Dear Editor,
We present here a case of idiopathic male infertility who developed deep vein thrombosis (DVT) with the use of tamoxifen, a selective estrogen receptor modulator, probably through a hypercoagulable state.
A selective estrogen receptor modulator (SERM) is a compound that can act as an estrogen agonist or antagonist, depending on the specific target tissue. At present, four SERMs are approved for clinical use: clomiphene, raloxifene, tamoxifen, and toremifene. In women, SERMs are widely used as adjuvant therapy for breast cancer. SERMs in males have been suggested as an empiric treatment for idiopathic infertility, but relatively few studies are currently available.1
Tamoxifen administration in males indirectly stimulates follicle stimulating hormone (FSH) and luteinizing hormone (LH) secretion by blocking estrogen receptors in the hypothalamus and pituitary gland, thus increasing the hypothalamic release of gonadotrophic releasing hormone (GnRH). The major effect of tamoxifen administration is the stimulation of Leydig cells to produce testosterone and of Sertoli cells to improve the testicular environment for spermatogenesis.2 Noteworthy, tamoxifen has also been reported to directly stimulate Leydig cells and 5α-dihydrotestosterone production in seminiferous tubules and epididymis.3 Tamoxifen increases the risk of venous thrombosis by 2- to 3-fold.4 The underlying mechanisms in patients receiving tamoxifen have not been completely elucidated yet, although a decrease of several coagulation inhibitors has been reported. Tamoxifen-associated changes of the coagulation system differ substantially from estrogen-induced alterations, still it has been hypothesized that tamoxifen also induces an activated protein C (APC) resistance phenotype.4
A 36-year-old Caucasian male was admitted for severe pain at his left lower limb. The patient was alert, fully oriented, and did not have fever. His left leg was disproportionately large by inspection, with warm, reddened, and edematous skin. The patient had taken tamoxifen 20 mg daily for 3 months for idiopathic male infertility in a program of assisted reproduction. Moreover, he had a recent history of long haul car and train trips. He was normal weight (body mass index, BMI 23.5 kg m−2), eugonadal at the beginning of tamoxifen treatment (total testosterone 4.3 ng ml−1), and a nonsmoker.
Blood tests performed on admission showed coagulation abnormalities, but no erythrocytosis nor thrombocytosis: D-dimer 11.12 μg ml−1 (normal values, n.v., <0.8), platelet count 177 000 μl−1 (n.v. 150 000–450 000), red blood cells 4 800 000 mm−3 (n.v. 4 500 000–6 000 000), hemoglobin 14.4 g dl−1 (n.v. 13.5–17.5), hematocrit 42.5% (n.v. 41–50), fibrinogen 362 mg dl−1 (n.v. 200–400), prothrombin time 15 s (n.v. 16–20), international normalized ratio 1.07 (n.v. 0.85–1.25), and activated partial thromboplastin time 1.13 s (n.v. 0.82–1.24).
Suspecting DVT, a lower limbs Doppler ultrasonography of the deep venous system was performed, showing a partial obstruction with internal thrombus from the left popliteal vein to the left external iliac vein.
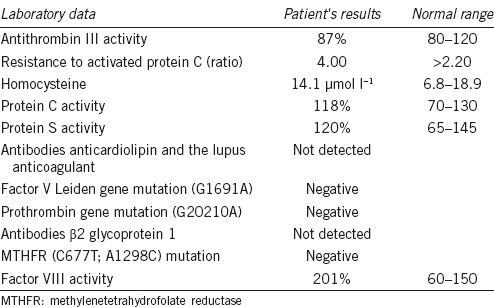
After diagnosis of DVT, a thrombophilia screening was performed, revealing an increased activity of clotting factor VIII (Table 1). Testosterone levels were not measured in the absence of signs and symptoms suggestive of testosterone deficiency.5
Table 1.
Thrombophilia screening results in the patient described in the text
Anticoagulant treatment with low-molecular-weight heparin (enoxaparin) was initiated along with warfarin for 5 days. After the therapeutic range was reached (i.e. prothrombin time 2–3 by international normalized ratio), treatment was continued with warfarin alone. On discharge, D-dimer levels were reduced (6.96 μg ml−1) and Doppler ultrasonography of the deep venous iliac – femoral – popliteal system showed signs of initial revascularization and thrombus dissolution.
Among different treatments proposed for idiopathic male infertility, anti-estrogens, like tamoxifen, may be taken into account. Tamoxifen seems to have a positive effect on sperm count and concentration in eugonadal patients, but it does not improve other semen parameters such as motility, morphology, and viability probably due to its effects on the first steps of spermatogenesis.6 Literature data suggest a better effect of tamoxifen in patients with low FSH levels, suggesting the need for a well-functioning hypothalamic-pituitary-gonadal axis. On the other hand, oxidative stress has well-recognized deleterious effects on sperm function. Estrogens have been shown to modulate the antioxidant system in human semen, and this provides a further rationale for antiestrogen administration in male infertility.7
Noteworthy, tamoxifen, which is widely used as adjuvant therapy for breast cancer in women, increases the risk of thromboembolic events.8 To the best of our knowledge, no known clotting abnormalities in males treated with tamoxifen for idiopathic infertility have been reported, also because it is used as an off-label treatment and rigorous safety analyses are lacking.
The potential mechanism for the procoagulant effect of tamoxifen has not been fully elucidated, although a reduction in antithrombin III and protein C levels or an APC resistance phenotype have been reported.9
We conclude that immobilization due to the recent long haul trips along with the use of tamoxifen-induced a hypercoagulable state, thus increasing the risk of thrombosis in this patient. We observed an increase in factor VIII activity which favors the propagation and amplification, rather than the initiation, of the coagulation cascade, increasing the risk of venous thrombosis.10
Although a single case in not sufficient to state a cause and effect relationship between tamoxifen and deep vein thrombosis, clinicians should be aware of the possibility of thromboembolic complications during tamoxifen treatment in males, even when it is administered for short periods of 3–6 months. Other treatments for idiopathic male infertility should be considered in male patients with an elevated risk of thromboembolism.
AUTHOR CONTRIBUTIONS
SA was the physician in charge of the patient described in the case study, drafted the manuscript and critical discussion; GM and MM participated in manuscript drafting and critical discussion; MPT participated in the critical discussion; FL participated in the critical discussion and revised the manuscript.
The authors have nothing to disclose about the data presented in this manuscript.
COMPETING FINANCIAL INTERESTS
We declare that we have no competing financial interests.
REFERENCES
- 1.Cocuzza M, Agarwal A. Nonsurgical treatment of male infertility: specific and empiric therapy. Biologics. 2007;1:259–69. [PMC free article] [PubMed] [Google Scholar]
- 2.Riggs BL, Hartmann LC. Selective estrogen receptor modulators – Mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 3.Zalata A, Hafez T, Verdonck L, Vermeulen L, Comhaire F. Androgens in seminal plasma: markers of the surface epithelium on the male genital tract. Int J Androl. 1995;18:271–7. [PubMed] [Google Scholar]
- 4.Cosman F, Baz-Hecht M, Cushman M, Vardy MD, Cruz JD, et al. Short term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literature. Thromb Res. 2005;116:1–13. doi: 10.1016/j.thromres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 6.Koukkou E, Billa E, Kapolla N, Pappa A, Venaki E, et al. An empiric treatment for idiopathic oligozoospermia revisited: a 20-year investigative saga. Andrologia. 2012;44:337–42. doi: 10.1111/j.1439-0272.2012.01286.x. [DOI] [PubMed] [Google Scholar]
- 7.Mancini A, Raimondo S, Persano M, Di Segni C, Cammarano M, et al. Estrogens as antioxidant modulators in human fertility. Int J Endocrinol. 2013;2013:607939. doi: 10.1155/2013/607939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onitilo AA, Doi SA, Engel JM, Glurich I, Johnson J, et al. Clustering of venous thrombosis events at the start of tamoxifen therapy in breast cancer: a population-based experience. Thromb Res. 2012;130:27–31. doi: 10.1016/j.thromres.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rühl H, Schröder L, Müller J, Fimmers R, Sukhitashvili S, et al. Tamoxifen induces resistance to activated protein C. Thromb Res. 2014;133:886–91. doi: 10.1016/j.thromres.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Kovac M, Kovac Z, Tomasevic Z, Vucicevic S, Djordjevic V, et al. Factor V Leiden mutation and high FVIII are associated with an increased risk of VTE in women with breast cancer during adjuvant tamoxifen – Results from a prospective, single center, case control study. Eur J Intern Med. 2015;26:63–7. doi: 10.1016/j.ejim.2014.12.015. [DOI] [PubMed] [Google Scholar]


