Abstract
Increasing evidence indicates that inflammation may play important roles in tumorigenesis and progression, and an elevated peripheral monocyte count predicts a poor prognosis in various types of malignancies. Here, we evaluate the roles of peripheral monocyte count in the diagnosis and prognosis for prostate cancer in Chinese patients. A total of 1107 consecutive patients who had undergone prostate biopsy and 290 prostate cancer patients receiving androgen deprivation therapy as first-line therapy were retrospectively analyzed. The parameters were measured at the time of diagnosis. Univariate and multivariate logistic regression analyses were performed to identify the independent predictors of a positive biopsy. Patients were categorized in two groups using a cutoff point of 0.425 × 109 l−1 as calculated by the receiver-operating curve analysis for prognosis. Univariate and multivariate Cox regression analyses were performed to determine the associations of monocyte count with progression-free survival, cancer-specific survival, and overall survival. Multivariate logistic regression analyses showed that monocyte count, age, prostate-specific antigen (PSA), free/total PSA, and prostate volume were independent predictors for prostate cancer. Multivariate Cox regression analyses identified an elevated monocyte count as an independent prognostic factor for worse cancer-specific survival (hazard ratio = 2.244, P < 0.05) and overall survival (hazard ratio = 1.995, P < 0.05), but not progression-free survival (P = 0.117). Our results indicated that an elevated monocyte count was an independent diagnostic biomarker for prostate cancer, and pretreatment peripheral monocyte count might play a significant role in the prognosis of prostate cancer patients treated with androgen deprivation therapy.
Keywords: diagnosis, monocyte, prognosis, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the most common diagnosed cancer and the second leading cause of cancer death in men in the United States.1 The incidence of PCa in China is increasing in recent years, and the proportion of advanced prostate cancer is higher than that in Western countries. Androgen deprivation therapy (ADT) is the mainstay of therapy for patients with locally advanced or metastatic PCa or patients with early-stage disease who are ineligible for local regional treatments due to health disparity.2
Growing evidence suggests that inflammation might have a major role in the tumorigenesis and progression of PCa.3,4,5 Many systemic inflammation-based parameters could distinguish PCa and benign prostate diseases and predict the prognosis of PCa.6,7 Low serum neutrophil count could predict a positive prostate biopsy.6 The neutrophil-to-lymphocyte ratio (NLR) seems to represent an independent prognostic marker in patients with PCa.7 Tumor-associated macrophages (TAMs) are critical modulators of the tumor microenvironment, and they are reported to be associated with a poor prognosis.8,9 TAMs originate from circulating bone marrow-derived monocytic precursors.10 Numerous studies have revealed that an elevated peripheral monocyte count independently predicts adverse clinical characteristics and poor prognosis in patients with tumors including follicular lymphoma,11 mantle cell lymphoma,12 lung adenocarcinoma,13 oropharyngeal cancer,14 and hepatocellular carcinoma.15 However, whether peripheral monocyte count plays important roles in the diagnosis and prognosis of PCa has not been reported.
Monocyte is a type of Leukocytes, and its measurement is routinely performed in most clinical laboratories worldwide. The purpose of this study was to evaluate whether peripheral monocyte count could be a useful diagnostic and prognostic biomarker for PCa.
MATERIALS AND METHODS
Study population
After obtaining approval from the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine, and informed consent of patients, 1107 consecutive patients who had undergone transrectal ultrasound-guided 12-core prostate needle biopsy and 290 prostate cancer (PCa) patients receiving androgen deprivation therapy (ADT) as first-line therapy at the Renji Hospital between January 2010 and December 2014 were retrospectively reviewed. We excluded patients with inflammatory diseases, autoimmune diseases, hematological, hepatic, and renal diseases, cardiovascular and cerebrovascular diseases, other types of cancer, and those patients lost to follow-up.
Clinical and pathological evaluation
Medical data on clinical characteristics including age, prostate-specific antigen (PSA), free PSA to total PSA ratio (f/tPSA), prostate volume (PV), PSA density (PSAD) and monocyte count at diagnosis, clinical tumor stage, biopsy Gleason score, and follow-up information were collected. The pathologic slides were re-reviewed by the urologic pathologists, and their Gleason scores were retrieved from the pathology records. For clinical tumor stage, the patients underwent pelvic Computed Tomography or Magnetic Resonance Imaging. Radionuclide bone scan was performed to determine whether there was bone metastasis. PCa patients were stratified into low-, intermediate-, and high-risk groups according to the EAU guidelines:16 low-risk group, PSA <10 μg l−1 and Gleason score <7 and cT1c-2a; intermediate-risk group, PSA 10–20 μg l−1 or Gleason score 7 or cT2b-2c; and high-risk group, PSA >20 μg l−1 or Gleason score 8–10 or > cT2c.
Eligible PCa patients were treated with continuous ADT as first-line therapy, including castration and anti-androgen therapy. Castration was achieved either by orchiectomy or luteinizing hormone-releasing hormone-a (LHRN-a), such as goserelin 3.6 mg (Zoladex, AstraZeneca, London, UK) subcutaneously monthly. Anti-androgen therapy was achieved either by bicalutamide tablets 50 mg (Casodex, AstraZeneca, London, UK) per day orally or flutamide tablets 3 times a day, each time 250 mg (Forward, Shanghai, China) orally.
Follow-up
Patients with PCa were followed for every 3 months. Duration of the follow-up was assessed from the date of treatment until the last follow-up (June 2015) or death, which was defined as cancer-related or a different cause. Progression was defined as castration-resistant or death, and the castration-resistant was judged according to the EAU guidelines:17 castrate serum testosterone <50 ng ml−1 or 1.7 nmol l−1 plus either: (1) biochemical progression: three consecutive rises of PSA, 1 week apart, resulting in two 50% increases over the nadir, with PSA >2 ng ml−1 or (2) radiological progression: the appearance of two or more bone lesions on bone scan or enlargement of a soft tissue lesion using RECIST (Response Evaluation Criteria in Solid Tumours).
Laboratory assays
Venous blood samples were collected before the prostate biopsy. Peripheral monocyte count was performed as part of the routine clinical procedure, and by a fully automated blood cell counting system (Sysmex 2100; Sysmex, Kobe, Japan). The normal range of peripheral monocyte count is (0.12–1.00) × 109 l−1.
Statistical analysis
For the prostate biopsy cohort, parameters were shown as median with interquartile ranges (IQRs), univariate and multivariate logistic regression analyses were performed to identify the independent predictors of a positive biopsy. The areas under the receiver-operator characteristics curve (AUC) of monocyte count and PSA for the probability of detecting cancer were evaluated. As to the PCa patients, for continuous variables, the Wilcoxon Signed Rank test was used to interrogate the median with IQRs between monocyte count and clinical characteristics, while Chi-squared test was used for categorical variables. The cutoff value for the continuous variable monocyte count was determined by applying a receiver-operating characteristic curve (ROC) analysis to test all possible cutoffs that would separate between patients’ survival and cancer-related death. The survival distributions, including progression-free survival (PFS), cancer-specific survival (CSS), and overall survival (OS), were estimated by the Kaplan–Meier method and compared by a log-rank test, and subgroup analyses were taken according to the Gleason score and incidence of metastasis. PFS was calculated from the date of prostate biopsy to the date of disease progression or the time of the last follow-up. The effect of all variables on PFS, CSS, and OS was examined using Cox proportional hazard regression models. All variables with a P < 0.05 on univariate analyses were entered into multivariate stepwise Cox regression analyses. Hazard ratio (HR) and 95% confidence interval (CI) were computed. Differences were considered to be statistically significant if P < 0.05. Statistical analysis was carried out using SPSS, version 19.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Prediction of a positive prostate biopsy
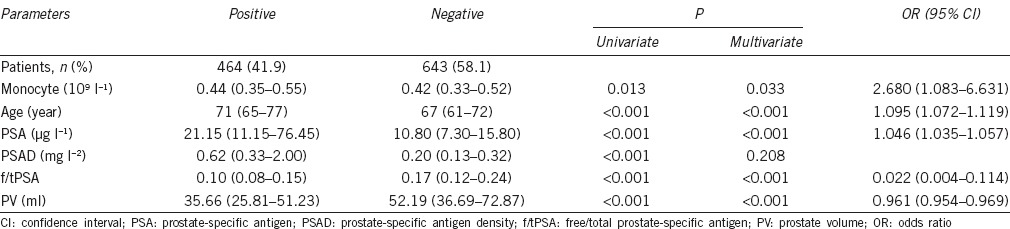
Of the 1107 men, 464 (41.9%) had a positive biopsy for PCa and 643 (58.1%) had a negative biopsy. The characteristics of the patients were summarized in Table 1. The median peripheral monocyte count of the positive biopsy group was 0.44 (0.35–0.55) × 109 l−1, while the corresponding level of the negative biopsy group was 0.42 (0.33–0.52) × 109 l−1. The peripheral monocyte count was significantly higher in the positive biopsy group compared with the negative biopsy group (P < 0.05). Univariate analyses showed that age, PSA, PSAD, f/tPSA, PV, and monocyte count had statistically significant relationships with PCa (P < 0.05). Multivariate analyses revealed that age, PSA, f/tPSA, PV, and monocyte count were predictive independently of a positive prostate biopsy (P < 0.05). AUC of monocyte count for the probability of detecting cancer was 0.544 (95% CI 0.509–0.578), whereas the AUC of PSA was 0.745 (95% CI 0.715–0.775).
Table 1.
The clinical factor results of univariate and multivariate logistic regression analyses in patients with positive or negative prostate biopsy (n=1107)
Clinical characteristics of PCa patients
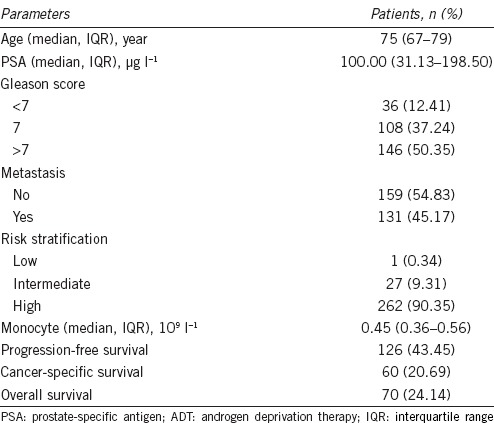
A total of 325 PCa patients who had received ADT as the first-line therapy were qualified for this study, of whom 35 patients were excluded according to the exclusion criteria in the materials and methods section, leaving 290 suitable for further analysis. The basic characteristics of the patients were shown in Table 2. The median age was 75 years (IQR, 67–79 years).
Table 2.
Clinical characteristics of prostate cancer patients treated with ADT (n=290)
Association between peripheral monocyte count and clinical and pathological characteristics
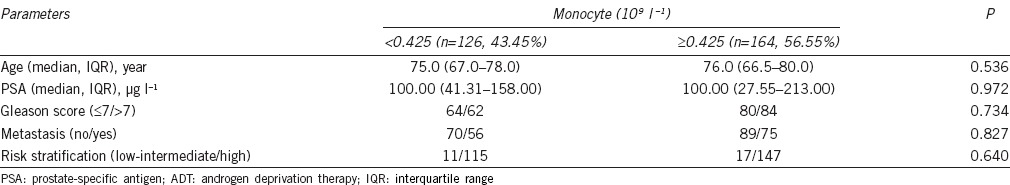
Based on ROC curve for CSS, the best cutoff value of monocyte was 0.425 × 109 l−1, AUC for monocyte count was 56.8% with a sensitivity of 70.0% and a specificity of 47.0% by the Youden index (Figure 1), and all the patients were divided into either low monocyte count group (n = 126, 43.45%) or high monocyte count group (n = 164, 56.55%). The differences of age, PSA, Gleason score, risk stratification, and incidence of metastasis between low monocyte count group and high monocyte count group were not significant (P > 0.05; Table 3).
Figure 1.
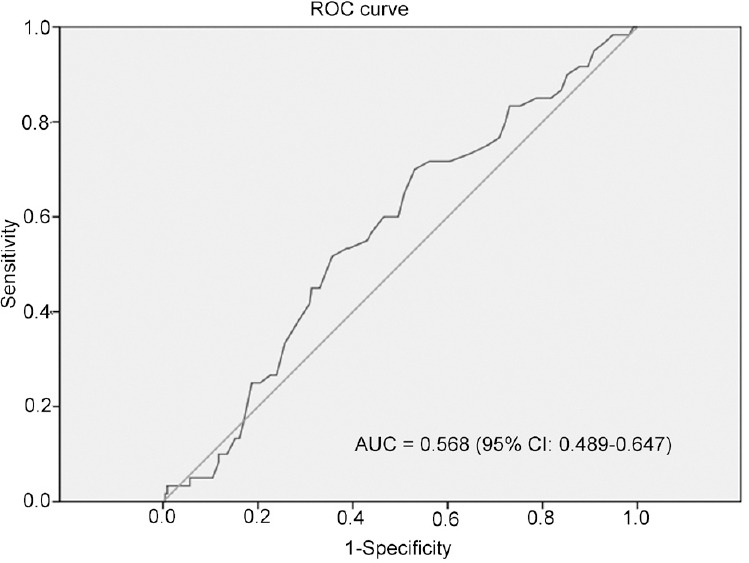
ROC analysis of optimal monocyte count cutoff value (0.425 × 109 l−1). The AUC for monocyte count was 56.8%, with a sensitivity of 70.0% and a specificity of 47.0% by the Youden index.
Table 3.
Clinical parameters of prostate cancer patients treated with ADT according to peripheral monocyte count
Association between peripheral monocyte count and prognosis of PCa
The median follow-up duration was 37.0 months (IQR, 24.0–50.3 months), out of 290 patients with usable follow-up information, 126 (43.45%) patients experienced disease progression and 70 (24.14%) patients died, including 60 (20.69%) patients died of PCa at the end of the last follow-up.
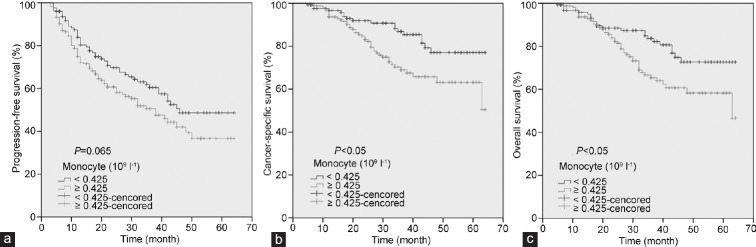
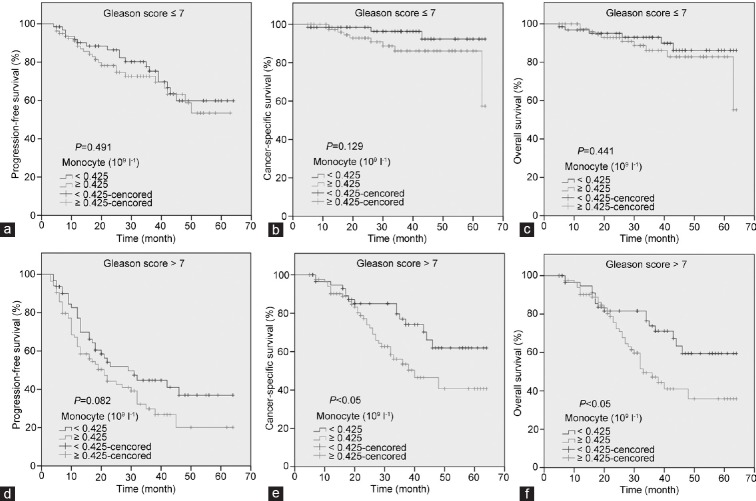
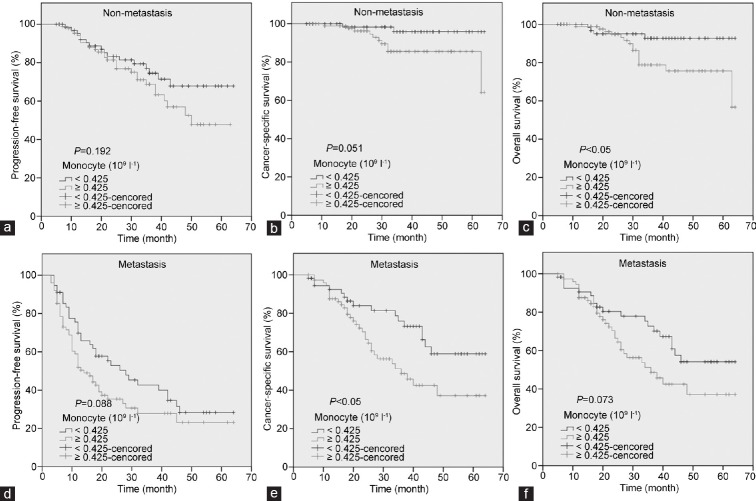
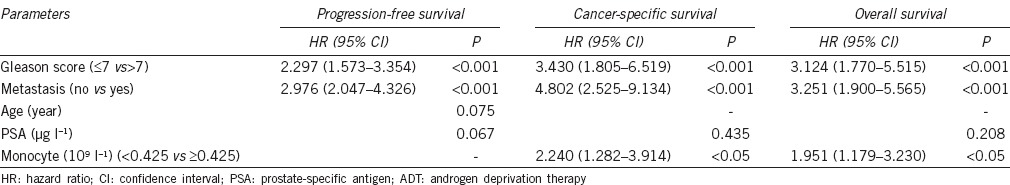
The patients with high monocyte count had a significantly worse survival than those with low monocyte count with regard to CSS (log-rank test, P < 0.05) and OS (P < 0.05), but not PFS (P = 0.065) (Figure 2). As shown in Figure 3, in the Gleason score >7 subgroup, high monocyte count group exhibited worse CSS and OS (each P < 0.05), but not PFS (P = 0.082); however, in the Gleason score ≤7 subgroup, the prognostic difference was not significant between high monocyte count group and low monocyte count group (P = 0.491 for PFS, P = 0.129 for CSS and P = 0.441 for OS). According to Figure 4, in the metastasis subgroup, high monocyte count group exhibited worse CSS (P < 0.05), but not PFS and OS (P = 0.088 for PFS and P = 0.073 for OS). Conversely, in the nonmetastasis subgroup, high monocyte count group exhibited worse OS (P < 0.05), but not PFS and CSS (P = 0.192 for PFS and P = 0.051 for CSS). Univariate and multivariate Cox regression analyses (stepwise analysis) of the factors influencing PFS, CSS, and OS were shown in Tables 4 and 5. Univariate analyses demonstrated that PSA, Gleason score, and incidence of metastasis were significant predictors for PFS, CSS, and OS (each P < 0.05), but age and risk stratification were the significant predictors for PFS, not for CSS and OS, and monocyte count was the significant predictor for CSS and OS, not for PFS. In the multivariate analyses, we identified that Gleason score and incidence of metastasis were the independent prognostic factors for PFS, whereas Gleason score, incidence of metastasis, and monocyte count were the independent prognostic factors for CSS and OS. The HRs of monocyte count were 2.240 (95% CI 1.282–3.914) for CSS and 1.951 (95% CI 1.179–3.230) for OS, respectively.
Figure 2.
Kaplan–Meier curves for survival of prostate cancer patients according to peripheral monocyte count. (a) Progression-free survival. (b) Cancer-specific survival. (c) Overall survival.
Figure 3.
Kaplan–Meier survival curves stratified by peripheral monocyte count in prostate cancer patients. In the Gleason score ≤7 subgroup, the progression-free survival (PFS), cancer-specific survival (CSS), and overall survival (OS) were shown in (a–c), respectively. In the Gleason score >7 subgroup, the PFS, CSS, and OS were shown in (d–f), respectively.
Figure 4.
Kaplan–Meier survival curves stratified by peripheral monocyte count in prostate cancer patients. In the nonmetastasis subgroup, the progression-free survival (PFS), cancer-specific survival (CSS), and overall survival (OS) were shown in (a–c), respectively. In the metastasis subgroup, the PFS, CSS, and OS were shown in (d–f), respectively.
Table 4.
Univariate analyses of various clinical parameters in prostate cancer patients treated with ADT
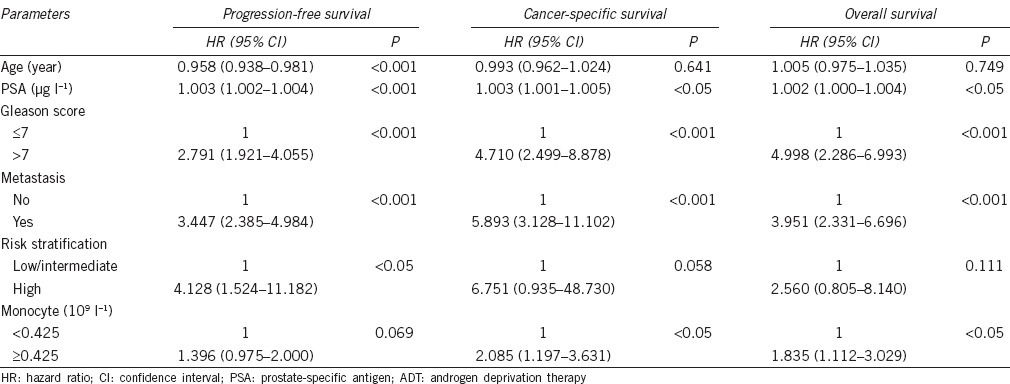
Table 5.
Multivariate analyses of various clinical parameters in prostate cancer patients treated with ADT
DISCUSSION
PSA level was the most important indicator to screen PCa, guide the prostate biopsy, and evaluate the prognosis of PCa, but it was a lack of sensitivity and specificity, thus looking for a marker for the diagnostic and prognostic assessment of PCa adjunctively is of great significance. In this large cohort of patients, we demonstrated that the monocyte counts in positive prostate biopsy patients were higher than that in negative prostate biopsy patients, and the high monocyte count was associated with an increased risk for cancer-specific and all-cause mortalities. These findings remained significant after adjusting for clinicopathological features, indicating independent associations of elevated monocyte count with PCa and adverse outcomes of PCa.
As there is recent progress in the identification of genetic and molecular alterations in PCa, many researchers started to search for relevant markers.18,19 Catalona et al.18 made a multicenter study and found that [-2]pro-PSA could aid PSA and free PSA to enhance specificity to detect overall and high-grade PCa in the 2.0 μg l−1 to 10.0 μg l−1 PSA range. Wei et al.19 conducted a prospective validation trial to assess the diagnostic performance of the prostate cancer antigen 3 (PCA 3) urinary assay for the detection of PCa among men screened with PSA and found that urinary PCA3 could reduce the burden of prostate biopsies among men undergoing a repeat prostate biopsy, and for biopsy-naive patients, a high PCA3 score (>60) significantly increased the probability that an initial prostate biopsy will identify cancer.
The total blood cell count is cheap and routinely measured, which gives us the counts of all types of blood cells, such as white blood cell, neutrophil, lymphocyte, and monocyte. These blood cell counts and derived indicators are systemic inflammation-based parameters, and a great deal of studies showed that the systemic inflammation-based parameters played important roles in the diagnostic and prognostic assessment of tumors. Kaynar et al.20 found that platelet-to-lymphocyte ratio (PLR) was a noninvasive inflammation biomarker, differentiating PCa and benign prostate hyperplasia. Huang et al.14 demonstrated that a high neutrophil count and a high monocyte count independently predicted inferior OS and recurrence-free survival (RFS), whereas a high lymphocyte count predicted better RFS and marginally better OS in HPV+ Oropharyngeal Cancer patients, this association was not apparent in HPV− patients. Multiple malignancies have been associated with an elevated monocyte count and adverse oncologic outcomes.11,12,13,14,15 On behalf of the systemic inflammation-based parameters, peripheral monocyte count is of great clinical research and application value.
To the best of our knowledge, to date, our analysis is the first study of peripheral monocyte count focusing on the diagnosis and prognosis of PCa. We found that the monocyte counts of positive and negative prostate biopsy were 0.44 (0.35–0.55) × 109 l−1 and 0.42 (0.33–0.52) × 109 l−1, respectively, in which positive prostate biopsy group was significantly higher than negative prostate biopsy group (P < 0.05), and the difference remained significant after adjusting for clinical features (age, PSA, PSAD, f/t PSA, and PV). Although monocyte count was not a better diagnostic factor than PSA (AUC: 0.544 vs 0.745), the difference of the peripheral monocyte count between the positive and negative biopsy groups gave us a mechanistic insight that monocyte might play a significant role in the tumorigenesis of prostate cancer. In the PCa patients treated with ADT, after a median follow-up of 37.0 months, we showed that an elevated monocyte count was an independent prognostic factor for worse CSS (HR = 2.244, P < 0.05) and OS (HR = 1.995, P < 0.05), but not PFS (P = 0.117), which might indicate that pretreatment peripheral monocyte count was a biomarker to predict clinical outcome for castration-resistant prostate cancer (CRPC). Thus, peripheral monocyte count could be added to the blood gene expression-based prognostic models for CRPC built by Olmos et al.,21 Ross et al.,22 and Wang et al.23 However, the differences of tumor features including PSA, Gleason score, risk stratification, and incidence of metastasis between high monocyte count group and low monocyte count group were not significant in our study.
The exact explanation for the association between high monocyte count and PCa and poor prognosis of PCa remains unclear and is yet to be elucidated. First, TAMs, which are mainly derived from circulating monocytes, played a key role in the tumor microenvironment, and affected tumor growth, angiogenesis, and metastasis. TAMs were divided into two categories: M1 (CD68) and M2 (CD163) macrophages according to their function.8 M1 macrophage was involved in the inflammatory response, pathogen clearance, and antitumor immunity. In contrast, the M2 macrophage influenced an anti-inflammatory response, wound healing, and pro-tumorigenic properties. TAMs generally exhibited an M2 phenotype known to promote angiogenesis, tumor growth, and metastasis. Lanciotti et al.24 investigated the causal connection between M1 and M2 phenotype macrophages’ occurrence with PCa and their correlation with tumor extension and biochemical recurrence after Radical Prostatectomy and found that patients with higher density of M count were associated with poor prognosis, and M2 phenotype was significantly associated with tumor extension. Second, the peripheral monocytes could promote PCa progression. Lindholm et al.25 showed that co-culture of PC-3, DU145, and LNCaP cells with isolated human monocytes significantly stimulated PCa cell invasion activity.
There are some limitations in this study. First, this study was a retrospective investigation. Despite the strict enrollment criteria used, we were unable to completely exclude conditions that might cause inflammatory changes in PCa. Second, peripheral monocyte count was a marker to reflect systemic inflammatory and immunological status, not specific to PCa, and there was a large overlap between the positive and negative biopsy groups. Third, the data were obtained from a single institution, our results should be validated by prospective research and data from multiple centers.
CONCLUSION
An elevated monocyte count was an independent diagnostic biomarker for PCa, and pretreatment peripheral monocyte count might play a significant role in the prognosis of PCa patients treated with ADT. Thus, we recommend that peripheral monocyte count should be an additional diagnostic and prognostic factor for the individual risk assessment of PCa.
AUTHOR CONTRIBUTIONS
YQW was responsible for the design of the study and participated in the data analysis and manuscript mapping. YJZ contributed to the manuscript composition, submission and revision, and participated in the data analysis and manuscript mapping. JHP carried out the data collection, interpretation, and discussion. FX involved in the data collection and interpretation. XGS made substantial contributions to the data collection and discussion. JJS participated in the data collection, interpretation, and discussion. QL reviewed the pathologic slides and re-made the Gleason scores. YRH contributed to the manuscript composition and the funding application. BJD involved in the design of the study and the funding application, the supervision, and management of the project. WX participated in its design and was responsible for the funding application and the supervision and management of the project. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (91129725, 81572536), Science and Technology Commission of Shanghai Municipality (14140901700), Shanghai Municipal Education Commission (15ZZ058), Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (PWZxq2014-05), the Joint research foundation of Shanghai Shenkang hospital development center on innovative medical technology (SHDC12015125), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20152215), and the Shanghai Jiao Tong University School of Medicine Translational Research Innovation Fund (15ZH4002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We sincerely thank the patients for their participation in this study.
REFERENCES
- 1.Siegel R, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Wein AJ, Kavaussi LR, Novick AC, Partin AW, Peters CA, et al. Campbell-Wash Urology. 10th ed. Philadelphia: Elsevier; 2012. pp. 2934–53. [Google Scholar]
- 3.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60:106–17. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taverna G, Pedretti E, Di Caro G, Borroni EM, Marchesi F, et al. Inflammation and prostate cancer: friends or foe? Inflamm Res. 2015;64:275–86. doi: 10.1007/s00011-015-0812-2. [DOI] [PubMed] [Google Scholar]
- 6.Fujita K, Imamura R, Tanigawa G, Nakagawa M, Hayashi T, et al. Low serum neutrophil count predicts a positive prostate biopsy. Prostate Cancer Prostatic Dis. 2012;15:386–90. doi: 10.1038/pcan.2012.27. [DOI] [PubMed] [Google Scholar]
- 7.Langsenlehner T, Thurner EM, Krenn-Pilko S, Langsenlehner U, Stojakovic T, et al. Validation of the neutrophil-to-lymphocyte ratio as a prognostic factor in a cohort of European prostate cancer patients. World J Urol. 2015;33:1661–7. doi: 10.1007/s00345-015-1494-7. [DOI] [PubMed] [Google Scholar]
- 8.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–90. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonomura N, Takayama H, Nakayama M, Nakai Y, Kawashima A, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107:1918–22. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 10.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, et al. The absolute peripheral monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leuk Lymphoma. 2012;53:575–80. doi: 10.3109/10428194.2011.637211. [DOI] [PubMed] [Google Scholar]
- 12.Von Hohenstaufen KA, Conconi A, De Campos CP, Franceschetti S, Bertoni F, et al. Prognostic impact of peripheral monocyte count at presentation in mantle cell lymphoma. Br J Haematol. 2013;162:465–73. doi: 10.1111/bjh.12409. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai S, Marumo S, Shoji T, Sakuramoto M, Hirai T, et al. Prognostic impact of preoperative monocyte counts in patients with resected lung adenocarcinoma. Lung Cancer. 2014;85:457–64. doi: 10.1016/j.lungcan.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Huang SH, Waldron JN, Milosevic M, Shen X, Ringash J, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121:545–55. doi: 10.1002/cncr.29100. [DOI] [PubMed] [Google Scholar]
- 15.Shen SL, Fu SJ, Huang XQ, Chen B, Kuang M, et al. Elevated preoperative peripheral blood monocyte count predicts poor prognosis for hepatocellular carcinoma after curative resection. BMC Cancer. 2014;14:744. doi: 10.1186/1471-2407-14-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng ml-1 prostate specific antigen range. J Urol. 2011;185:1650–5. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei JT, Feng ZD, Partin AW, Brown E, Thompson I, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J Clin Oncol. 2014;32:4066–72. doi: 10.1200/JCO.2013.52.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaynar M, Yildirim ME, Gul M, Kilic O, Ceylan K, et al. Benign prostatic hyperplasia and prostate cancer differentiation via platelet to lymphocyte ratio. Cancer Biomark. 2015;15:317–23. doi: 10.3233/CBM-150458. [DOI] [PubMed] [Google Scholar]
- 21.Olmos D, Brewer D, Clark J, Danila DC, Parker C, et al. Prognostic value of blood mRNA expression signatures in castration-resistant prostate cancer: a prospective, two-stage study. Lancet Oncol. 2012;13:1114–24. doi: 10.1016/S1470-2045(12)70372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross RW, Galsky MD, Scher HI, Maqidson J, Wassmann K, et al. A whole-blood RNA transcript-based prognostic model in men with castration-resistant prostate cancer: a prospective study. Lancet Oncol. 2012;13:1105–13. doi: 10.1016/S1470-2045(12)70263-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Gong Y, Chippada-Venkata U, Heck MM, Retz M, et al. A robust blood gene expression-based prognostic model for castration-resistant prostate cancer. BMC Med. 2015;13:201. doi: 10.1186/s12916-015-0442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanciotti M, Masieri L, Raspollini MR, Minervini A, Mari A, et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res Int. 2014;2014:486798. doi: 10.1155/2014/486798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindholm PF, Lu Y, Adley BP, Vladislav T, Jovanovic B, et al. Role of monocyte-lineage cells in prostate cancer cell invasion and tissue factor expression. Prostate. 2010;70:1672–82. doi: 10.1002/pros.21202. [DOI] [PubMed] [Google Scholar]