Abstract
Polychlorinated biphenyls (PCBs) are common environmental contaminants that represent a considerable risk to reproductive toxicity in exposed human populations. Although some experimental studies have suggested an association between the levels of PCBs and semen quality, the direct effects of PCBs on human sperm parameters remain largely unexplored. To this aim, a short-term in vitro incubation experiment that better imitated the putative exposure of sperm to Aroclor 1254 (a commercial PCB mixture) in male reproduction tissue was conducted. Human sperm were incubated with various concentrations (0, 1, 5, or 25 mg l−1) of Aroclor 1254 for different amounts of time (3 and 6 h) in vitro. Sperm motility parameters were analyzed with computer-assisted sperm analysis (CASA). The proportion of sperm with high mitochondrial membrane potential (ΔΨm) and the levels of intracellular reactive oxygen species (ROS) were detected to explore the probable cause of sperm impairment. Human sperm exposed to continuous Aroclor 1254 exhibited: (i) reduced sperm motility and kinematic parameters, (ii) a proportion of sperm with high ΔΨm that decreased in a dose-dependent manner (P < 0.05), and (iii) increased levels of ROS compared with controls (P < 0.05). In conclusion, Aroclor 1254 can decrease sperm motility, which may culminate in increased ROS and general mitochondrial dysfunction, thus affecting the fertilization potential of sperm. Our findings suggest a broader understanding of the effect of Aroclor 1254 on human sperm.
Keywords: Aroclor 1254, CASA, human sperm, mitochondrial membrane potential, reactive oxygen species, sperm motility
INTRODUCTION
In recent decades, a decline in human semen quality has been observed.1 Despite some regional and ethnic differences, various studies have shown a global decline in sperm parameters.2,3 A possible explanation could be an increased exposure to environmental contaminants. One class of persistent organic pollutant, polychlorinated biphenyls (PCBs), was originally manufactured for industrial applications due to its insulating and flame-retardant properties. Several recent studies on PCBs have found that they possess endocrine disrupting activity. As a result of their lipophilicity and chemical stability, PCBs have been detected all over the world in remote regions,4,5 in dairy and fishery products,6,7 and even in human breast milk.8 PCBs have attracted considerable attention due to their ability to concentrate in food chains and their persistence in organisms.
During the past few decades, there has been a decline of organism PCBs levels associated with the production ban on PCBs.9 Nevertheless, there remains concern about human exposure due to the growing number of poisoning incidents, such as the inadvertent ingestion of dairy products or seafood contaminated by PCBs. These toxic compounds also appear to accumulate in organisms and can cause endocrine disruption and reproductive disorders, such as infertility and miscarriage.10,11
Several recent studies have evaluated the impact of PCBs on the male reproductive system, most of which were carried out using animal models. A 20-day intraperitoneal injection exposure to 3 mg kg−1 Aroclor 1254 reduced the weight of the testes and epididymis in rats.12 PCBs can also inhibit germ cell proliferation and induce germ cell apoptosis in mice.13 In contrast, another study found no toxic impact of different single PCB congeners on the percentage of living acrosomes that react with spermatozoa and vitality in humans.14 However, PCBs exist in the environment as a mixture of congeners and isomers with different positions of the biphenyl moiety substituted by different numbers of chlorine atoms, and organisms are rarely exposed to single PCB congeners.15 Therefore, more accurate information on the effects of mixtures of congeners on the male reproductive system is needed to perform an exhaustive toxicological evaluation of PCBs. Aroclor 1254, a commercial PCB mixture containing 54% chlorine by weight, has been widely used in PCB toxicity research. This mixture of more than 60 PCB congeners was chosen in this study because homologous compounds have been used in industry and organisms are never exposed to a single congener in the natural environment.16
Despite previous findings, the data on the PCB load in human sperm are lacking in terms of studies on the effects of PCBs on the male human reproduction system. The purpose of the present study was to analyze the changes in motility parameters, mitochondrial membrane potential, and intracellular reactive oxygen species (ROS) levels of human sperm treated with Aroclor 1254 for an exhaustive toxicological assessment of this PCB mixture.
MATERIALS AND METHODS
Chemicals
Aroclor 1254 was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). All other chemicals used in the present study were obtained from Sigma-Aldrich (St. Louis, MO, USA), unless indicated otherwise.
Human biological samples
Sperm samples with ≥15 × 106 spermatozoa ml−1, ≥32% progressive sperm and ≥4% morphologically normal forms,17 were obtained from healthy donors (n = 12) undergoing routine semen analysis in the Qilu Hospital of Shandong University (China). Semen samples were collected by masturbation after a recommended sexual abstinence of 3–5 days.
The study was approved by the Ethics Committee of Qilu Hospital at Shandong University, and there is no conflict of interest. Donors were informed about the purpose of the study and asked for their consent.
Sperm preparation and Aroclor 1254 treatment
Stock solutions of Aroclor 1254 at 50 mg l−1 dissolved in olive oil were stored at 4°C and diluted to 1, 5, or 25 mg l−1 at the time of treatment. The final olive oil concentrations in the culture medium did not exceed 0.3% (v/v), which did not affect sperm motility. As a control, sperm suspensions were diluted with 0.3% (v/v) olive oil.
Sperm were collected by density-gradient preparation and prepared as described previously.18 Sperm suspensions were diluted with fresh G-mops (added 5% HSA, produced by Vitrolife, Sweden) to yield an approximate concentration of 1 × 106 sperm l−1. The samples were centrifuged, and motile sperm were resuspended in G-mops containing Aroclor 1254 at different concentrations. The sperm suspensions were incubated for 3 and 6 h at 37°C in a humidified atmosphere of 5% CO2. Each experiment was performed in duplicate.
Assessment of sperm motility
Sperm motility was analyzed using computer-assisted sperm analysis (CASA) as described previously.19 The following sperm motility parameters were determined: curvilinear velocity (VCL, μm s−1), average path velocity (VAP, μm s−1), straight-line velocity (VSL, μm s−1), beat cross frequency (BCF, Hz), linearity (LIN, %), and straightness (STR, %). An aliquot of 10 μl of each sample was transferred to a Makler chamber that had been prewarmed to 37°C. The motility of 200 spermatozoa was evaluated by CASA. The sperm motility parameters were evaluated after 3 and 6 h of incubation with 0, 1, 5, or 25 mg l−1 Aroclor 1254.
Mitochondrial membrane potential (ΔΨm)
The ΔΨm was measured using the fluorescent dye 5,5’,6,6’- tetra-chloro-1,1’,3,3’- tetraethylben-zimidazolyl-carbocyanine iodide (JC-1), a cell permeable cationic dye that can accumulate in energized mitochondria based on the highly negative ΔΨm. Essentially, JC-1 changes its fluorescence from green (monomeric status) to red (multimeric status) when the ΔΨm is high.20 A stock solution was prepared at 1 mg l−1 in olive oil. The stock solution was divided into small aliquots and stored at −20°C until use. The sperm samples were exposed to a series of Aroclor 1254 concentrations (0, 1, 5, or 25 mg l−1) for 3 or 6 h. After co-incubation with Aroclor 1254, the sperm in different groups were stained in JC-1 solution at 37°C in a humidified atmosphere of 5% CO2 for 20 min. The stained samples were then analyzed by flow cytometry to determine the sperm with high ΔΨm (red stained) using the appropriate gating parameters. Ten thousand sperm from each group were used in the evaluation of ΔΨm.
Intracellular ROS production
DCFH-DA, an oxidation-sensitive fluorescent probe, was used to analyze ROS generation.21 Sperm were treated as described in section 2.3 and then incubated with 5 μmol l−1 DCFH-DA for 30 min at 37°C. The cellular mean fluorescence intensity (MFI) was measured using flow cytometry. The relative ROS production was calculated as the ratio of the MFI of the treated sample to the MFI of the vehicle control.
Flow cytometry
All samples were analyzed using a FACScan flow cytometer (FACSCalibur, Becton Dickinson, San Jose, CA, USA), and the data were obtained with CellQuest software (Becton Dickinson, San Jose, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). All values are presented as the means ± s.d., and the statistical analyses were performed by ANOVA, followed by LSD multiple comparisons when appropriate. Variables were checked for normal distribution, and comparisons among the different concentrations and controls were carried out by paired t-tests. The values of P < 0.05 were considered statistically significant.
RESULTS
Effects of Aroclor 1254 on sperm movement
Overall, the total motility and progressive motility of sperm treated with 5 or 25 mg l−1 Aroclor 1254 were lower (P < 0.05) than those of the control group. There were no differences in motility between the control samples and the samples treated with 1 mg l−1 Aroclor 1254 (Figure 1 and Supplementary Table 1 (207.6KB, tif) ).
Figure 1.
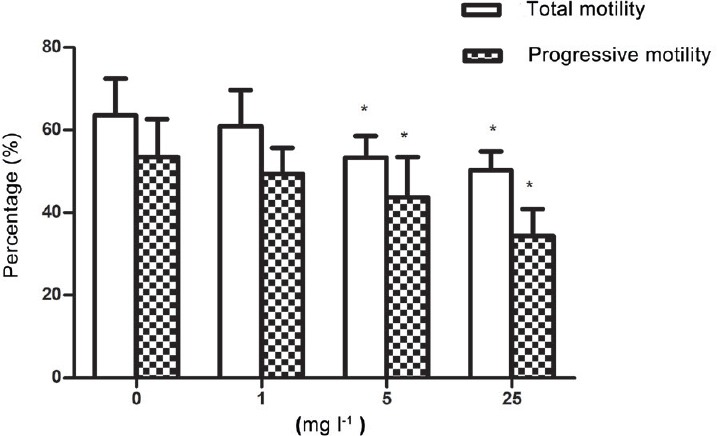
Effect of Aroclor 1254 on the total and progressive sperm motility after 6 h of incubation. Data are expressed as the mean ± s.d. *Statistical significance compared with the control (n = 12, P < 0.05).
Sperm motility
Effect of Aroclor 1254 on the kinematic parameters of sperm
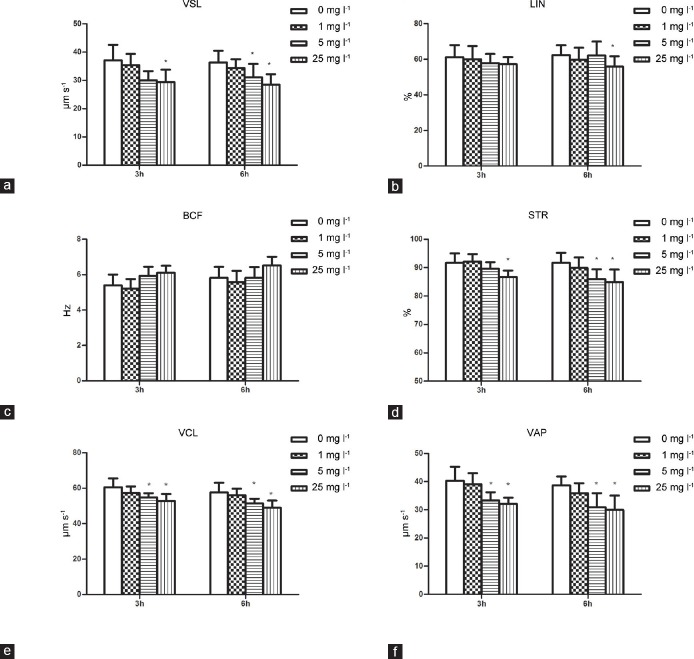
To determine the effects of Aroclor 1254 on different motility patterns, CASA was performed for each concentration (0, 1, 5, and 25 mg l−1). In general, Aroclor 1254 reduced the kinematic parameters of sperm in a concentration-dependent manner. Decreases in VSL and STR were observed after 3 and 6 h of incubation at 25 mg l−1 Aroclor 1254, and these decreases were also observed after 6 h of incubation at 5 mg l−1 Aroclor 1254. After 3 h of incubation, decreases in VSL and STR were observed only at 25 mg l−1 Aroclor 1254. VCL and VAP decreased after 3 and 6 h of incubation at 5 and 25 mg l−1 Aroclor 1254 (P < 0.05). No differences in BCF were observed among the different concentrations or between the incubation periods (3 and 6 h). In addition, a significant reduction of LIN was observed in the 25 mg l−1 group after 6 h of incubation compared with the untreated control (Figure 2 and Supplementary Table 2 (754.7KB, tif) ).
Figure 2.
Effect of Aroclor 1254 on the kinematic parameters of human sperm analyzed by CASA. Straight-line velocity (VSL, a), linearity (LIN, b), beat cross frequency (BCF, c), straightness (STR, d), curvilinear velocity (VCL, e) and average path velocity (VAP, f) were measured. Data are expressed as the mean ± s.d. *Statistical significance compared with the control (n = 6, P < 0.05). CASA: computer-assisted sperm analysis.
Kinematic parameters
Effect of Aroclor 1254 on the mitochondrial membrane potential of sperm
Microscopic evaluation revealed the vitality of the sperm mitochondria: sperm with a high mitochondrial membrane potential were stained bright orange whereas sperm with a low mitochondrial membrane potential were stained green. Flow cytometric analysis demonstrated that the percentages of sperm stained bright orange were higher (P < 0.05) in the 5 and 25 mg l−1 Aroclor 1254 groups than in the other groups, but there was no difference between the 1 mg l−1 group and the untreated control group (Figures 3, 4 and Supplementary Table 3 (105.4KB, tif) ).
Figure 3.
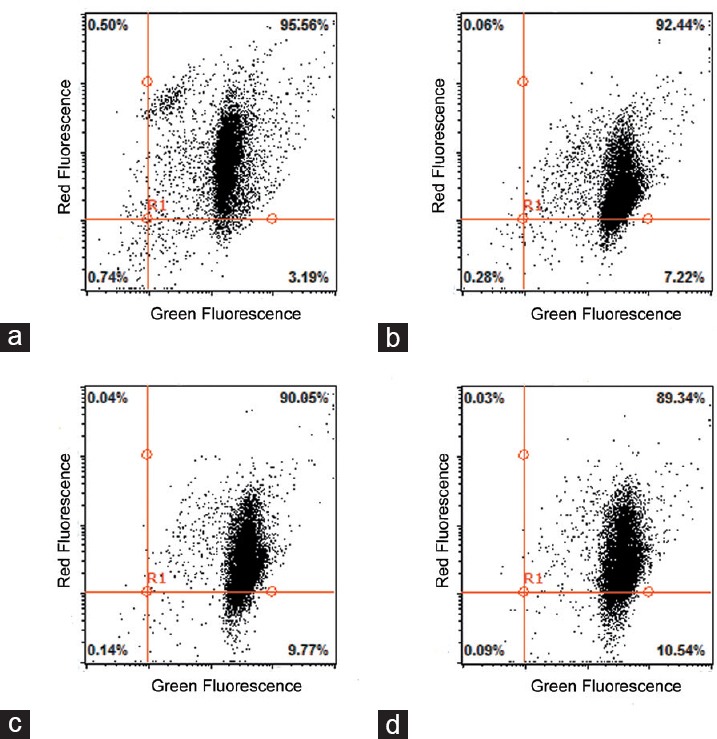
Flow cytometry plots of human sperm stained with JC-1. Flow cytometry plots of untreated sperm as a control (a) and after exposure to 1, 5, and 25 μg ml−1 Aroclor 1254 for 6 h (b–d).
Figure 4.
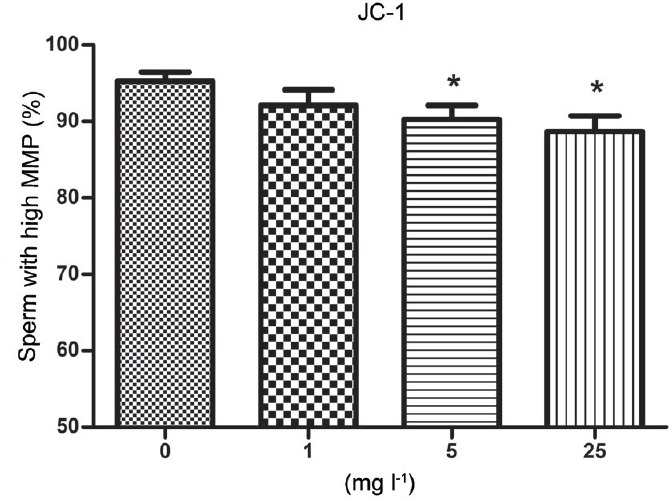
Proportion of human sperm with strong JC-1 fluorescence, indicating high MMP, when incubated with 0, 1, 5, and 25 μg ml−1 Aroclor 1254 after 6 h. Data are expressed as the mean ± s.d. *Statistical significance compared with the control (n = 6, P < 0.05).
Mitochondrial membrane potential (ΔΨm)
Effect of Aroclor 1254-induced ROS production
In the present study, we used the fluorescence probe DCFH-DA to determine the intracellular ROS levels in human sperm after exposure to Aroclor 1254. As shown in Figure 5 and Supplementary Table 4 (105.4KB, tif) , the sperm presented higher fluorescence intensity (112.7% and 128.1% after 6 h in 5 and 25 mg l−1 Aroclor 1254, respectively). Although the ROS levels of sperm after exposure to 1 mg l−1 Aroclor 1254 increased (101.5% after 6 h in 1 mg l−1 Aroclor 1254), this change was not statistically significant. These results indicate that Aroclor 1254 can induce ROS generation in human sperm.
Figure 5.
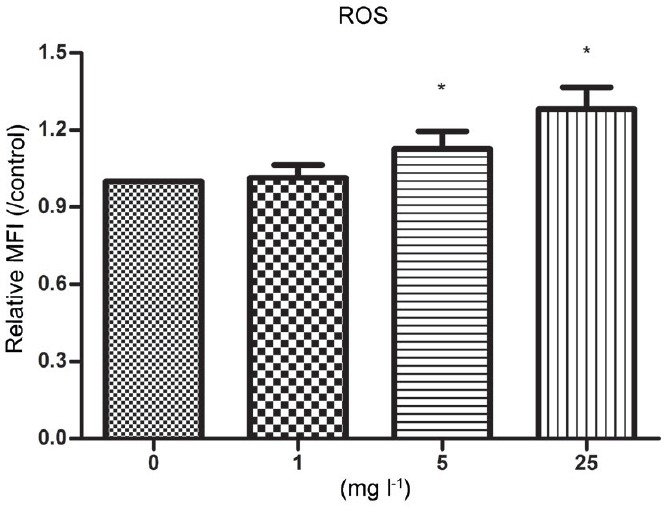
Effect of Aroclor 1254-induced ROS production after treatment with 0, 1, 5, and 25 μg ml−1 Aroclor 1254 for 6 h. Data are presented as the MFI relative of the control. Each result is presented as mean ± s.d. *Indicates P < 0.05, compared with the vehicle control. ROS: reactive oxygen species; MFI: mean fluorescence intensity.
Reactive oxygen species
DISCUSSION
There has been increasing concern over the potential toxic effects of environmental pollutants on male fertility. PCBs, a typical environmental pollutant, exhibit endocrine disrupting activity. Several recent studies have found that considerable risk still exists from exposure to PCBs.10 The known perniciousness of PCBs to the health of populations calls for a better understanding of their action on target cells, especially gametes. However, information concerning the effects of PCBs on human reproduction remains limited and inconclusive. Therefore, the objective of the current study was to evaluate the effects of brief exposure to Aroclor 1254 on in vitro human sperm motility parameters.
In our experimental design, human sperm were exposed to Aroclor 1254 doses corresponding to a series of Aroclor 1254 concentrations (0, 1, 5, and 25 mg l−1) for 3 or 6 h. The doses were selected on the basis of previously published articles14,22 in which mammalian cells were treated with different concentrations of PCBs (ranging from nanograms to micrograms per ml). At these or similar concentration levels, PCBs also exert cytotoxic effects on human cells, such as osteoblasts23 and astrocytoma cells.24 More importantly, the concentrations of Aroclor 1254 used in this study resembled the levels of PCBs found in plasma or reproductive tissue samples from humans.25,26 Using concentrations consistent with previous reference concentrations, we observed a decline in sperm motility after exposure to Aroclor 1254. However, interestingly, previous research found that sperm exhibited toxicity impairment when exposed to lower Aroclor 1254 doses (10−9 mol l−1 for 3 h) in mice.27 However, no significant diversity in human sperm motility parameters at such low concentration was observed in our preliminary experiments. Thus, we conjectured that human sperm may be less sensitive to environmental contaminants than other species, but more evidence is needed.
Sperm motility parameters, such as the VCL, LIN, BCF, STR, VSL, and VAP, can indirectly reflect the fertilizability of human sperm. Sperm motility is considered as an independent predictor of the probability of sperm-oocyte fusion and achieving pregnancy. Among these motility parameters, LIN, STR, VSL, and VAP are indicators of sperm progression, whereas VCL and BCF are markers of sperm viability and vigor. LIN and STR are also used to describe the swimming pattern of sperm.28 In the present study, we found an overall pattern of decline in the CASA parameters of VCL, LIN, STR, VSL, and VAP after exposure to 5 and 25 mg l−1 Aroclor 1254, which indicates that human sperm exposed to Aroclor 1254 in vitro were characterized by reduced motility. Moreover, VSL, VCL, VAP, and STR may be more sensitive to Aroclor 1254-induced male reproductive toxicity, whereas BCF appeared to be less sensitive. Because the energetic motility of sperm depends on the energy produced in the mitochondria, Aroclor 1254 may injure the mitochondrial function of sperm, degrading their motility and ultimately leading to sperm death. To further study the cause of the damage, we investigated the effects of Aroclor 1254 on the mitochondrial membrane potential (ΔΨm) of sperm and the levels of cellular ROS.
Mitochondria, as the main cellular energy producers, are potential targets of drugs or environmental contaminants.29 The motility of sperm depends on the functional completeness of the mitochondria.30 Therefore, the changes of ΔΨm in sperm could be a good indicator of functional impairment. ΔΨm can be quickly measured using JC-1 staining and flow cytometry. Overall, our experimental results suggest that Aroclor 1254 exposure is associated with a decrease in ΔΨm. This information suggests that Aroclor 1254 exposure is likely to induce a loss of intracellular ATP, leading to visible impairment of human sperm. Previous research determined that the disruption of ΔΨm might be due to ROS generation.31 We conjectured that the ROS levels of human sperm would be impacted by Aroclor 1254, and further experiments were performed in our study to evaluate the levels of ROS change in sperm after exposure to Aroclor 1254.
ROS is a common collective term that includes highly oxidative radicals and nonradical species.32 Due to their short half-life, ROS are comparatively innocuous in sperm under normal circumstances. Low levels of ROS are required in many cellular processes. However, our results demonstrate that the levels of ROS were increased in human sperm exposed to Aroclor 1254. These data are in agreement with the observations of Aly,27 which revealed increased levels of hydrogen peroxide (H2O2) and lipid peroxidation (LPO) after rat sperm were exposed to Aroclor 1254. Aroclor 1254 exposure is associated with an increase in ROS. Sperm membranes contain a high concentration of polyunsaturated lipids, which are sensitive to ROS and oxidative damage. ROS can result in free radical attack of membrane phospholipids and decreased ΔΨm. Therefore, we can assume that the mitochondrial dysfunction upon exposure to Aroclor 1254 may be caused by high ROS levels. In addition, Ca2+ plays an influential role in cellular events. A previous study found that the effects of PCBs on neutrophil function can be explained by effects on Ca2+/calmodulin-dependent action.33 The disturbance of intracellular Ca2+ may increase ROS production.34,35 Therefore, it is reasonable to hypothesize that Aroclor 1254 can give rise to a disruption of Ca2+ homeostasis that leads to increased cellular ROS levels and mitochondrial dysfunction, ultimately resulting in low sperm motility. Although we cannot exclude potential effects on other components/pathways, more pioneering work is needed.
These data extend the results of our previous study that determined the exposure-related reproductive toxicity of Aroclor 1254.36 The results of this work can inform future research on the study of the reproductive toxicity of Aroclor 1254 in human males; however, further in vivo experiments are needed.
AUTHOR CONTRIBUTIONS
LGJ and LC conceived and designed the study and wrote the main part of the manuscript. YJS and LC contributed to data acquisition. CC carried out the analysis of sperm motility. LYC carried out the immunoassays and flow cytometry. SHK, YY, SZL, and XHD participated in the study design and performed the statistical analyses. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
All authors declared no competing financial interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant Numbers 81571511, 81370711, and 30901603), the Science and Technology Foundation of Shandong (Grant Number 2010GSF10814), the Shandong Provincial Natural Science Foundation (Grant Number ZR2013HM090), and the Science Foundation of Qilu Hospital of Shandong University, Fundamental Research Funds of Shandong University (Grant Numbers 2015QLMS24, 2016QLQN24 and 2015QLQN50).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Bonde JP, Ramlau-Hansen CH, Olsen J. Commentary: trends in sperm counts: the saga continues. Epidemiology. 2011;22:617–9. doi: 10.1097/EDE.0b013e318223442c. [DOI] [PubMed] [Google Scholar]
- 2.Geoffroy-Siraudin C, Loundou AD, Romain F, Achard V, Courbière B, et al. Decline of semen quality among 10932 males consulting for couple infertility over a 20-year period in Marseille, France. Asian J Androl. 2012;14:584–90. doi: 10.1038/aja.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Moal J, Rolland M, Goria S, Wagner V, De Crouy-Chanel P, et al. Semen quality trends in French regions are consistent with a global change in environmental exposure. Reproduction. 2014;147:567–74. doi: 10.1530/REP-13-0499. [DOI] [PubMed] [Google Scholar]
- 4.Van Emon JM, Chuang JC, Bronshtein A, Altstein M. Determination of polychlorinated biphenyls in soil and sediment by selective pressurized liquid extraction with immunochemical detection. Sci Total Environ. 2013;463:326–33. doi: 10.1016/j.scitotenv.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Fromberg A, Cleemann M, Carlsen L. Review on persistent organic pollutants in the environment of Greenland and Faroe Islands. Chemosphere. 1999;38:3075–93. doi: 10.1016/s0045-6535(98)00514-1. [DOI] [PubMed] [Google Scholar]
- 6.Ramos L, Eljarrat E, Hernandez LM, Rivera J, Gonzalez MJ. Levels of PCBs, PCDDs and PCDFs in commercial butter samples in Spain. Chemosphere. 1999;38:3141–53. doi: 10.1016/s0045-6535(98)00521-9. [DOI] [PubMed] [Google Scholar]
- 7.Storelli MM, Barone G, Perrone VG, Giacominelli-Stuffler R. Polychlorinated biphenyls (PCBs), dioxins and furans (PCDD/Fs): occurrence in fishery products and dietary intake. Food Chem. 2011;127:1648–52. [Google Scholar]
- 8.Czaja K, Ludwicki JK, Góralczyk K, Struciński P. Effect of changes in excretion of persistent organochlorine compounds with human breast milk on related exposure of breast-fed infants. Arch Environ Contam Toxicol. 1999;36:498–503. doi: 10.1007/pl00006623. [DOI] [PubMed] [Google Scholar]
- 9.Wittsiepe J, Fobil JN, Till H, Burchard GD, Wilhelm M, et al. Levels of polychlorinated dibenzo-p-dioxins, dibenzofurans (PCDD/Fs) and biphenyls (PCBs) in blood of informal e-waste recycling workers from Agbogbloshie, Ghana, and controls. Environ Int. 2015;79:65–73. doi: 10.1016/j.envint.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Norström K, Czub G, McLachlan MS, Hu D, Thorne PS, et al. External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ Int. 2010;36:855–61. doi: 10.1016/j.envint.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukimori K, Tokunaga S, Shibata S, Uchi H, Nakayama D, et al. Long-term effects of polychlorinated biphenyls and dioxins on pregnancy outcomes in women affected by the Yusho incident. Environ Health Perspect. 2008;116:626–30. doi: 10.1289/ehp.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aly HA, Oscar D, Abdel-Naim AB. Aroclor 1254 impairs spermatogenesis and induces oxidative stress in rat testicular mitochondria. Food Chem Toxicol. 2009;47:1733–8. doi: 10.1016/j.fct.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Wang C, Wu T, Moreno JM, Zhong Y, et al. Disruption of spermatogenesis and differential regulation of testicular estrogen receptor expression in mice after polychlorinated biphenyl exposure. Toxicology. 2011;287:21–8. doi: 10.1016/j.tox.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Sybille PB, Sandra H, Wolfgang K, Volker H, Frank-Michael K, et al. Effects of single non-ortho, mono-ortho, and di-ortho chlorinated biphenyls on human sperm functions in vitro. Reprod Toxicol. 2006;21:280–4. doi: 10.1016/j.reprotox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Pocar P, Brevini TA, Antonini S, Gandolfi F. Cellular and molecular mechanisms mediating the effect of polychlorinated biphenyls on oocyte in vitro maturation. Reprod Toxicol. 2006;22:242–9. doi: 10.1016/j.reprotox.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Stack AS, Altman-Hamamdzic S, Morris PJ, London SD, London L. Polychlorinated biphenyl mixtures (Aroclors) inhibit LPS-induced murine splenocyte proliferation in vitro. Toxicology. 1999;139:137–54. doi: 10.1016/s0300-483x(99)00118-3. [DOI] [PubMed] [Google Scholar]
- 17.Menkveld R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010;12:47–58. doi: 10.1038/aja.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denny S. Novel technologies for selecting the best sperm for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2013;99:1023–9. doi: 10.1016/j.fertnstert.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Grizard G, Ouchchane L, Roddier H, Artonne C, Sion B, et al. In vitro alachlor effects on reactive oxygen species generation, motility patterns and apoptosis markers in human spermatozoa. Reprod Toxicol. 2007;23:55–62. doi: 10.1016/j.reprotox.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Tavares RS, Amaral S, Paiva C, Baptista M, Ramalho-Santos J. In vitro exposure to the organochlorine p, p′-DDE affects functional human sperm parameters. Chemosphere. 2015;120:443–6. doi: 10.1016/j.chemosphere.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y, Zhong R, Chen L, Feng C, Sun H, et al. Effects of astaxanthin supplementation on the sperm quality and antioxidant capacity of ram semen during liquid storage. Small Ruminant Res. 2015;130:178–82. [Google Scholar]
- 22.Ptak G, Zacchini F, Czernik M, Fidanza A, Palmieri C, et al. A short exposure to polychlorinated biphenyls deregulates cellular autophagy in mammalian blastocyst in vitro. Hum Reprod. 2012;27:1034–42. doi: 10.1093/humrep/der454. [DOI] [PubMed] [Google Scholar]
- 23.Herlin M, Öberg M, Ringblom J, Joseph B, Korkalainen M, et al. Inhibitory effects on osteoblast differentiation in vitro by the polychlorinated biphenyl mixture Aroclor 1254 are mainly associated with the dioxin-like constituents. Toxicol In Vitro. 2015;29:876–83. doi: 10.1016/j.tiv.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, et al. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Campagna C, Sirard MA, Ayotte P, Bailey JL. Impaired maturation, fertilization, and embryonic development of porcine oocytes following exposure to an environmentally relevant organochlorine mixture. Biol Reprod. 2001;65:554–60. doi: 10.1095/biolreprod65.2.554. [DOI] [PubMed] [Google Scholar]
- 26.Pocar P, Brevini TA, Fischer B, Gandolfi F. The impact of endocrine disruptors on oocyte competence. Reproduction. 2003;125:313–25. doi: 10.1530/rep.0.1250313. [DOI] [PubMed] [Google Scholar]
- 27.Aly HA. Aroclor 1254 induced oxidative stress and mitochondria mediated apoptosis in adult rat sperm in vitro. Environ Toxicol Pharmacol. 2013;36:274–83. doi: 10.1016/j.etap.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Duty SM, Calafat AM, Silva MJ, Brock JW, Ryan L, et al. The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl. 2004;25:293–302. doi: 10.1002/j.1939-4640.2004.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 29.Pereira SP, Pereira GC, Moreno AJ, Oliveira PJ. Can drug safety be predicted and animal experiments reduced by using isolated mitochondrial fractions? Altern Lab Anim. 2009;37:355–65. doi: 10.1177/026119290903700406. [DOI] [PubMed] [Google Scholar]
- 30.Paoli D, Gallo M, Rizzo F, Baldi E, Francavilla S, et al. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil Steril. 2011;95:2315–9. doi: 10.1016/j.fertnstert.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 31.Wang CL, Xia Y, Nie JZ, Zhou M, Zhang RP, et al. Musca domestica larva lectin induces apoptosis in BEL-7402 cells through a Ca2+/JNK-mediated mitochondrial pathway. Cell Biochem Biophys. 2012;66:319–29. doi: 10.1007/s12013-012-9489-0. [DOI] [PubMed] [Google Scholar]
- 32.Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Olivero J, Ganey PE. Participation of Ca 2+ /calmodulin during activation of rat neutrophils by polychlorinated biphenyls. Biochem Pharmacol. 2001;62:1125–32. doi: 10.1016/s0006-2952(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 34.Kawai Y, Nakao T, Kunimura N, Kohda Y, Gemba M. Relationship of intracellular calcium and oxygen radicals to Cisplatin-related renal cell injury. J Pharmacol Sci. 2006;100:65–72. doi: 10.1254/jphs.fp0050661. [DOI] [PubMed] [Google Scholar]
- 35.Allen DG, Whitehead NP. Duchenne muscular dystrophy - what causes the increased membrane permeability in skeletal muscle? Int J Biochem Cell B. 2011;43:290–4. doi: 10.1016/j.biocel.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Jiang L, Meng X, Han X, Cheng D, et al. Effects of Aroclor 1254 on in vivo oocyte maturation in the mouse. PLoS One. 2014;9:e102064. doi: 10.1371/journal.pone.0102064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sperm motility
Kinematic parameters
Mitochondrial membrane potential (ΔΨm)
Reactive oxygen species