Abstract
The effects of the combination of exercise and TRT on symptoms of late-onset hypogonadism (LOH) and the durability of response after cessation of TRT were investigated. A total of fifty patients with erectile dysfunction (ED) who had a sedentary lifestyle and low serum total testosterone (T) levels were enrolled and followed for 20 weeks. Patients were randomly divided into two groups; all of them received T gel for 12 weeks and it was discontinued for 8 weeks. Patients assigned to Group II were offered a supervised exercise program for 20 weeks. Measurement of serological testing was performed and self-assessment questionnaires and Global Assessment Question (GAQ) were asked. Baseline characteristics and the initial symptom scores showed no significant difference between the two groups. Serum total T levels and the symptom scores were increased at 12 weeks in both groups, and Group II showed better results with statistical significance. There was a decrease in T levels and worsening of symptom scores at week 20 compared to week 12 in both groups, and Group II showed better results with statistical significance. On the GAQ, Group II showed higher ratio of “yes” at week 12 and the same tendency was sustained at week 20 with significant difference between two groups. The combination of exercise and TRT showed significant improvements in serum T levels and LOH symptoms compared to TRT alone. In addition, these improvements were maintained in the combination group with continuous exercise, even after cessation of TRT.
Keywords: erectile dysfunction, exercise, hypogonadism, testosterone
INTRODUCTION
It is well-known that testosterone replacement therapy (TRT) can improve many symptoms of hypogonadism in patients with late-onset hypogonadism (LOH), including effects on mood, energy level, sense of well-being, sexual function, muscle strength, erythropoiesis, bone mineral density, and cognition.1 However, TRT is not a risk-free treatment because several possible adverse effects, such as polycythemia, liver dysfunction, worsening of sleep apnea, and prostate disease, have been seen in specific situations.1,2,3 Moreover, the safety of TRT over a long period has not yet been shown conclusively. In addition, few evidence-based information is available on certain aspects of TRT, such as duration of treatment and durability of response after discontinuation of TRT. In fact, there have been few reports discussing whether symptoms of LOH return after cessation of TRT.4,5 Moreover, in those studies, serum testosterone levels and symptoms of LOH were shown to be worsened after cessation of TRT. As a result of these reports, we do not know whether stopping TRT is possible or TRT must be continued life-long.
Some studies have found that the combination of TRT and exercise showed better efficacy for specific LOH symptoms than TRT alone.6,7 However, in those studies, the type of exercise was mainly resistive strengthening exercise and the effects on the sexual function and the durability of response were not evaluated. Based on the results of these studies, we made our hypothesis as follows: (1) the combination of TRT and exercise is better than TRT alone for improvement of sexual functions as well as other LOH symptoms and (2) continuous combination exercise is helpful in maintaining improvement in LOH symptoms and elevated serum testosterone levels after the cessation of TRT.
Therefore, we designed and performed the present study to determine the effects of a combination of exercise and TRT on serum testosterone levels, symptoms of LOH, and the durability of response after cessation of TRT.
MATERIALS AND METHODS
From January 2013, fifty patients with erectile dysfunction (ED) and a sedentary lifestyle (a men who do not engage in at least 1 h per week of moderate-intensity physical activity) with low serum total testosterone levels (<350 ng dl−1) were consecutively enrolled; each patient was followed for 20 weeks. Patients received a full explanation of the content and purpose of the study before the trial and were enrolled after signing informed consent. All of the men included in the study were more than 40 years of age; each had a normal digital rectal examination (DRE) and a prostate-specific antigen (PSA) level of <3.0 ng ml−1. Exclusion criteria of the study were as follows: YES to one or more questions of physical activity readiness questionnaire (PAR-Q), previous treatment for hypogonadism, the presence of congestive heart failure, unstable angina, myocardial infarction, cerebrovascular events, prostate or breast cancer, psychosis or sleep apnea, and infection with human immunodeficiency virus or any active systemic disease.
Patients were block-randomized into two groups (Groups I and II). Testosterone replacement therapy was administered for 12 weeks in both groups using 2% testosterone gel (50 mg) applied once in the morning. In addition, a supervised 20-week physical activity program was provided for Group II only. The program was performed 3 times per week, 80 min per time and consisted of 20 min of aerobic exercise, followed by 10 min of whole body stretching and 30 min of strength exercise with the last 20 min consisting of aerobic exercise again. The intensity was adjusted to the individuals’ exercise capacity as measured by seven baseline exercise tests (ergometer, sit and reach test, curl-up test, squeeze and standing high jump test, whole body reaction test, and one leg balance with eyes close test), which measured cardiopulmonary function, flexibility, muscular endurance, muscular strength, agility, and balance.8 Both groups of patients were instructed to have three meals per day that had a low glycemic load, were low in saturated fats, and were rich in omega-3 fatty acids. Patients were also instructed to attempt to eat approximately 10% fewer calories per meal compared with their pretreatment diet.
Serum testosterone levels were measured by radioimmunoassay during the first visit, at week 12, and at 8 weeks after treatment cessation (week 20). Blood samples were obtained between 8 am and 11 am. Serum biochemistry for safety profiles, hemoglobin (Hb), and hematocrit (Hct) were assessed during the first visit and at week 12; glucose and lipid profiles were also checked during the first visit and at week 12. Prostate-specific antigen (PSA) was measured at baseline and at 12 and 20 weeks. The height and weight of each patient were measured and body mass index (BMI) was calculated at the first visit, at 12 weeks, and at week 20.
To determine the clinical efficacy of treatment, the International Index of Erectile Function (IIEF) questionnaire and the Aging Males’ Symptoms (AMS) questionnaire, which were translated into Korean and validated for use in our population,9,10 were administered to patients at baseline and at weeks 12 and 20. Sexual Encounter Profile (SEP) questions 2 (“Were you able to insert your penis into your partner's vagina?”) and 3 (“Did your erection last long enough for you to have a successful intercourse?”) were also administered to patients at baseline and at 12 and 20 weeks. The following Global Assessment Question (GAQ) was asked at weeks 12 and 20, “Has there been any improvement in your erectile function since the start of therapy?” Possible answers included “improved” and “not improved.”
The primary efficacy outcome was total serum testosterone levels at weeks 12 and 20. The secondary efficacy outcomes were changes in IIEF and AMS scores, changes in answers to SEP questions 2 and 3, and response to the GAQ at the same visits. Means of data were calculated for each group and reported as mean ± 95% confidence interval (CI) of the standard deviation of the mean.
For statistical analysis, we used PASW Statistics for Windows, version 18.0 (SPSS, Inc., Chicago, IL, USA). Statistical analyses were conducted using the Mann–Whitney U-test, Wilcoxon signed-rank test, and Fisher's exact test. P < 0.05 was considered statistically significant.
RESULTS
There were no significant differences in baseline characteristics between two groups (Table 1). During the treatment period, two patients in Group I and four patients in Group II dropped out of the study. Two patients in Groups I and II did not continue treatment due to the expense of treatment medications, and the other two patients in Group II did not complete the exercise program due to individual schedule conflicts. No patient was withdrawn from the study for not meeting the detailed exclusion criteria or from complications related to treatment. The compliance to testosterone gel showed no significant difference between two groups.
Table 1.
Comparison of baseline characteristics, serological parameters, and symptom severities before and 12 weeks after testosterone replacement therapy in both groups
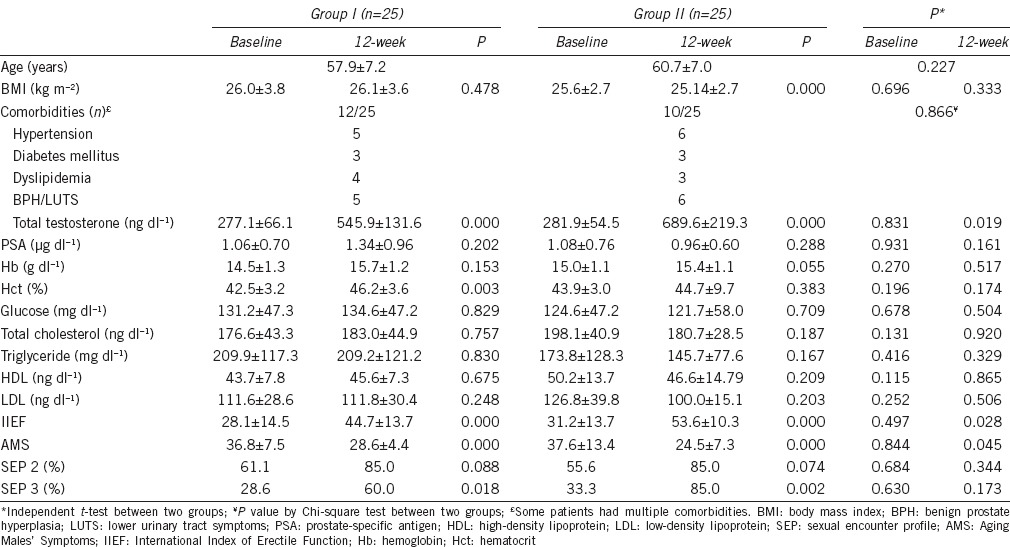
Figure 1 shows the change in serum testosterone levels through the treatment periods. Serum testosterone levels increased significantly at 12 weeks in both groups. However, testosterone levels in Group II were significantly higher than Group I (P = 0.019). At 20 weeks, both groups showed a significant decrease in testosterone levels compared to 12 weeks data; however, Group II showed significantly higher testosterone levels than Group I (P < 0.001). For the change in serum testosterone levels from baseline to at 12 weeks, Group II showed a significantly wider range of increase than Group I (P = 0.029). For the change in serum testosterone levels from 12 to 20 weeks, the change of Group II was less than Group I without statistical significance (P = 0.415).
Figure 1.
Changes of serum total testosterone levels and comparison between two groups (P value by independent t-test, P = 0.019 at 12 weeks, P < 0.001 at 20 weeks). ¥¥P < 0.001 by paired t-test compared to 3 months in Group I, *P < 0.01 by paired t-test compared to 3 months in Group II.
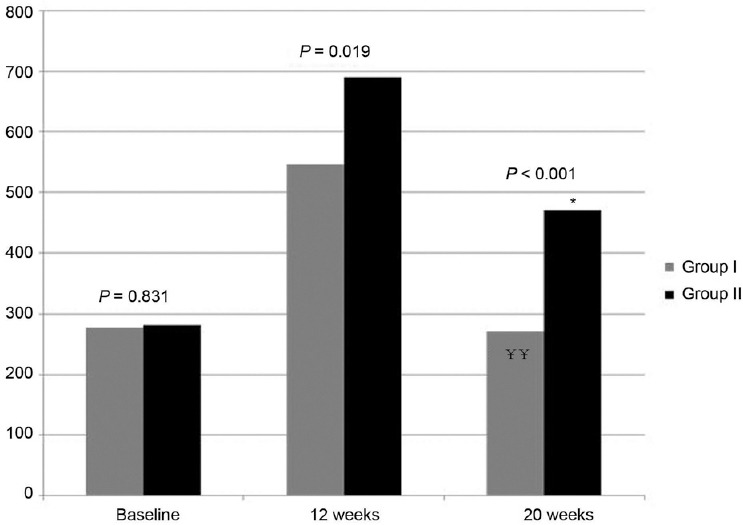
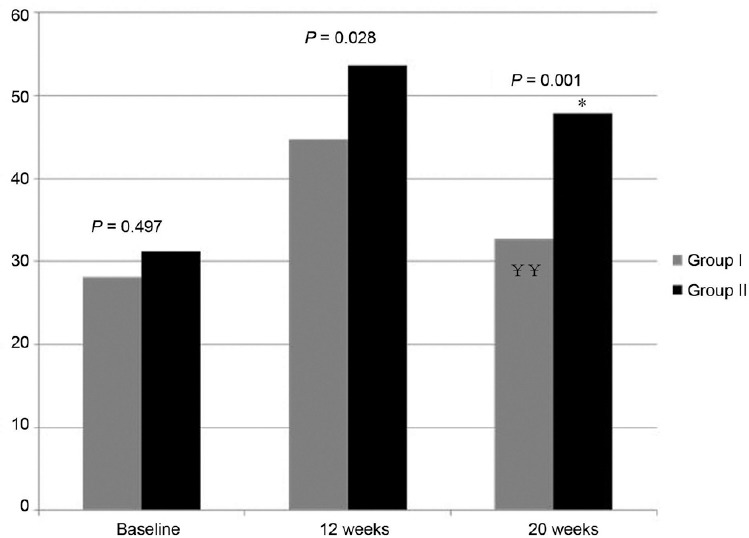
The changes in symptom severity also showed similar pattern. In both groups, total IIEF scores improved significantly at 12 weeks. However, symptom scores for Group II were more improved than Group I with statistical significance (P = 0.028). Similar to testosterone levels, symptoms worsened after cessation of TRT but Group II showed better symptom scores than Group I with statistical significance (P = 0.001) (Figure 2). For total AMS scores, the same pattern was observed as with IIEF; Group II showed more improved symptom scores than Group I at 12 weeks (P = 0.045) and improvement was maintained in Group II after cessation of TRT at 20 weeks (P = 0.007) (Figure 3). In the analysis of AMS subscale scores (psycho, somatic, and sexual symptoms), a similar pattern was observed; in psycho symptoms, there was no significant difference between the two groups at baseline or at 12 weeks, but the improvement was well-maintained in Group II at 20 weeks (P = 0.004). In somatic symptoms, there was no difference at baseline, but Group II showed a better symptom score at 12 and 20 weeks, which was not statistically significant (P = 0.343, P = 0.114). In the sexual symptom subscale, Group II showed a significantly better symptom score at 12 and 20 weeks (P = 0.000, P = 0.005), the same pattern as seen in the total AMS score.
Figure 2.
Changes of total score of IIEF and comparison between two groups (P value by independent t-test, P = 0.028 at 12 weeks, P = 0.001 at 20 weeks). ¥¥P < 0.001 by paired t-test compared to 3 months in Group I, *P < 0.01 by paired t-test compared to 3 months in Group II.
Figure 3.
Changes of total score of AMS and comparison between two groups (P value by independent t-test, P = 0.045 at 12 weeks, P = 0.007 at 20 weeks). ¥P < 0.01 by paired t-test compared to 3 months in Group I, *P < 0.01 by paired t-test compared to 3 months in Group II.
For the ratio of “yes” responses to SEP questions 2 and 3, there were significant increases in both groups at 12 weeks compared to baseline and there were no significant differences between the two groups at week 12 (P = 0.344 and P = 0.173 for questions 2 and 3, respectively). For the ratio of “yes” responses to SEP question 2, there was no significant difference between two groups at 20 weeks, but for SEP question 3, the results at week 20 showed a higher ratio of “yes” in Group II compared to Group I with statistical significance (P = 0.031).
With respect to BMI, Group II showed a continuous decrease in BMI throughout the 20 weeks with statistical significance. However, in Group I, there was a tendency for an increase with no statistical significance.
We also investigated patient satisfaction to treatment. At 3 months and 5 months, the ratio of “yes” responses to the GAQ “Has there been any improvement in your erectile function since the start of therapy” was investigated. The results showed a higher ratio of “yes” responses in Group II than in Group I at week 12 without statistical significance. At 20 weeks, the satisfaction rate in Group II was significantly higher than Group I after cessation of TRT (P = 0.043).
For changes in serologic parameters during the 12 weeks of treatment, Group II showed a trend toward improving metabolic status, such as glucose and lipid profiles, but there were no changes in Group I compared to baseline status (Table 1). For Hb and Hct, both of which had increased during the first 12 weeks of testosterone administration without statistical significance, no values were observed above the upper limit of the normal range in either group (Table 1).
For PSA levels, there was no significant increase in either group at 12 weeks compared to baseline and no significant difference was observed between the two groups. After TRT, PSA levels in both groups showed nonsignificant decreases, with no significant difference between the two groups. No disorders or abnormalities were identified by follow-up DRE or based on clinical symptoms. There were two and three patients who complained of redness and itching sense at the skin of inner thigh, respectively, but these events were not serious and resolved after changing the site of gel application to the lower abdomen.
DISCUSSION
The criteria for an optimal treatment modality might have included a shorter treatment period and a longer durability of response after completion of treatment. We are already aware of the risks associated with testosterone treatment. Still, controversies about the cardiovascular risk of treatment exist,11,12 and there is concern about developing prostate cancer during testosterone replacement,2,13 although the incidence of these adverse events is not significant.13 In other words, we do not know the real effects of TRT on the cardiovascular system and the prostate so far. Hence, it cannot yet be clearly stated that “testosterone treatment is safe.” Therefore, it is necessary to find a way to maintain efficacy of TRT with shortened treatment periods to reduce the potential risks associated with TRT.
There have been several efforts to determine the durability of response after completion of TRT. Tsujimura et al.4 evaluated LOH-related symptoms and endocrinologic markers at 3 months after discontinuation of TRT in patients with LOH, who had benefited from TRT. The results showed that improvements in symptoms may remain after cessation of TRT, but endocrinologic status declines to baseline level.4 According to Francomano et al.,14 withdrawal of long-acting injectable testosterone therapy provokes a return to baseline status for all cardiac and hormonal parameters within 6 months. Another study by O’Connell et al.5 showed a similar pattern; the effects of testosterone treatment on body composition, muscle strength, and quality of life did not persist by 6 months after treatment withdrawal in intermediate-frail and frail elderly men. Based on the results of these aforementioned studies, it appears that the effects of TRT are not maintained after cessation of treatment. If so, a continuous supply of exogenous testosterone is needed to maintain constant serum testosterone levels and its positive effects. If there is a way to augment the durability of response after stopping treatment, shorter treatment periods can be used, which may then lower the risks associated with TRT. This was the reason behind our present pilot study.
Meanwhile, according to several studies, exercise has positive effects on LOH patients. In a 52-week, double-blind clinical trial, the effects of supervised exercise and diet with or without testosterone treatment on components of metabolic syndrome and newly diagnosed type 2 diabetes in hypogonadal men were assessed by Heufelder et al.15 Serum testosterone, glycated hemoglobin (A1C), fasting glucose, high-density lipoproteins (HDL), triglycerides (TG), and waist circumference were significantly improved after 52 weeks of supervised diet and exercise without testosterone treatment. Addition of testosterone significantly improved these parameters compared with supervised diet and exercise alone.15
For ED, we already know about the effects of exercise on erectile function.16,17 There is epidemiologic evidence that frequent vigorous exercise is associated with a 30% lower risk of ED.18 Furthermore, men who are sedentary are 3 times as likely to have ED whereas moderate physical activity reduces the risk of ED by two-thirds, and in men with high physical activity, ED is reduced by over 80%.18 It is known that moderate exercise increases nitric oxide (NO) production by mechanical shear forces of blood flow.19 Acute exercise increases NO release for only 48 h while regular daily exercise induces 4-fold higher levels of NO lasting for about 1 week.20
Because of these findings, we selected exercise as the method to augment the effects of TRT on ED. However, it is unknown what type of exercise is best in improving symptoms and endocrinologic status in patients with LOH. The effects of resistive exercise on serum testosterone levels have been mainly investigated. It has been shown that acute vigorous resistance exercise can elevate serum testosterone levels, but there is insufficient information about the effects of long-term chronic exercise on serum testosterone.21,22,23,24 Smilios et al.25 showed that acute elevation of testosterone is not always observed after vigorous resistance exercise; it seems that the total amount of exercise over several levels is important for increasing testosterone. In addition, exercise using large muscles can result in an acute testosterone surge compared with exercise with small muscles, according to Cho et al.26
Most studies have tried to determine the relationship between resistive exercise and elevation of serum testosterone levels. In our present study, we did not focus on resistive exercise only. The background of our exercise program was based on unpublished data of our experience. According to our experience, cardiopulmonary function (measured by ergometer) has a significant correlation with the serum testosterone levels in LOH patients. However, six other basic exercise function tests, such as “sit and reach” (flexibility), “curl-up” (muscular endurance), “squeeze” and “standing high jump” (muscular strength), “whole body reaction test” (agility), and “one leg balance with eyes close” (balance) tests, showed no significant correlation with serum testosterone levels. With respect to erectile function and LOH symptom severity, there was a significant correlation not only with cardiopulmonary function but also with muscular endurance and strength. Therefore, the exercise program of our study consisted of 20 min of aerobic exercise, followed by 10 min of whole body stretching, 30 min of strength exercise, and 10 min of aerobic exercise.
Based on changes in BMI during treatment, we can confirm a significant decrease in BMI in Group II. Of course, long-term testosterone treatment can decrease BMI and fat according to several studies,27,28 but in our present study, the treatment periods were very short; hence, the main component affecting BMI decrease might have been exercise. If the treatment periods were longer, we might be able to confirm synergistic effects of TRT and exercise, which was observed in the study by Heufelder et al.15
One of the mechanisms of higher serum testosterone levels at 12 weeks and maintaining serum testosterone levels after cessation of TRT in Group II is thought to be due to a decrease in adipose tissue by continuous exercise and also by a decrease in the conversion of testosterone to estradiol by aromatase in adipose tissue,29 although this theory has not been confirmed and many controversies are present. Serum estradiol level was not measured in this study, but according to a recent study by Monteagudo et al.,30 depression, which is one of the LOH symptoms, was more severe in obese patients in whom there was a greater imbalance between serum testosterone and estradiol level. In this study, symptom improvement at week 12 was more substantial in the exercise-TRT-combination group than in the TRT-only group, and a further analysis is in need to examine whether or not it was because the exercise-treated group maintained the serum testosterone/estradiol balance better through the reduction of adipose tissue.
With respect to another mechanisms, Kumagai et al.31 reported that the serum testosterone levels of obese men increased significantly following a 12-week course of regular aerobic exercise and dietary regulation. They explained that the aerobic exercise and dietary regulation reduced the insulin levels of obese males, leading to increased serum testosterone levels; this finding corresponded with the results of the previous study, which demonstrated that as the serum insulin level decreased, the serum testosterone level increased. In the present study, as the glucose level tended to decrease in Group II after the 3-month treatment period although the serum insulin level was not measured, we speculated that a decrease in the serum insulin level consequent to continuous aerobic exercise and dietary improvements over a 5-month period prevented a reduction in serum testosterone levels. In addition, we already know the positive effects of weight loss in obese men on erectile function; reduction in adipose tissue by continuous exercise can lower oxidative stress by decreasing free radical formation, which is associated with deactivation of NO availability.32
There have also been some studies evaluating the association between oxidative stress and testosterone levels. Sajjadian et al.33 increased oxidative stress via buthionine sulfoximine (BSO) in an animal experiment involving mice. As a result, histological changes were induced in the testicles that reduced testosterone levels and affected semen parameters. The result of that study suggests that antioxidant activity obtained from steady exercise positively affects testicular function, possibly leading to an increase in the serum testosterone level. Furthermore, Chigurupati et al.34 divided 6-month-old mice into two groups: one group ran a distance of 1.75 km every day whereas the other group performed no exercise until reaching an age of 20 months. At that point, testicles were examined histologically and metabolites associated with oxidative stress were quantitated, and the findings revealed that the testicular tissue in the group with steady exercise maintained a normal appearance, despite the increase in age, whereas the levels of metabolites associated with oxidative stress were significantly high in spermatogenic and Leydig cells from mice not exposed to exercise.
In addition, data from a previously reported animal experiment support the idea that regular exercise directly increases the levels of steroidogenesis-related enzymes and, thus, increases DHT in the muscles and serum. According to studies by Aizawa et al. and Sato et al.,35,36 DHT concentrations increased significantly in the skeletal muscle and plasma of mice that had participated in endurance aerobic exercise on a treadmill for 25–30 min sessions, 5 times per week, when compared with mice that had not exercised.
The higher serum testosterone level and small range of serum testosterone reduction, even after the discontinuation of testosterone treatment in the exercise-treated group in the present study, could be considered due to mechanisms such as reductions in the serum insulin level, oxidative stress, and the conversion of testosterone to estradiol by aromatase in adipose tissue, and a direct increase in the muscular and plasma DHT concentration resulting from exercise and diet regulation.
Our present study has many limitations. This was a pilot study, so the study population was small and treatment and observation periods were not long enough to assess the real effects of TRT. The absence of an exercise-only group also limits the interpretation of our results. Furthermore, the patients enrolled in both groups were relatively healthy; the results of PAR-Q were used as exclusion criteria. There were also several patients who did not complete the entire study due to the economic burden of the treatment medication. In addition, although we evaluated psycho and somatic symptoms with the AMS subscale analysis, the main focus of the study was sexual function such as erectile function, and such important evaluative elements in LOH symptoms as muscle strength and quality of life were excluded. Finally, because our analysis of symptom severity of ED and TDS relied on self-assessment questionnaires, bias in reporting is a concern. The lack of objective parameters, such a penile Doppler ultrasound, is also a limitation of this study.
However, in this study, the combination of exercise and TRT resulted in significant improvements in serum testosterone levels and LOH symptoms compared with TRT only. The differences between the two groups in serum testosterone, IIEF and AMS scores, and responses to the GAQ at 12 weeks reflect the synergistic effects of combined testosterone and exercise. Moreover, these improvements were well-maintained in the combination group with continuous exercise even after cessation of TRT. Higher serum testosterone levels, better symptom scores for both IIEF and AMS questionnaires, a higher ratio of “yes” to SEP question 3, and better responses to the GAQ at week 20 (8 weeks after stopping treatment) in Group II confirmed that exercise is a way to augment the durability of response after stopping TRT and shorten the treatment duration so as to lower the risks of treatment.
There is a need for large clinical trials including an exercise-only group, and longer treatment and longer observation periods are necessary. In addition, we need to investigate which exercise is better for improving testosterone levels and LOH symptoms.
CONCLUSIONS
The combination of exercise and TRT showed significant improvements in serum testosterone levels and LOH symptoms compared to TRT alone. In addition, these improvements were well-maintained in the combination group with continuous exercise even after cessation of TRT. Exercise can augment the durability of response to TRT after stopping treatment, and it may be the solution to shorten the treatment duration with a lower risk from testosterone therapy. Further large-scale clinical trials including an exercise-only group with long treatment periods are necessary to support the conclusion of this present study.
AUTHORS’ CONTRIBUTIONS
DYC and MGP participated in conceiving of the study and drafted the manuscript. JKY, JKH, JGK, and MGP participated in the study design. SIC, JEJ, SJY, DHK, and MGP performed the studies and collected the data. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159:507–14. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons JK, Carter HB, Platz EA, Wright EJ, Landis P, et al. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14:2257–60. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimura A, Takada S, Matsuoka Y, Hirai T, Takao T, et al. Is discontinuation of hormone replacement therapy possible for patients with late-onset hypogonadism? Int J Urol. 2008;15:625–9. doi: 10.1111/j.1442-2042.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell MD, Roberts SA, Srinivas-Shankar U, Tajar A, Connolly MJ, et al. Do the effects of testosterone on muscle strength, physical function, body composition, and quality of life persist six months after treatment in intermediate-frail and frail elderly men? J Clin Endocrinol Metab. 2011;96:454–8. doi: 10.1210/jc.2010-1167. [DOI] [PubMed] [Google Scholar]
- 6.Katznelson L, Robinson MW, Coyle CL, Lee H, Farrell CE. Effects of modest testosterone supplementation and exercise for 12 weeks on body composition and quality of life in elderly men. Eur J Endocrinol. 2006;155:867–75. doi: 10.1530/eje.1.02291. [DOI] [PubMed] [Google Scholar]
- 7.Hildreth KL, Barry DW, Moreau KL, Vande Griend J, Meacham RB, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab. 2013;98:1891–900. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang GT, Choi DH, Park H, Ko YW, Lee DT, et al. Advanced Fitness Assessment & Exercise Program. Seoul: Hanmi Medical Publishing Co; 2000. p. 322. [Google Scholar]
- 9.Chung T, Lee T, Chung S, Lee M, Kim Y, et al. The Korean version of the international index of erectile function (IIEF): reliability and validation study. Korean J Urol. 1990;40:1334–43. [Google Scholar]
- 10.Daig I, Heinemann L, Kim S, Leungwattanakij S, Badia X, et al. The aging males’ symptoms (AMS) scale: review of its methodological characteristics. Health Qual Life Outcomes. 2003;1:77. doi: 10.1186/1477-7525-1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–20. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Francomano D, Bruzziches R, Barbaro G, Lenzi A, Aversa A. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest. 2014;37:401–11. doi: 10.1007/s40618-014-0066-9. [DOI] [PubMed] [Google Scholar]
- 15.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 16.La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero A. Aerobic physical activity improves endothelial function in the middle-aged patients with erectile dysfunction. Aging Male. 2011;14:265–72. doi: 10.3109/13685538.2010.544344. [DOI] [PubMed] [Google Scholar]
- 17.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–84. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 18.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–8. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–93. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 20.Haram PM, Adams V, Kemi OJ, Brubakk AO, Hambrecht R, et al. Time-course of endothelial adaptation following acute and regular exercise. Eur J Cardiovasc Prev Rehabil. 2006;13:585–91. doi: 10.1097/01.hjr.0000198920.57685.76. [DOI] [PubMed] [Google Scholar]
- 21.Hakkinen K, Pakarinen A, Newton RU, Kraemer WJ. Acute hormone responses to heavy resistance lower and upper extremity exercise in young versus old men. Eur J Appl Physiol Occup Physiol. 1998;77:312–9. doi: 10.1007/s004210050339. [DOI] [PubMed] [Google Scholar]
- 22.Linnamo V, Pakarinen A, Komi PV, Kraemer WJ, Hakkinen K. Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. J Strength Cond Res. 2005;19:566–71. doi: 10.1519/R-15404.1. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, et al. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol (1985) 1990;69:1442–50. doi: 10.1152/jappl.1990.69.4.1442. [DOI] [PubMed] [Google Scholar]
- 24.Hakkinen K, Pakarinen A, Kraemer WJ, Newton RU, Alen M. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. J Gerontol A Biol Sci Med Sci. 2000;55:B95–105. doi: 10.1093/gerona/55.2.b95. [DOI] [PubMed] [Google Scholar]
- 25.Smilios I, Pilianidis T, Karamouzis M, Tokmakidis SP. Hormonal responses after various resistance exercise protocols. Med Sci Sports Exerc. 2003;35:644–54. doi: 10.1249/01.MSS.0000058366.04460.5F. [DOI] [PubMed] [Google Scholar]
- 26.Choi HC, Cho BL, Oh B, Kim HS. Physical activity and exercise for men with late onset hypogonadism. Korean J Androl. 2011;29:181–90. [Google Scholar]
- 27.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–81. doi: 10.1002/oby.20407. [DOI] [PubMed] [Google Scholar]
- 28.Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11:1567–76. doi: 10.1111/jsm.12523. [DOI] [PubMed] [Google Scholar]
- 29.Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health. 2013;31:136–40. doi: 10.5534/wjmh.2013.31.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monteagudo PT, Falcao AA, Verreschi IT, Zanella MT. The imbalance of sex-hormones related to depressive symptoms in obese men. Aging male. 2016;19:20–6. doi: 10.3109/13685538.2015.1084500. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai H, Zempo-Miyaki A, Yoshikawa T, Tsujimoto T, Tanaka K, et al. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr J. 2015;62:423–30. doi: 10.1507/endocrj.EJ14-0555. [DOI] [PubMed] [Google Scholar]
- 32.Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002;106:2530–2. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- 33.Sajjadian F, Roshangar L, Hemmati A, Nori M, Soleimani-Rad S, et al. The effect of BSO-induced oxidative stress on histologic feature of testis: testosterone secretion and semen parameters in mice. Iran J Basic Med Sci. 2014;17:606–12. [PMC free article] [PubMed] [Google Scholar]
- 34.Chigurupati S, Son TG, Hyun DH, Lathia JD, Mughal MR, et al. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008;199:333–41. doi: 10.1677/JOE-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aizawa K, Iemitsu M, Maeda S, Mesaki N, Ushida T, et al. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med Sci Sports Exerc. 2011;43:2072–80. doi: 10.1249/MSS.0b013e31821e9d74. [DOI] [PubMed] [Google Scholar]
- 36.Sato K, Iemitsu M, Aizawa K, Mesaki N, Fujita S. Increased muscular dehydroepiandrosterone levels are associated with improved hyperglycemia in obese rats. Am J Physiol Endocrinol Metab. 2011;301:E274–80. doi: 10.1152/ajpendo.00564.2010. [DOI] [PubMed] [Google Scholar]


