Abstract
Constraining photosynthetic energy conversion efficiency in nature is challenging. In principle, two yield measurements must be made simultaneously: photochemistry, fluorescence and/or thermal dissipation. We constructed two different, extremely sensitive and precise active fluorometers: one measures the quantum yield of photochemistry from changes in variable fluorescence, the other measures fluorescence lifetimes in the picosecond time domain. By deploying the pair of instruments on eight transoceanic cruises over six years, we obtained over 200 000 measurements of fluorescence yields and lifetimes from surface waters in five ocean basins. Our results revealed that the average quantum yield of photochemistry was approximately 0.35 while the average quantum yield of fluorescence was approximately 0.07. Thus, closure on the energy budget suggests that, on average, approximately 58% of the photons absorbed by phytoplankton in the world oceans are dissipated as heat. This extraordinary inefficiency is associated with the paucity of nutrients in the upper ocean, especially dissolved inorganic nitrogen and iron. Our results strongly suggest that, in nature, most of the time, most of the phytoplankton community operates at approximately half of its maximal photosynthetic energy conversion efficiency because nutrients limit the synthesis or function of essential components in the photosynthetic apparatus.
This article is part of the themed issue ‘Enhancing photosynthesis in crop plants: targets for improvement’.
Keywords: phytoplankton, photosynthesis, fluorescence, oceans, nutrient limitation
1. Introduction
The conversion of one form of energy to another inevitably comes at a thermodynamic cost of heat. The energy conversion efficiency in photosynthesis has been inferred from several different experimental protocols, including photoacoustics [1–3], amplitude-based variable fluorescence [4–6] and fluorescence lifetimes [7], yet seldom has there been closure on the energy budget in model organisms, let alone in nature. Fundamentally, closure can be achieved by measuring any two of the three rate constants that ultimately determine the overall quantum yield. The basic argument is that photons absorbed by a photosynthetic reaction centre have only three possible fates: they can be used to drive an electrochemical reaction, reradiated as fluorescence, or dissipated as heat [8,9]. In unicellular algae, the first two reactions can be precisely measured using two active fluorescence techniques, namely amplitude and lifetime analyses, respectively (figure 1). Moreover, by using very sensitive detectors, these two signals can be obtained from natural phytoplankton communities in the global oceans [12].
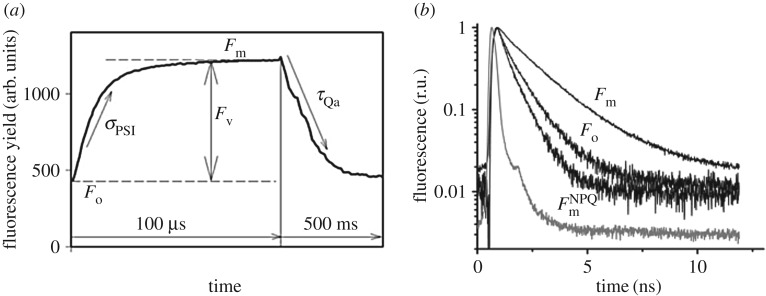
Figure 1.
The concept of amplitude-based variable fluorescence (a) and picosecond lifetime (b) measurements. (a) Amplitude-based variable fluorescence techniques, such as fast repetition rate (FRR) or fluorescence induction and relaxation (FIRe), rely on recording the changes in fluorescence intensities induced by saturating flashes of excitation light on micro- and milli-second time scales [10,11]. The quantum yield of photochemistry in photosystem II (PSII) (i.e. photosynthetic efficiency) is deduced from the relative change in fluorescence yield (Fv/Fm). The effective absorption cross section of PSII (σPSII) is derived from the rate of fluorescence rise during the first 100 µs of the induction curve. The subsequent relaxation in fluorescence yield on millisecond time scale reflects the rates of photosynthetic electron transport delivered on the acceptor side of PSII. Most of those electrons are used in carbon fixation. All amplitude-based fluorometers record fluorescence yields in arbitrary units and do not measure absolute quantum yields of fluorescence. (b) Picosecond-resolved measurements of fluorescence lifetimes—fluorescence is induced by an ultra-short laser pulse (grey line) and the decay kinetics is recorded on pico- and nano-second time scales. The rate of fluorescence decay (i.e. lifetime) is directly proportional to the absolute quantum yield of fluorescence. The three profiles show fluorescence kinetics of phytoplankton at different physiological states of photosystem II reaction centres. Fo was recorded in dark-adapted cells with open reaction centres (when the quantum yield of photochemistry is maximum), Fm in dark-adapted cells with closed centres (when photochemistry is nil), 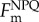 under high light exposure (1500 µmol quanta m−2 s−1) with closed centres. The reduction in the fluorescence lifetime under high light is due to non-photochemical quenching (NPQ) [12,13]. The grey trace is the instrument response function. Both variable fluorescence and lifetime instruments (namely a mini-FIRe and picosecond lifetime fluorometer (PicoLiF), respectively [12]) have extremely high sensitivity; they can accurately measure fluorescence signals in samples as low as 0.01 mg chlorophyll/m3—effectively the lowest concentration of chlorophyll recorded anywhere in the upper ocean.
under high light exposure (1500 µmol quanta m−2 s−1) with closed centres. The reduction in the fluorescence lifetime under high light is due to non-photochemical quenching (NPQ) [12,13]. The grey trace is the instrument response function. Both variable fluorescence and lifetime instruments (namely a mini-FIRe and picosecond lifetime fluorometer (PicoLiF), respectively [12]) have extremely high sensitivity; they can accurately measure fluorescence signals in samples as low as 0.01 mg chlorophyll/m3—effectively the lowest concentration of chlorophyll recorded anywhere in the upper ocean.
Phytoplankton are a highly diverse group of unicellular photosynthetic algae, including cyanobacteria, capable of oxygenic photosynthesis [14]. One clade of these organisms gave rise to higher plants, but phytoplankton are much more diverse: there are one prokaryotic and thirteen eukaryotic phyla containing oxygenic photosynthetic species [15]. All of these have the same two photosynthetic reaction centres as found in plants, but are highly diverse in the pigmentation in their light harvesting antenna systems. Regardless of antenna pigmentation however, the maximum photosynthetic energy conversion efficiency in photosystem II (PSII), obtained when grown under optimal conditions in cultures, is remarkably constant. When measured with a single turnover saturating flash (e.g. approx. 100 µs duration), variable fluorescence normalized to the maximal fluorescence (Fv/Fm) is about 0.65 [16]. Assuming that the first order loss processes to fluorescence have the quantum yield of ca. 0.05, this suggests that about 30% of the absorbed excitation energy is dissipated as heat in PSII. The efficiency of photosystem I (PSI), which does not fluoresce at room temperature, is much more difficult to assess in phytoplankton [3] because their concentration is usually so low in natural waters that absorption spectra cannot be easily obtained [17]. Regardless, as PSI operates in series with PSII, its efficiency can only reduce the overall calculation of photosynthetic energy conversion efficiency.
2. Variability and controls of photosynthetic energy conversion efficiency in the ocean
Based on numerous experiments with cultured phytoplankton, if allowed sufficient time to photoacclimate, Fv/Fm is insensitive to growth irradiance, but not to nutrient insufficiency [16,18]. Nitrate and iron, in particular, are two nutrients that can lead to marked reductions in Fv/Fm. Based on laboratory experiments, analyses of natural variations in Fv/Fm strongly indicate that in every major ocean gyre there is significant nutrient limitation—but it is highly patchy [14]. That is, in some regions of the ocean, injection of nutrients from deep waters due to (e.g.) storm events or eddy pumping, can temporarily lead to elevated Fv/Fm values, approaching those obtained under optimal growth conditions in the laboratory or observed in nutrient-rich coastal waters. This interpretation was further supported by deliberate iron fertilization experiments in high-nutrient-low-chlorophyll (HNLC) regions of the open ocean [19–22]. To date, thirteen iron fertilization experiments have been conducted in the three major iron limited regions of the world's oceans: the Subarctic Pacific, the eastern Equatorial Pacific and the Southern Ocean [23,24]. An increase in Fv/Fm was the first detectable signal of release from iron deficiency in every iron enrichment experiment.
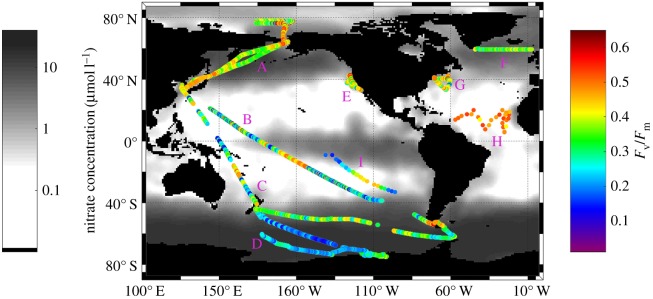
We obtained more than 200 000 discrete measurements of variable fluorescence from the North and South Pacific, North Atlantic, Arctic and Southern Oceans. These measurements comprise the global map of photosynthetic energy conversion efficiencies in phytoplankton in the upper ocean (figure 2). The nighttime in situ measurements of Fv/Fm ranged from 0.12 to 0.60 with a mean of 0.35 ± 0.11. These values span virtually the entire range of published values of photosynthetic conversion efficiencies obtained from cultured phytoplankton by single turnover flashes, and reflect extraordinary variability in phytoplankton photophysiology in the global ocean. Fv/Fm values in ocean phytoplankton communities are two- to three-fold lower than those observed in higher plants, suggesting the fundamental difference in nutrient availability between terrestrial and aquatic ecosystems.
Figure 2.
Global distribution of ship-based measurements of the photosynthetic energy conversion efficiencies (Fv/Fm) in the upper ocean superimposed on the climatological map of surface nitrate concentrations in the world ocean. Periods of in situ sampling were (A) July–Aug 2011; (B) Oct–Nov 2011; (C) Oct–Nov 2010; (D) Jan 2012; (E) July 2014; (F) Sept 2010; (G) May 2014; (H) Aug 2008; (I) Nov. 2004.
The highest Fv/Fm values (more than 0.4) were observed along continental margins, in the Antarctic convergence, the Subtropical Atlantic and Pacific oceans. In contrast the lowest photosynthetic efficiencies were observed in HNLC regions of the equatorial Pacific Ocean and the Southern Ocean, where primary production is limited by a paucity of iron [25,26], a micronutrient that is critical for the function of photosystem II [18]. The exceptionally low Fv/Fm values (less than 0.20) in these regions are consistent with extremely long fluorescence lifetimes and suggest that there is a significant fraction of non-functional PSII reaction centres and/or energetically uncoupled antenna pigment–protein complexes [18,27,28].
With literally hundreds of thousands of measurements of Fv/Fm, and other parameters that can be derived from variable fluorescence analysis of single and multiple turnover flashes, including the effective absorption cross section of PSII and the electron transport rate on the acceptor side of the reaction centre, we turned to fluorescence lifetime analyses as a possible approach to retrieving the absolute quantum yields of fluorescence and to closing the energy budget for phytoplankton in nature.
3. Theoretical bases of fluorescence yields and lifetimes
The quantum yield of fluorescence (ϕf) is defined as the ratio of the photons reradiated to those absorbed. The biophysical basis of fluorescence measurements derives from the three possible fates of solar energy absorbed by any photosynthetic organism [9]. Absorbed photons can (i) generate photochemical reactions (with the rate kp), (ii) be dissipated as heat (kt), or (iii) be emitted back to the environment as fluorescence (kf). The rate kp is at first order proportional to the fraction of open or active reaction centres. The rate kt is the sum of dark component (kD) and light-dependent component (kNPQ), driven by non-photochemical quenching.
In a dark-adapted state or under low irradiance (when kNPQ is nil and kt is constant), the quantum yield of chlorophyll fluorescence, ϕf (=kf/(kp + kt + kf)), is inversely related to the quantum yield of photochemistry in PSII, ϕp = kp/(kp + kt + kf) = Fv/Fm:
| 3.1 |
where ϕfm ( = kf/(kt + kf)) is the maximum fluorescence yield obtained when the quantum yield of photochemistry is nil (e.g. at saturating background light).
This biophysical model predicts an inverse linear relationship between the quantum yield of photochemistry and that of chlorophyll fluorescence. However, by the early 1980s it was realized that exposure to high irradiance can generate a suite of thermal dissipation mechanisms, collectively called non-photochemical quenching (NPQ) [29,30]. This photoprotective response markedly decreases the quantum yield of chlorophyll fluorescence at high background light. Hence, the relationship between fluorescence yield and photochemistry becomes highly nonlinear as NPQ phenomena play an increasingly larger role in energy dissipation.
Fluorescence is a delayed light emission process, which is described by one or more exponential decay functions that can be parameterized by the lifetime, which is the e-folding time of the decay function. The fluorescence lifetime can be quantitatively related to the absolute quantum yield of fluorescence [31]:
| 3.2 |
where τ is the observed lifetime and τn is the intrinsic (or ‘natural’) lifetime constant for the molecule. Thus, the longer the lifetime, the higher the quantum yield of fluorescence.
The ‘natural’ lifetime (τn) is that which would be observed if fluorescence emission were the only path of dissipation of excited state energy; this number cannot be measured directly. The calculated value of τn (which is a constant for a specific molecule) for chlorophyll a is 15 ns [32]. In a population of molecules, the actual measured lifetimes are inevitably shorter than the ‘natural’ lifetime due to intra-molecular conversion (i.e. energy dissipation as heat) and triplet state formation. The actual measured lifetimes of isolated chlorophyll a molecules range from ca. 3.0 to 5.1 ns, depending on the solvent polarity. These measured lifetimes correspond to quantum yields ranging from 20 to 32%. Fluorescence lifetimes in living cells are even shorter (ca. 0.3 to approx. 1.5 ns), as a significant fraction of the absorbed energy is used in photochemical reactions [7], and reflect the physiological state of the cells.
4. Lifetimes and quantum yields of chlorophyll fluorescence in the ocean
Accurate, optical measurements of the quantum yield of fluorescence are among the most complicated measurements to obtain in applied physics. By definition, such measurements require precise determination of the number of quanta absorbed by the sample and the number of quanta emitted over the entire fluorescence spectrum integrated over 4π steradians. In living phytoplankton cells, the measurements are further complicated by the ‘packaging’ effect (i.e. self-shading) of photosynthetic pigments embedded within layers of thylakoid membranes, as well as scattering and refraction of light by cell walls and membranes [32]. The measurement of fluorescence lifetimes conveniently circumvents all these potential obstacles.
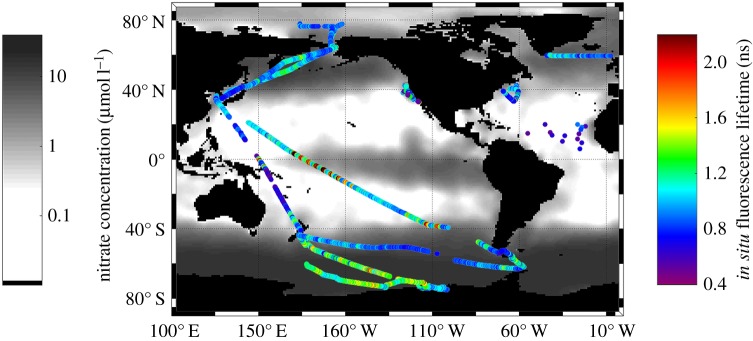
To that end, we constructed a sea-going picosecond lifetime instrument that employs a time-correlated single photon counting detector [12]. The instrument has extremely high sensitivity; it can measure lifetimes in samples as low as 0.01 mg chlorophyll/m3—effectively the lowest concentration of chlorophyll recorded anywhere in the upper ocean. Furthermore, this instrument employs a multi-component kinetic analysis, which is crucial for interpretation of fluorescence kinetics from a living cell [7]. We obtained more than 200 000 discrete chlorophyll fluorescence lifetime measurements from the Pacific, Atlantic, Arctic and Southern (Antarctic) Oceans. These comprise the first map of in situ measurements of quantum yields of chlorophyll fluorescence from phytoplankton in the upper ocean (figure 3). The nighttime in situ lifetimes ranged from 0.5 to 2.7 ns with a mean of 1.13 ± 0.33 ns [12]. These values span the entire range of published lifetimes of in vivo chlorophyll fluorescence obtained from cultured phytoplankton and reflect extraordinary variability in phytoplankton photophysiology and nutrient status in the global ocean. The general pattern of the fluorescence lifetimes in the central gyres of the global ocean is rather featureless [12], although phytoplankton growth is subject to both macro- and micronutrient limitation [15]. The shortest fluorescence lifetimes (less than 1 ns) were observed along continental margins, in the Antarctic convergence, the Subtropical Atlantic and Pacific oceans. These lifetime distributions support the hypothesis that phytoplankton in the central gyres are acclimated to broad scale and persistent nutrient limitation [25,33]. In contrast, the longest fluorescence lifetimes were observed in HNLC regions of the equatorial Pacific Ocean and the Southern Ocean where primary production is limited by a paucity of iron [25,26]. The exceptionally long fluorescence lifetimes (greater than 2.0 ns) in these areas of the global ocean exceed the typical maximum lifetime values observed in algal cultures with fully closed reaction centres [34] and thus cannot, entirely, be explained by the presence of inactive PSII reaction centres alone. Therefore, our lifetime measurements suggest the presence of both a large fraction of non-functional PSII reaction centres and energetically uncoupled antenna pigment–protein complexes in Fe-limited phytoplankton communities. The combination of variable fluorescence and lifetime measurements allows us to quantify the contributions of these two modifications in the photosynthetic apparatus under nutrient limitation. This analysis revealed that up to ca. 50% of reaction centres are inactive and up to 30% of light-harvesting antennae become uncoupled in iron-limited phytoplankton in the Southern Ocean [35].
Figure 3.
Global distribution of ship-based measurements of chlorophyll fluorescence lifetimes in the upper ocean superimposed on the climatological map of surface nitrate concentrations in the world ocean. (Data from [12]).
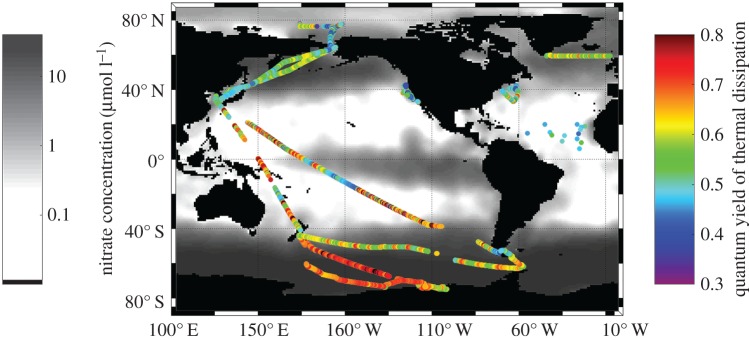
Simultaneous measurements of amplitude-based fluorescence yields and lifetimes were startling. While there was significant spatial and temporal variability, Fv/Fm in the upper ocean averaged ca. 0.35 at night, when non-photochemical quenching is nil. These results indicate that most of the contemporary open surface waters are extremely nutrient deficient. Moreover, the average lifetime obtained was about 1 ns, and only varied by ca. 10% between day, when NPQ is maximal, and night, when NPQ is nil but photochemical quenching is maximal. Assuming a natural lifetime of 15 ns, these results suggest that approximately 7% of the absorbed photosynthetic energy is reradiated as fluorescence (figure 3). Combining the two measurements suggests that approximately 60% of the absorbed excitation energy in natural phytoplankton is converted to heat (figure 4). Thus, photosynthetic energy conversion efficiency in the open ocean is almost the exact opposite of that obtained under optimal growth conditions for unicellular algae in culture.
Figure 4.
Global distribution of the quantum yields of thermal dissipation in photosynthetic reactions in the upper ocean superimposed on the climatological map of surface nitrate concentrations in the world ocean. These values were reconstructed from in situ variable fluorescence and lifetime measurements.
5. Phytoplankton physiology from space
Variable fluorescence signals can be readily measured remotely in both phytoplankton and terrestrial plants using shipboard or airborne LIght Detection And Ranging (LIDAR) instruments, such as pump-and-probe LIDARs [36,37]. Chlorophyll fluorescence lifetimes can potentially be recorded in terrestrial plants, but not from phytoplankton in the water column, using Picosecond LIDARs [38,39]. In terrestrial ecosystems however, the operational use of such instruments is restrained by heterogeneity of the plant canopy, which makes it extremely difficult to deconvolute kinetic profiles generated by multiple layers of leaves within the canopy [38].
Variable fluorescence signals cannot be recorded from space without high-power lasers or some other source of light—which is not practical, let alone potentially dangerous. An alternative approach to infer phytoplankton physiology and photosynthetic rates is based on measurements of the absolute quantum yields of chlorophyll fluorescence. With the launch of the MOderate Resolution Imaging Spectroradiometer (MODIS) and MEdium Resolution Imaging Spectrometer (MERIS) satellites, which possess the capability of detecting solar induced chlorophyll fluorescence signals from the global ocean, it became theoretically possible to calculate the quantum yield of chlorophyll fluorescence from space [40–42]. The MODIS/MERIS analytical algorithms retrieve the quantum yields of chlorophyll fluorescence from the ratio of two independent variables, namely the magnitude of solar-induced fluorescence and the number of quanta absorbed by phytoplankton. Solar induced fluorescence (SIF, also called passive fluorescence) from chlorophyll a is detected as a red peak (centred at ca. 683 nm) in spectra of water-leaving radiance [43–45]. Although the presence of phytoplankton in natural waters alters the entire visible spectrum of water-leaving radiance [44–48], SIF is the only signal emitted from the ocean and detectable from space that can be unambiguously ascribed to phytoplankton.
The natural variations of fluorescence yields are the sources of both errors and useful information. SIF yield is highly variable in nature [49–54]. While the apparently huge variability of chlorophyll fluorescence yield in the ocean (ca. 10 fold) is often correlated with environmental forcing [12,41,50,54], the mechanisms and interpretation of this relationship remain to be elucidated.
The development of remote sensing algorithms for interpretation of solar-induced fluorescence yields crucially depends on comparison with accurate in situ measurements. Our sea-going lifetime fluorometer provides a unique operational tool for ground-truthing of satellite-based retrievals of the quantum yields of solar-induced chlorophyll fluorescence [12]. Our analysis of more than 20 000 matchups revealed a weak, but significant correlation between satellite-derived estimates of the quantum yields of chlorophyll fluorescence with corresponding in situ measurements [12].
6. Applications and limitations to terrestrial plants
Unlike marine unicellular organisms, which are optically thin, terrestrial plants concentrate their photosynthetic pigments in leaves, which are optically thick and spatially very heterogeneous. This leads to three fundamental problems in applying active fluorescence techniques in terrestrial ecosystems. First, the pigment packaging effect in leaves leads to reabsorption of fluorescent photons, thereby significantly reducing the observed fluorescence yields and red-shifting the emission spectrum of chlorophyll. The application of active fluorescence techniques to terrestrial plants potentially can resolve variable fluorescence parameters on leaf surfaces [55]. However, the packaging effect greatly complicates the interpretation of fluorescence signals, and therefore the conversion of lifetimes to absolute quantum yields [38]. Second, under natural irradiance conditions, the spatial heterogeneity in terrestrial canopies presents a significant problem in sampling fluorescence parameters under controlled irradiance. Hence, perhaps paradoxically, although chlorophyll concentrations in the upper ocean are 3 to 5 orders of magnitude smaller on an areal basis compared to vegetated terrestrial ecosystems, the biophysical signals obtained from active fluorescence techniques, while harder to measure, are easier to interpret within the context of current models of photosynthetic energy conversion efficiency.
7. Conclusion
The results of our extensive field measurements of variable fluorescence and lifetimes strongly imply that most of the time the phytoplankton in the world oceans operate at a much lower photosynthetic efficiency than they are potentially capable of achieving.
Indeed, it would appear that approximately 60% of the absorbed solar energy in the photosynthetically useable portion of the spectrum is dissipated as heat. Regardless, these organisms, which account for less than 1% of the global photosynthetic biomass, contribute about 45% of the global carbon fixation on Earth [34]. One of the fundamental limitations to an even higher contribution to carbon fixation is nutrients, especially fixed inorganic nitrogen and, in HNLC regions, iron. Deliberate iron fertilization experiments in the latter regions clearly show that photosynthetic energy conversion efficiency can be rapidly increased to its maximum potential. In the former, mesoscale (e.g. tens to hundreds of kilometres) measurements reveal spatial variations in photosynthetic energy conversion efficiency associated with eddy turbulence, storms and wind driven processes that advect nutrients from deep waters into the euphotic zone. In effect, phytoplankton ‘body surf’, waiting for a wave of nutrients to allow the synthesis of key components of their photosynthetic apparatus, ultimately stimulating bursts of growth.
Acknowledgements
We thank Drs E. Boyle, S.H. Lee, P. Quinn, K. Thamatrakoln and V. Fadeev for providing shiptime, and the captains and crews of RV Oceanus, RV Knorr, RV Melville, RV Akademik Yoffe and RV Araon. We thank Young Nam Kim, Fedor Kuzminov and Jisoo Park for assistance.
Data accessibility
All data are deposited at PANGAEA (Publishing Network for Geoscientific and Environmental Data) under accession number PDI-11228.
Authors' contributions
P.G.F. and M.Y.G. designed the research. All authors analysed data. P.G.F. and M.Y.G. wrote the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by NASA Ocean Biology and Biogeochemistry Program (grant no. NNX16AT54G).
References
- 1.Malkin S, Cahen D. 1979. Photoacoustic spectroscopy and radiant energy conversion: theory of the effect with special emphasis on photosynthesis. Photochem. Photobiol. 29, 803–813. ( 10.1111/J.1751-1097.1979.Tb07770.X) [DOI] [Google Scholar]
- 2.Mauzerall DC. 1990. Determination of oxygen emission and uptake in leaves by pulsed, time resolved photoacoustics. Plant Physiol. 94, 278–283. ( 10.1104/Pp.94.1.278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan CY, Schofield O, Dubinsky Z, Mauzerall D, Falkowski PG, Gorbunov MY. 2011. Photosynthetic energy storage efficiency in Chlamydomonas reinhardtii, based on microsecond photoacoustics. Photosynth. Res. 108, 215–224. ( 10.1007/s11120-011-9682-9) [DOI] [PubMed] [Google Scholar]
- 4.Bradbury M, Baker NR. 1983. Analysis of the induction of chlorophyll fluorescence in leaves and isolated thylakoids: contributions of photochemical and non-photochemical quenching. Proc. R. Soc. Lond. B 220, 251–264. ( 10.1098/rspb.1983.0098) [DOI] [Google Scholar]
- 5.Falkowski P, Kiefer DA. 1985. Chlorophyll a fluorescence in phytoplankton: relationship to photosynthesis and biomass. J. Plankton Res. 7, 715–731. ( 10.1093/plankt/7.5.715) [DOI] [Google Scholar]
- 6.Krause GH, Weis E. 1991. Chlorophyll fluorescence and photosynthesis: the basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 313–349. ( 10.1146/annurev.pp.42.060191.001525) [DOI] [Google Scholar]
- 7.Holzwarth AR. 1986. Fluorescence lifetimes in photosynthetic systems. Photochem. Photobiol. 43, 707–725. ( 10.1111/j.1751-1097.1986.tb05650.x) [DOI] [Google Scholar]
- 8.Butler WL. 1972. On the primary nature of fluorescence yield changes associated with photosynthesis. Proc. Natl Acad. Sci. USA 69, 3420–3422. ( 10.1073/pnas.69.11.3420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler WL. 1978. Energy distribution in the photochemical apparatus of photosynthesis. Annu. Rev. Plant Biol. 29, 345–378. ( 10.1146/annurev.pp.29.060178.002021) [DOI] [Google Scholar]
- 10.Kolber ZS, Prasil O, Falkowski PG. 1998. Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Biochim. Biophys. Acta 1367, 88–106. ( 10.1016/S0005-2728(98)00135-2) [DOI] [PubMed] [Google Scholar]
- 11.Gorbunov M, Falkowski P. 2005. Fluorescence induction and relaxation (FIRe) technique and instrumentation for monitoring photosynthetic processes and primary production in aquatic ecosystems. In Photosynthesis fundamental aspects to global perspectives, International Society of Photosynthesis (eds van der Est A, Bruce D), pp. 1029–1031. London, UK: Allen Press. [Google Scholar]
- 12.Lin H, Kuzminov FI, Park J, Lee S, Falkowski PG, Gorbunov MY. 2016. The fate of photons absorbed by phytoplankton in the global ocean. Science 351, 264–267. ( 10.1126/science.aab2213) [DOI] [PubMed] [Google Scholar]
- 13.Kuzminov FI, Gorbunov MY. 2016. Energy dissipation pathways in Photosystem 2 of the diatom, Phaeodactylum tricornutum, under high-light conditions. Photosynth. Res. 127, 219–235. ( 10.1007/s11120-015-0180-3) [DOI] [PubMed] [Google Scholar]
- 14.Falkowski PG, Koblezek M, Gorbunov M, Kolber Z. 2004. Development and application of variable chlorophyll fluorescence techniques in marine ecosystems. In Chlorophyll a fluorescence: a signature of photosynthesis (eds Papageorgiou GC, Govindjee J), pp. 757–778. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 15.Falkowski P, Raven JA. 2007. Aquatic photosynthesis, 2nd edn Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Kolber Z, Zehr J, Falkowski P. 1988. Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II. Plant Physiol. 88, 923–929. ( 10.1104/Pp.88.3.923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkowski PG. 1983. Light–shade adaptation and vertical mixing of marine phytoplankton: a comparative field study. J. Mar. Res. 41, 215–237. ( 10.1357/002224083788520199) [DOI] [Google Scholar]
- 18.Vassiliev IR, Kolber Z, Wyman KD, Mauzerall D, Shukla VK, Falkowski PG. 1995. Effects of iron limitation on photosystem II composition and light utilization in Dunaliella tertiolecta. Plant Physiol. 109, 963–972. ( 10.1104/pp.109.3.963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolber ZS, Barber RT, Coale KH, Fitzwateri SE, Greene RM, Johnson KS, Lindley S, Falkowski PG. 1994. Iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean. Nature 371, 145–149. ( 10.1038/371145a0) [DOI] [Google Scholar]
- 20.Behrenfeld MJ, Bale AJ, Kolber ZS, Aiken J, Falkowski PG. 1996. Confirmation of iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean. Nature 383, 508–511. ( 10.1038/383508a0) [DOI] [Google Scholar]
- 21.Gervais F, Riebesell U, Gorbunov MY. 2002. Changes in primary productivity and chlorophyll a in response to iron fertilization in the Southern Polar Frontal Zone. Limnol. Oceanogr. 47, 1324–1335. ( 10.4319/lo.2002.47.5.1324) [DOI] [Google Scholar]
- 22.Coale KH, et al. 2004. Southern ocean iron enrichment experiment: carbon cycling in high- and low-Si waters. Science 304, 408–414. ( 10.1126/science.1089778) [DOI] [PubMed] [Google Scholar]
- 23.de Baar HJW, et al. 2005. Synthesis of iron fertilization experiments: from the iron age in the age of enlightenment. J. Geophys. Res. Oceans 110, C09S16 ( 10.1029/2004JC002601) [DOI] [Google Scholar]
- 24.Boyd PW, et al. 2007. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315, 612–617. ( 10.1126/science.1131669) [DOI] [PubMed] [Google Scholar]
- 25.Behrenfeld MJ, Worthington K, Sherrell RM, Chavez FP, Strutton P, McPhaden M, Shea DM. 2006. Controls on tropical Pacific Ocean productivity revealed through nutrient stress diagnostics. Nature 442, 1025–1028. ( 10.1038/Nature05083) [DOI] [PubMed] [Google Scholar]
- 26.Moore CM, et al. 2013. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710. ( 10.1038/ngeo1765) [DOI] [Google Scholar]
- 27.Falkowski PG, Greene RM, Geider RJ. 1992. Physiological limitations on phytoplankton productivity in the ocean. Oceanography 5, 84–91. ( 10.5670/oceanog.1992.14) [DOI] [Google Scholar]
- 28.Schrader PS, Milligan AJ, Behrenfeld MJ. 2011. Surplus photosynthetic antennae complexes underlie diagnostics of iron limitation in a cyanobacterium. PLoS ONE 6, e0018753 ( 10.1371/journal.pone.0018753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falkowski PG, Wyman K, Ley AC, Mauzerall DC. 1986. Relationship of steady-state photosynthesis to fluorescence in eucaryotic algae. Biochim Biophys. Acta 849, 183–192. ( 10.1016/0005-2728(86)90024-1) [DOI] [Google Scholar]
- 30.Schreiber U, Schliwa U, Bilger W. 1986. Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62. ( 10.1007/bf00024185) [DOI] [PubMed] [Google Scholar]
- 31.Lakowicz JR. 2006. Principles of fluorescence spectroscopy, 3rd edn, pp. 8–10. New York, NY: Springer Science+Business Media. [Google Scholar]
- 32.Brody SS, Rabinowitch E. 1957. Excitation lifetime of photosynthetic pigments in vitro and in vivo. Science 125, 555 ( 10.1126/science.125.3247.555) [DOI] [PubMed] [Google Scholar]
- 33.Suggett DJ, Moore CM, Hickman AE, Geider RJ. 2009. Interpretation of fast repetition rate (FRR) fluorescence: signatures of phytoplankton community structure versus physiological state. Mar. Ecol. Prog. Ser. 376, 1–19. ( 10.3354/meps07830) [DOI] [Google Scholar]
- 34.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. ( 10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 35.Park J, Kuzminov FI, Bailleul B, Yang EJ, Lee SH, Falkowski PG, Gorbunov MY. 2017. Light availability rather than Fe controls the magnitude of phytoplankton blooms in the Amundsen Sea polynyas, Antarctica. Limnol. Oceanogr. ( 10.1002/lno.10565) [DOI]
- 36.Gorbunov MY, Chekalyuk AM. 1994. Lidar implementation of pump-and-probe technique for remote sensing estimates of photosynthesis efficiency in plants. In Geoscience and Remote Sensing Symposium, 1994 IGARSS '94 Surface and Atmospheric Remote Sensing: Technologies, Data Analysis and Interpretation, International, pp. 120–122. New York, NY: IEEE Publishing. [Google Scholar]
- 37.Gorbunov MY, Fadeev VV, Chekalyuk AM. 1991. Method of remote laser monitoring of photosynthesis efficiency in phytoplankton. Mosc. U. Phys. B+ 46, 59–65. [Google Scholar]
- 38.Moya I, Goulas Y, Morales F, Camenen L, Guyot G, Schmuck G. 1995. Remote sensing of time-resolved chlorophyll fluorescence and back-scattering of the laser excitation by vegetation. EARSeL Adv. Remote Sens. 3, 188–197. [Google Scholar]
- 39.Cerovic ZG, Goulas Y, Gorbunov M, Briantais JM, Camenen L, Moya I. 1996. Fluorosensing of water stress in plants: diurnal changes of the mean lifetime and yield of chlorophyll fluorescence, measured simultaneously and at distance with a τ-LIDAR and a modified PAM-fluorimeter, in maize, sugar beet, and kalanchoë. Remote Sens. Environ. 58, 311–321. ( 10.1016/S0034-4257(96)00076-4) [DOI] [Google Scholar]
- 40.Abbott MR, Letelier RM. 1999. Algorithm theoretical basis document: chlorophyll fluorescence (MODIS Product Number 20). (Ocean Biology Processing Group, NASA's Earth Observing System).
- 41.Behrenfeld MJ, et al. 2009. Satellite-detected fluorescence reveals global physiology of ocean phytoplankton. Biogeosciences 6, 779–794. ( 10.5194/bg-6-779-2009) [DOI] [Google Scholar]
- 42.Huot Y, Franz BA, Fradette M. 2013. Estimating variability in the quantum yield of sun-induced chlorophyll fluorescence: a global analysis of oceanic waters. Remote Sens. Environ. 132, 238–253. ( 10.1016/j.rse.2013.01.003) [DOI] [Google Scholar]
- 43.Neville RA, Gower JFR. 1977. Passive remote sensing of phytoplankton via chlorophyll α fluorescence. J. Geophys. Res. 82, 3487–3493. ( 10.1029/JC082i024p03487) [DOI] [Google Scholar]
- 44.Gordon HR, Brown OB, Evans RH, Brown JW, Smith RC, Baker KS, Clark DK. 1988. A semianalytic radiance model of ocean color. J. Geophys. Res. Atmos. 93, 10 909–10 924. ( 10.1029/JD093iD09p10909) [DOI] [Google Scholar]
- 45.Gower JFR, Doerffer R, Borstad GA. 1999. Interpretation of the 685 nm peak in water-leaving radiance spectra in terms of fluorescence, absorption and scattering, and its observation by MERIS. Int. J. Remote Sens. 20, 1771–1786. ( 10.1080/014311699212470) [DOI] [Google Scholar]
- 46.Moral A, Prieur L. 1977. Analysis of variations in ocean color. Limnol. Oceanogr. 22, 709–722. ( 10.4319/lo.1977.22.4.0709) [DOI] [Google Scholar]
- 47.Gordon HR, Morel AY. 1983. In-water algorithms. In Remote assessment of ocean color for interpretation of satellite visible imagery: a review (eds Gordon HR, Morel AY), pp. 24–67. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 48.Esaias WE, et al. 1998. An overview of MODIS capabilities for ocean science observations. IEEE Trans. Geosci. Remote Sens. 36, 1250–1265. ( 10.1109/36.701076) [DOI] [Google Scholar]
- 49.Cullen JJ, Ciotti AM, Davis RF, Neale PJ. 1997. Relationship between near-surface chlorophyll and solar-stimulated fluorescence: biological effects. Proc. SPIE 2963, 272–277. ( 10.1117/12.266454) [DOI] [Google Scholar]
- 50.Letelier RM, Abbott MR, Karl DM. 1997. Chlorophyll natural fluorescence response to upwelling events in the Southern Ocean. Geophys. Res. Lett. 24, 409–412. ( 10.1029/97GL00205) [DOI] [Google Scholar]
- 51.Abbott MR, Letelier RM. 1998. Decorrelation scales of chlorophyll as observed from bio-optical drifters in the California Current. Deep Sea Res. Pt II 45, 1639–1667. ( 10.1016/S0967-0645(98)80011-8) [DOI] [Google Scholar]
- 52.Maritorena S, Morel A, Gentili B. 2000. Determination of the fluorescence quantum yield by oceanic phytoplankton in their natural habitat. Appl. Opt. 39, 6725–6737. ( 10.1364/Ao.39.006725) [DOI] [PubMed] [Google Scholar]
- 53.Morrison JR. 2003. In situ determination of the quantum yield of phytoplankton chlorophyll a fluorescence: a simple algorithm, observations, and a model. Limnol. Oceanogr. 48, 618–631. ( 10.4319/lo.2003.48.2.0618) [DOI] [Google Scholar]
- 54.Huot Y, Brown CA, Cullen JJ. 2005. New algorithms for MODIS sun-induced chlorophyll fluorescence and a comparison with present data products. Limnol. Oceanogr. Methods 3, 108–130. ( 10.4319/lom.2005.3.108) [DOI] [Google Scholar]
- 55.Ananyev G, Kolber ZS, Klimov D, Falkowski PG, Berry JA, Rascher U, Martin R, Osmond B. 2005. Remote sensing of heterogeneity in photosynthetic efficiency, electron transport and dissipation of excess light in Populus deltoides stands under ambient and elevated CO2 concentrations, and in a tropical forest canopy, using a new laser-induced fluorescence transient device. Glob. Chang. Biol. 11, 1195–1206. ( 10.1111/j.1365-2486.2005.00988.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are deposited at PANGAEA (Publishing Network for Geoscientific and Environmental Data) under accession number PDI-11228.