Abstract
Purpose
To study the accuracy and reliability of optical pachymetry using the Alcon WaveLight EX500 during laser-assisted in situ keratomileusis (LASIK).
Materials and methods
This was a retrospective chart review of 90 eyes from 45 patients who had undergone LASIK (mean age 35.2±8.2 years; 19 males, 26 females). The WaveLight FS200 femtosecond laser was programmed to cut LASIK flaps at a desired depth of 120 μm. Optical low-coherence reflectometry (WaveLight EX500) was used to measure central corneal thickness prior to lifting the flap, and the residual stromal bed immediately after excimer ablation. Flap thickness (FT) was calculated using simple subtraction. Optical coherence tomography (OCT) was used to measure central corneal thickness, flap thickness, and residual stromal bed in the postoperative period and the results compared to intraoperative measurements.
Results
Mean programmed FS200 FT was 119 μm. Mean FT using EX500 optical pachymetry was 109 μm. The difference between FS200- programmed and EX500-measured FT was 9 μm (P<0.001). There was also a significant difference between the EX500 and OCT FT (109 μm vs 119 μm, respectively; P<0.001).
Conclusion
FT values calculated using intraoperative EX500 optical pachymetry were significantly lower than programmed FS200 values or OCT measurements.
Keywords: flap thickness, optical coherence tomography, femtosecond laser
Introduction
Excellent laser-assisted in situ keratomileusis (LASIK) outcomes require safe, predictable, and reproducible flap parameters.1,2 Excessively thick flaps may predispose patients to biomechanical instability and possible ectasia. Femtosecond-LASIK flaps that are too thin may be prone to gas breakthrough or lifting complications, such as flap tears, striae, or buttonhole formation.3 Intraoperative measurements may help surgeons determine the accuracy of programmed versus achieved flap thickness (FT). Postoperative measurements using imaging technology, such as optical coherence tomography (OCT), are less commonly used, but also may offer useful information. The WaveLight® EX500 (Alcon Laboratories, Fort Worth, TX, USA) has built-in optical pachymetry that is generally quick, easy to use, and does not interrupt the cadence of surgery. Previous studies have shown similar residual stromal bed (RSB) values when comparing measurement with intraoperative optical pachymetry and ultrasound pachymetry.4 High-definition OCT can also be used to measure central corneal thickness (CCT), FT, and RSB by directly visualizing the parameters in high-resolution cross-sectional images. Anterior-segment pachymetric mapping by OCT has been shown to be repeatable and reproducible.5 Studies have shown that OCT is comparable and similar to ultrasound.6–8
We consider ultrasonic pachymetry, while useful, to be less than ideal for intraoperative measurements during LASIK. Our concerns include risk of introduction of contaminants or even infectious agents into the interface, interruption of the surgical cadence, and possible challenges with technical aspects of the measurement itself, including exact centration and perpendicularity of the probe. The purpose of this study was to compare intraoperative noncontact optical pachymetry with the EX500 to programmed FS200 FT and postoperative OCT.
Materials and methods
Approval was obtained from the University of Utah Health Sciences Institutional Review Board. Data from consecutive LASIK eyes from a single surgeon (MDM) for whom intraoperative EX500 optical pachymetry was performed was reviewed. Due to the retrospective nature of this chart review, the review board waived the requirement for written informed consent. Standard precautions to keep patient data confidential were instituted during the course of the study. Data from 90 eyes of 45 patients treated with the Allegretto WaveLight FS200 and EX500 lasers at the John A Moran Eye Center were analyzed. The mean age of the patient cohort was 35.2±8.2 years (19 males, 26 females). A total of 88 eyes (98%) had myopic ablations, and two eyes (2%) had hyperopic ablations.
Intraoperative CCT was obtained by optical low-coherence reflectometry (WaveLight EX500) just prior to flap lifting and according to manufacturer recommendations, centered on the pupil and focused on the corneal apex.9 The RSB was measured immediately after excimer-laser ablation, using the same alignment strategy. All measurements were made by the same experienced surgical technician, and required no more than 2–3 seconds to acquire. FT values were calculated by subtracting RSB from CCT.
Postoperative measurements were obtained by OCT. Anterior-segment spectral domain OCT has been shown to be more accurate and reproducible than time-domain OCT.10 FT and RSB were measured manually with the caliper tool in the cross-line scan at the corneal apex. These measurements were always manually performed at the center of the cornea (Figure 1). To guard against subjectivity, five different measurements were taken of FT with a 1.5 mm distance between each measurement (Figure 2). The individual performing the manual measurements was blinded from the EX500 measurements and programmed FT. Each OCT measurement was performed on each eye included in the study at one time point only after LASIK had been performed. Most OCT measurements were performed 1 month after LASIK surgery, although there was some variability in the timing of measurements (Table 1). RSB measured with EX500 optical was also compared to the estimated RSB from the EX500 planning software (EX500 programmed RSB).
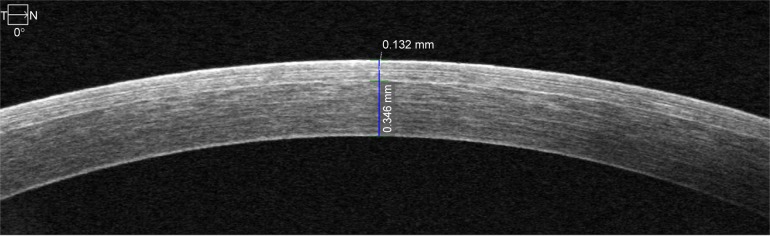
Figure 1.
High-definition optical coherence tomography at center of cornea.
Note: Top caliper measures flap thickness at 132 μm and bottom caliper measures residual stromal bed thickness at 346 μm.
Abbreviations: T, temporal; N, nasal.
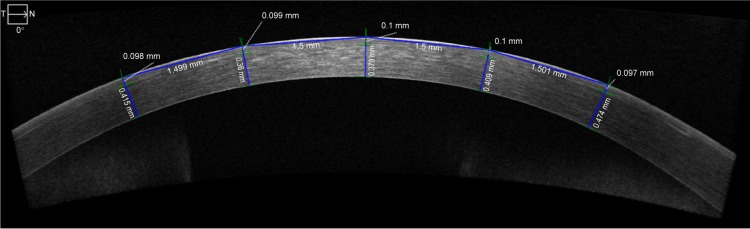
Figure 2.
High-definition optical coherence tomography measurements.
Notes: Example of measurements across the cornea. Five measurements are spaced approximately 1.5 mm apart from each another, with the center measurement at the center of the cornea. Top calipers measure flap thickness. Bottom calipers measure residual stromal bed thickness.
Abbreviations: T, temporal; N, nasal.
Table 1.
Timing of OCT measurements
| Months after surgery | Eyes, n | Percentage |
|---|---|---|
| 1 | 24 | 53% |
| 2–3 | 7 | 16% |
| 4–6 | 6 | 13% |
| 14–17 | 8 | 18% |
| Total | 45 | 100% |
Abbreviation: OCT, optical coherence tomography.
Statistical analysis
A multilevel mixed-effect linear regression was used to compare the two measurement methods. This model accounts for the lack of independence in the data introduced by the measurements from the two methods being nested within the same eye, in a paired-sample fashion, and eyes being nested within the same patient. For data analysis and significance testing, the data included the standard number of decimal places. These values were rounded to the nearest integer to simplify the presentation of results.
Results
Mean FT was 119 μm (95% CI 117–120 μm) and 110 μm (95% CI 107–112 μm, P<0.001) by FS200 and EX500, respectively. The mean difference between programmed FS200 and the EX500 FT was 9 μm (95% CI 6–12 μm, P<0.001), with EX500 values being lower. There was no significant difference between OCT FT and programmed FS200 FT (P=0.87). The average EX500-programmed RSB was 380 μm (95% CI 369–390 μm, P<0.001), while the average postoperative EX500 RSB measurement was 365 μm (95% CI 353–377 μm) and average OCT RSB measurement 380 μm (95% CI 368–392 μm). The mean difference between EX500-programmed RSB and EX500 measurement was 15 μm (95% CI 8–22 μm, P<0.001), with the measured value being lower, while there was no statistically significant difference between OCT RSB measurements and EX500-programmed RSB thickness (P=0.89) (Table 2).
Table 2.
Flap- and RSB-thickness comparison
| Measure | Mean (mm) | 95% CI | P-value |
|---|---|---|---|
| FT difference: FS200 – EX500 | 9 | 6–12 | <0.001 |
| FT difference: FS200 – OCT | −0.2 | −2 to 2 | 0.87 |
| RSB difference: EX500 (programmed) – EX500 | 15 | 8–22 | <0.001 |
| RSB difference: EX500 (programmed) – OCT | −0.3 | −5 to 4 | 0.89 |
Note: Comparison of difference in flap and RSB measurements between FS200 and EX500 using OCT.
Abbreviations: FT, flap thickness; OCT, optical coherence tomography; RSB, residual stromal bed.
There was no correlation between time interval after LASIK surgery and measurement difference between OCT and EX500 calculations (95% CI -8 to 8 μm). There was no correlation either between FT measurements by EX500 or OCT and postoperative visual acuity (95% CI -0.6 to 0.9 logMAR). A summary of the overall clinical outcomes is shown in Table 3.
Table 3.
Summary of overall clinical outcome of LASIK patients
| Measure | n* | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| UCVA: logMAR (Snellen) | 88 | −0.024 (20/19) | 0.17 | −0.165 (20/15 +2) | 0.66 (20/80 −1) |
| BCVA: logMAR (Snellen) | 71 | −0.08 (20/17) | 0.07 | −0.125 (20/15) | 0.14 (20/25 −2) |
| Spherical equivalent | 79 | −0.18 | 0.44 | −2 | 0.5 |
| Sphere | 77 | −0.26 | 0.24 | 0 | 1 |
| Cylinder | 77 | 0.15 | 0.45 | −2 | 0.25 |
Note:
Number of individual eyes measured.
Abbreviations: LASIK, laser-assisted in situ keratomileusis; UCVA, uncorrected visual acuity; BCVA, best-corrected visual acuity; logMAR, logarithm of minimum angle of resolution.
Discussion
The femtosecond laser has revolutionized the way corneal flaps are created during LASIK surgery. Accurate FT creation may have importance in visual quality, prevention or avoidance of striae, epithelial ingrowth, and ease of flap lifting at primary or enhancement surgeries. The addition of the EX500 excimer laser now allows the additional advantage of pre-, intra-, and postsurgical noncontact pachymetry.
Integrated optical pachymetry is a convenient and useful feature of the EX500 excimer laser. Significant advantages of this modality include the ability to measure important CT values quickly without interrupting the normal cadence of surgery. When compared to contact ultrasound pachymetry, there is less risk for nonuniform SB hydration, although CT measurements can be difficult to obtain if the stromal surface is too dry or wet.
The EX500 was found to have lower calculated mean FT when compared to both the measured OCT and the FS200 laser, while these latter two measurements were very consistent with each other. Intraoperative hydration status may contribute to the measurement differences between modalities. Capturing optical pachymetry measurements intraoperatively can be affected by small pupil size and opacities, such as an opaque bubble layer. This can increase the time it takes to capture a measurement, which could lead to drying of the flap and RSB and artificially low measurements. The 9 μm FT difference between the EX500 and other modalities was significantly different, but still predictable and clinically acceptable. To avoid thin-flap complications, FT should be programmed to be more than 110 μm.
Future studies should aim to explain the higher programmed FS200 and OCT FT measurements in comparison to the EX500. Swept-source OCT has been shown to allow improved sensitivity and signal:noise ratio when compared to standard-definition OCT.11 This may allow better measurement of the cornea epithelium to explain our study’s results further. Additionally, there may be slight variations in measurements in individual EX500 lasers, and evaluating one’s own laser may show different pachymetry-reading variations than our study.
Conclusion
Calculated FT using the EX500 was found to have a statistically significant lower mean when compared to FT programmed into the FS200 laser and measured postoperatively by OCT. This was also observed when comparing RSB measurements by the same methods. Furthermore, the observation that OCT-calculated FT showed no statistically significant difference when compared to programmed FS200-laser values, unlike the EX500-calculated measurements, implies that postoperative measurements by OCT are more accurate than intraoperative EX500 measurements. However, the difference between OCT and the EX500 is still clinically acceptable with a predictable trend, and the advantages of noncontact optical low-coherence reflectometry interferometer-based pachymetry may have the potential to outweigh other intraoperative measurement methods. In order to avoid complications, surgeons should choose an FT of 110 or more.
Acknowledgments
This study was supported in part by an unrestricted grant from Research to Prevent Blindness Inc, New York, NY, USA to the Department of Ophthalmology and Visual Sciences, University of Utah, Salt Lake City, UT, USA.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Durairaj VJ, Balentine J, Kouyoumdjian G, et al. The predictability of corneal flap thickness and tissue laser ablation in laser in situ keratomileusis. Ophthalmology. 2000;107:2140–2143. doi: 10.1016/s0161-6420(00)00407-3. [DOI] [PubMed] [Google Scholar]
- 2.Kymionis GD, Kontadakis GA, Grentzelos MA, et al. Thin-flap laser in situ keratomileusis with femtosecond-laser technology. J Cataract Refract Surg. 2013;39:1366–1371. doi: 10.1016/j.jcrs.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Salomão MQ, Wilson SE. Femtosecond laser in laser in situ keratomileusis. J Cataract Refract Surg. 2010;36:1024–1032. doi: 10.1016/j.jcrs.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoughi MM, Einollahi B, Einollahi N, Rezaei J, Roshandel D, Feizi S. Measurement of central corneal thickness using ultrasound pachymetry and Orbscan II in normal eyes. J Ophthalmic Vis Res. 2015;10:4–9. doi: 10.4103/2008-322X.156084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed S, Lee GK, Rao SK, et al. Repeatability and reproducibility of pachymetric mapping with Visante anterior segment-optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:5499–5504. doi: 10.1167/iovs.07-0591. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Lee D, Rhee KI. Flap thickness reproducibility in laser in situ keratomileusis with a femtosecond laser: optical coherence tomography measurement. J Cataract Refract Surg. 2008;34:132–136. doi: 10.1016/j.jcrs.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Ye C, Yu M, Jhanji V. Stromal bed thickness measurement during laser in situ keratomileusis using intraoperative optical coherence tomography. Cornea. 2015;34:387–391. doi: 10.1097/ICO.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda S, Kawana K, Yasuno Y, Oshika T. Anterior ocular biometry using 3-dimensional optical coherence tomography. Ophthalmology. 2009;116:882–889. doi: 10.1016/j.ophtha.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Soeken TA, Apsey DA, Townley JR, Haas RW, Caldwell MC. Comparison of pachymetry measurements between the Alcon WaveLight EX500 and Sonogage Corneo-Gage plus platforms. J Cataract Refract Surg. 2015;31:328–332. doi: 10.3928/1081597X-20150423-06. [DOI] [PubMed] [Google Scholar]
- 10.Keane PA, Bhatti RA, Brubaker JW, Liakopoulos S, Sadda SR, Walsh AC. Comparison of clinically relevant findings from high-speed Fourier-domain and conventional time-domain optical coherence tomography. Am J Ophthalmol. 2009;148:242–248. doi: 10.1016/j.ajo.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim LS, Cheung G, Lee SY. Comparison of spectral domain and swept-source optical coherence tomography in pathological myopia. Eye (Lond) 2014;28:488–491. doi: 10.1038/eye.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]