, et al. Androgen receptor polymorphisms and testicular cancer risk. Andrology. 2015;3:27–33. doi: 10.1111/j.2047-2927.2014.00252.x. [DOI] [PubMed] [Google Scholar]
- 9.Gendt VD. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–32. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddocks S, Hargreave TB, Reddie K, Fraser HM, Kerr JB, et al. Intratesticular hormone levels and the route of secretion of hormones from the testis of the rat, guinea pig, monkey and human. Int J Androl. 1993;16:272–8. doi: 10.1111/j.1365-2605.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 11.Giagulli VA, Carbone MD, De Pergola G, Guastamacchia E, Resta F, et al. Could androgen receptor gene CAG tract polymorphism affect spermatogenesis in men with idiopathic infertility? J Assist Reprod Genet. 2014;31:689–97. doi: 10.1007/s10815-014-0221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giagulli VA, Carbone D. Hormonal control of inhibin B in men. J Endocrinol Invest. 2006;29:706–13. doi: 10.1007/BF03344180. [DOI] [PubMed] [Google Scholar]
- 13.Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab. 1997;82:3777–82. doi: 10.1210/jcem.82.11.4385. [DOI] [PubMed] [Google Scholar]
- 14.Hsing AW, Gao YT, Wu G, Wang X, Deng J, et al. Polymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2000;60:5111–6. [PubMed] [Google Scholar]
- 15.von Eckardstein S, Syska A, Gromoll J, Kamischke A, Simoni M, et al. Inverse correlation between sperm concentration and number of androgen receptor CAG repeats in normal men. J Clin Endocrinol Metab. 2001;86:2585–90. doi: 10.1210/jcem.86.6.7608. [DOI] [PubMed] [Google Scholar]
- 16.Giwercman A, Giwercman YL. [The androgen receptor and effects of testosterone] Lakartidningen. 2009;106:2465–8. Article in Swedish. [PubMed] [Google Scholar]
- 17.Nenonen H, Bjork C, Skjaerpe PA, Giwercman A, Rylander L, et al. CAG repeat number is not inversely associated with androgen receptor activity in vitro. Mol Hum Reprod. 2010;16:153–7. doi: 10.1093/molehr/gap097. [DOI] [PubMed] [Google Scholar]
- 18.Nenonen HA, Giwercman A, Hallengren E, Giwercman YL. Non-linear association between androgen receptor CAG repeat length and risk of male subfertility - a meta-analysis. Int J Androl. 2011;34:327–32. doi: 10.1111/j.1365-2605.2010.01084.x. [DOI] [PubMed] [Google Scholar]
- 19.Mouritsen A, Hagen CP, Sorensen K, Aksglaede L, Mieritz MG, et al. Androgen receptor CAG repeat length is associated with body fat and serum SHBG in boys: a prospective cohort study. J Clin Endocrinol Metab. 2013;98:E605–9. doi: 10.1210/jc.2012-3778. [DOI] [PubMed] [Google Scholar]
- 20.Pan B, Li R, Chen Y, Tang Q, Wu W, et al. Genetic association between androgen receptor gene CAG repeat length polymorphism and male infertility: a meta-analysis. Medicine (Baltimore) 2016;95:e2878. doi: 10.1097/MD.0000000000002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhayel Y, Lundin K, Giwercman Y, Hallden C, Willen M, et al. Androgen receptor gene GGN and CAG polymorphisms among severely oligozoospermic and azoospermic Swedish men. Hum Reprod. 2004;19:2076–83. doi: 10.1093/humrep/deh349. [DOI] [PubMed] [Google Scholar]
- 22.Lundin KB, Giwercman A, Richthoff J, Abrahamsson PA, Giwercman YL. No association between mutations in the human androgen receptor GGN repeat and inter-sex conditions. Mol Hum Reprod. 2003;9:375–9. doi: 10.1093/molehr/gag048. [DOI] [PubMed] [Google Scholar]
- 23.Aschim EL, Nordenskjold A, Giwercman A, Lundin KB, Ruhayel Y, et al. Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene. J Clin Endocrinol Metab. 2004;89:5105–9. doi: 10.1210/jc.2004-0293. [DOI] [PubMed] [Google Scholar]
- 24.Radpour R, Rezaee M, Tavasoly A, Solati S, Saleki A. Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients. Andrology. 2007;28:164–9. doi: 10.2164/jandrol.106.000927. [DOI] [PubMed] [Google Scholar]
- 25.Gandini L, Sgro P, Lombardo F, Paoli D, Culasso F, et al. Effect of chemo- or radiotherapy on sperm parameters of testicular cancer patients. Hum Reprod. 2006;21:2882–9. doi: 10.1093/humrep/del167. [DOI] [PubMed] [Google Scholar]
- 26.Hansen PV, Trykker H, Helkjoer PE, Andersen J. Testicular function in patients with testicular cancer treated with orchiectomy alone or orchiectomy plus cisplatin-based chemotherapy. J Natl Cancer Inst. 1989;81:1246–50. doi: 10.1093/jnci/81.16.1246. [DOI] [PubMed] [Google Scholar]
- 27.Pont J, Albrecht W, Postner G, Sellner F, Angel K, et al. Adjuvant chemotherapy for high-risk clinical stage I nonseminomatous testicular germ cell cancer: long-term results of a prospective trial. J Clin Oncol. 1996;14:441–8. doi: 10.1200/JCO.1996.14.2.441. [DOI] [PubMed] [Google Scholar]
- 28.Brydoy M, Fossa SD, Klepp O, Bremnes RM, Wist EA, et al. Paternity and testicular function among testicular cancer survivors treated with two to four cycles of cisplatin-based chemotherapy. Eur Urol. 2010;58:134–40. doi: 10.1016/j.eururo.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 29.Sedlmayer F, Joos H, Deutschmann H, Rahim H, Merz F, et al. [Long-term tumor control and fertility after para-aortic limited radiotherapy of stage I seminoma] Strahlenther Onkol. 1999;175:320–4. doi: 10.1007/s000660050018. [Article in German] [DOI] [PubMed] [Google Scholar]
- 30.Fossa SD, De Angelis P, Kraggerud SM, Evenson D, Theodorsen L, et al. Prediction of posttreatment spermatogenesis in patients with testicular cancer by flow cytometric sperm chromatin structure assay. Cytometry. 1997;30:192–6. [PubMed] [Google Scholar]
- 31.Fossa SD, Ous S, Abyholm T, Norman N, Loeb M. Post-treatment fertility in patients with testicular cancer. II. Influence of cis-platin-based combination chemotherapy and of retroperitoneal surgery on hormone and sperm cell production. Br J Urol. 1985;57:210–4. doi: 10.1111/j.1464-410x.1985.tb06426.x. [DOI] [PubMed] [Google Scholar]
- 32.Fossa SD, Theodorsen L, Norman N, Aabyholm T. Recovery of impaired pretreatment spermatogenesis in testicular cancer. Fertil Steril. 1990;54:493–6. doi: 10.1016/s0015-0282(16)53768-6. [DOI] [PubMed] [Google Scholar]
- 33.Davis-Dao CA, Tuazon ED, Sokol RZ, Cortessis VK. Male infertility and variation in CAG repeat length in the androgen receptor gene: a meta-analysis. J Clin Endocrinol Metab. 2007;92:4319–26. doi: 10.1210/jc.2007-1110. [DOI] [PubMed] [Google Scholar]
- 34.Olofsson SE, Tandstad T, Jerkeman M, Dahl O, Stahl O, et al. Population-based study of treatment guided by tumor marker decline in patients with metastatic nonseminomatous germ cell tumor: a report from the Swedish-Norwegian Testicular Cancer Group. J Clin Oncol. 2011;29:2032–9. doi: 10.1200/JCO.2010.29.1278. [DOI] [PubMed] [Google Scholar]
- 35.WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 36.Cooper TG, Bjorndahl L, Vreeburg J, Nieschlag E. Semen analysis and external quality control schemes for semen analysis need global standardization. Int J Androl. 2002;25:306–11. doi: 10.1046/j.1365-2605.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 37.Romerius P, Giwercman A, Moell C, Relander T, Cavallin-Stahl E, et al. Estrogen receptor alpha single nucleotide polymorphism modifies the risk of azoospermia in childhood cancer survivors. Pharmacogenet Genomics. 2011;21:263–9. doi: 10.1097/FPC.0b013e328343a132. [DOI] [PubMed] [Google Scholar]
- 38.Meistrich ML, Wilson G, Shuttlesworth G, Huhtaniemi I, Reissmann T. GnRH agonists and antagonists stimulate recovery of fertility in irradiated LBNF1 rats. Andrology. 2001;22:809–17. [PubMed] [Google Scholar]
- 39.Meistrich ML, Wilson G, Huhtaniemi I. Hormonal treatment after cytotoxic therapy stimulates recovery of spermatogenesis. Cancer Res. 1999;59:3557–60. [PubMed] [Google Scholar]
- 40.Shetty G, Wilson G, Huhtaniemi I, Shuttlesworth GA, Reissmann T, et al. Gonadotropin-releasing hormone analogs stimulate and testosterone inhibits the recovery of spermatogenesis in irradiated rats. Endocrinology. 2000;141:1735–45. doi: 10.1210/endo.141.5.7446. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen N, Auger J, Giwercman A, Irvine DS, Jensen TK, et al. Semen analysis performed by different laboratory teams: an intervariation study. Int J Androl. 1997;20:201–8. doi: 10.1046/j.1365-2605.1997.00052.x. [DOI] [PubMed] [Google Scholar]
- 42.Giwercman A, Spano M, Lahdetie J, Bonde JP. Quality assurance of semen analysis in multicenter studies. Asclepios. Scand J Work Environ Health. 1999;25(Suppl 1):23–5. discussion 76-8. [PubMed] [Google Scholar]


