Abstract
To meet the growing demand for food, substantial improvements in yields are needed. This is particularly the case for wheat, where global yield has stagnated in recent years. Increasing photosynthesis has been identified as a primary target to achieve yield improvements. To increase leaf photosynthesis in wheat, the level of the Calvin–Benson cycle enzyme sedoheptulose-1,7-biphosphatase (SBPase) has been increased through transformation and expression of a Brachypodium distachyon SBPase gene construct. Transgenic lines with increased SBPase protein levels and activity were grown under greenhouse conditions and showed enhanced leaf photosynthesis and increased total biomass and dry seed yield. This showed the potential of improving yield potential by increasing leaf photosynthesis in a crop species such as wheat. The results are discussed with regard to future strategies for further improvement of photosynthesis in wheat.
This article is part of the themed issue ‘Enhancing photosynthesis in crop plants: targets for improvement’.
Keywords: sedoheptulose-1,7-biphosphatase; Calvin–Benson cycle; transgenic; biomass; yield
1. Introduction
To meet the future demands of an increasing world population for food and fuel, crop yields need to be improved substantially. During the latter half of the twentieth century, agricultural yields largely rose in line with demand, mainly due to advances through breeding and farming practices. However, wheat yield increases in many parts of the world have plateaued in recent years, while at the same time a predicted 70% increase in yield will be needed over the next four decades to feed the growing population [1,2]. It is unlikely that more land can be used for the production of food, stressing the need to improve crop yields that can be obtained from existing arable land. Moreover, these improvements will have to be realized in a changing climate, where [CO2] is expected to increase from the current level of 400–550 ppm in the next four decades [3,4].
To meet this challenge it is necessary to increase the yield potential of crops (Yp) which is the product of primary production over a growing season (Pn) and harvest index (η) when grown under optimal conditions [5]. The harvest index, η, is the partitioning of biomass into harvestable product and is already close to its theoretical limit in most cereals [6–10]. Pn is the product of the sum of solar irradiance over the growing season (St), the efficiency of canopy light interception (ɛi) and the efficiency of conversion of captured light into biomass (ɛc) divided by the energy content of the plant mass (k). Given that S is determined by the geographical location and k by the crop species, Yp is predominantly determined by the efficiencies ɛi and ɛc. Similar to η, ɛi has been improved substantially and is also near its theoretical maximum and is therefore not expected to greatly increase Yp, e.g. through breeding [11,12]. This leaves ɛc, which is not yet near its theoretical maximum of 4.6% [13] and there is therefore potential to improve this parameter in wheat [14] and in other crop species [11,12,15,16]. The primary targets for crop improvement would therefore be enhancement of photosynthesis per unit leaf area and optimization of canopy light distribution. Evidence for increased yield in response to CO2 enrichment has been shown consistently [17], providing compelling evidence that yields can be increased through the improvement of photosynthesis. For wheat, this is substantiated by the positive relationship between photosynthesis and biomass [18] and yield [19]. However, it should be noted that for CO2 enrichment studies of both wheat and other crops, measured yields in the field were lower than predicted [15,20,21]. This discrepancy indicates that, among potential other factors, the ratio of source (CO2 assimilation) to sink (demands of plant organs for assimilates) is also sub-optimal. In summary, there is evidence that improving photosynthesis can achieve significant enhancement of Yp [14,16,22]; however, in the future it is likely that optimization of the source–sink ratio will be required in order to maximize the potential gains [7].
The Calvin–Benson cycle is the primary CO2 assimilation pathway and is co-limited by the maximum carboxylation efficiency of Rubisco (Vc,max) and the regeneration of the substrate RuBP driven by photosynthetic electron transport (Jmax). Under current atmospheric [CO2] and saturating light intensity, CO2 assimilation operates at the transition between the Rubisco and the RuBP limited phases. However, CO2 assimilation will move towards being RuBP limited as a result of the predicted increase in atmospheric [CO2]. Both Rubisco and sedoheptulose-1,7-biphosphatase (SBPase) have been shown to exert control over flux of CO2 through the Calvin–Benson cycle. SBPase is the first step in the Calvin–Benson cycle that commits intermediates to the regeneration of RuBP through the dephosphorylation of sedoheptulose-1,7-biphosphate (SBP) to sedoheptulose-7-phosphate (S7P). The importance of SBPase in the control of the regenerative phase of the Calvin cycle has been shown by several studies; modelling and simulation studies have shown that SBPase exerts control over the rate of RuBP regeneration [23,24]. In studies that decreased SBPase levels in tobacco plants, reduced photosynthesis and growth were observed [25–27], with similar results found for rice [28]. Conversely, increases in the activity levels of SBPase led to increased photosynthesis and biomass in tobacco under controlled conditions [29] as well as in the field under elevated [CO2] [30]. In food crops such as tomato, increased SBPase activities resulted in similar increases in photosynthesis and growth, as well as improved chilling tolerance [31]. In rice, photosynthesis and growth were less affected by drought and heat stress in plants overexpressing SBPase, but no changes in biomass and yield were found [32,33]. This shows that the effects of increased SBPase activity on photosynthesis are positive, but effects on plant growth or yield may vary, particularly in important food crop species. Clearly, these results provide evidence to suggest that increasing the activity of SBPase has the potential to improve photosynthesis.
For one of the most important food crops in the world, the effect of increasing SBPase has not been investigated. Wheat provides more than 20% of calories consumed by the global population [34]. However, increases in wheat productivity (1.1% per year [35]) are below the predicted global demand in coming decades (1.7% per year [36]). Increasing photosynthesis in wheat is seen as one of the ways to meet this challenge, and increasing SBPase has been proposed as a means to achieve this [7]. Therefore, this study investigates whether Yp in wheat can be realized through increases in SBPase. The effects of increased SBPase in wheat were studied through generation of transgenic wheat plants expressing a Brachypodium distachyon gene for SBPase, to elevate the total SBPase levels and activity. These plants were not only analysed for effects on photosynthetic capacity and growth, but also for their biomass and yield components under controlled conditions. The results are discussed in relation to the possibilities of increasing levels of SBPase for increasing Yp in wheat.
2. Material and methods
(a). Production and selection of transgenic plants
A functional Brachypodium distachyon SBPase genomic and cDNA hybrid sequence was generated and cloned into a modified pBract302 vector, inserted in between the rice tungro virus promoter (RTVP) and 35S terminator (electronic supplementary material, figure S1). The plasmid contained the nptI kanamycin resistance gene for selection of bacteria and the bar gene for phosphinothricin resistance under control of the Zea mays ubiquitin promoter for plant selection. This was co-transformed with pAHC20 which contains the bar gene under the control of the Ubiquitin1 promoter sequence + intron [37] due to the inefficient selection provided by the pBract302 bar gene. The recombinant plasmids were introduced into wheat cv. Cadenza by particle bombardment of wheat embryos, as described by Sparks and Jones [38,39]. From bombardments, 25 independent transgenic plants were identified by PCR, self-fertilized, and T1 and T2 plants were used for further selection of stably transformed plants. In parallel, particle bombardment was done using an empty vector to generate wild-type (WT) lines that had gone through the transformation process but had no foreign DNA inserted.
(b). Growth conditions
Seeds of T3 and T4 generations were germinated and seedlings were grown in compost (Levington F2S, Fisons, Ipswich, UK) in a climate controlled room for three weeks (22°C, 12 h photoperiod). Selected seedlings were then transferred into 4 l pots (1 plant per pot), to a controlled environment greenhouse (25–32°C day/18°C night), with a 16 h photoperiod of natural irradiance (supplemented with high pressure sodium lamps, to a minimum light level of 175 µmol m−2 s−1 PAR). Plants of the T3 generation were grown in the greenhouse at the University of Essex (Colchester, UK) during the period of April to September (2014) at a constant density of 25 plants m−2. To reduce the effect of canopy-induced shading and increase the chance of tillering, plants of the T4 generation were grown in the same greenhouse from March to August (2016) at a lower initial density of 18.75 plant m−2 and from Zadoks stage 4.1 at a density of 15.6 plants m−2. A constant, high plant density (T3 plants) and a lower density (T4 plants) allowed assessment of plant growth with and without the influence of canopy-induced shading. All plants were regularly watered and moved to minimize spatial variation of growth conditions.
(c). DNA extraction, RNA isolation and cDNA synthesis
A high throughput DNA extraction method was used on the first leaf of two week old seedlings. Leaf material (ca 0.1 g fresh weight) was collected in 1.2 ml micro-collection tubes in 96-tube racks (Starlab UK Ltd, Milton Keynes, UK) on dry ice and subsequently stored at −80°C. Frozen samples were dried overnight in a freeze dryer. To each tube, one stainless steel ball bearing was added, sealed well and ground in a Retch mill (TissueLyser, Qiagen, Manchester, UK) for 5 min at a frequency of 25 s−1. To the finely ground tissue, 600 µl of extraction buffer (0.1 M Tris-HCl, 0.05 M EDTA, 1.25% SDS, pre-heated to 65°C) was added per tube. Tubes were closed, mixed well and incubated for 30 min at 65°C. Tubes were cooled to below room temperature in the fridge for 15–30 min. then, 300 µl of cold 6 M ammonium acetate was added to each tube, closed, mixed well and incubated for 15 min at 4°C. Tubes were centrifuged for 15 min at 4°C at 13 000 g and 600 µl of supernatant added to new micro-collection tubes containing 360 µl of isopropanol and incubated for 5 min at room temperature. Tubes were centrifuged for 15 min at 13 000 g and the supernatant was discarded. 500 µl of 70% ethanol was added to the pellet, centrifuged for 15 min at 13 000 g and supernatant discarded. The pellet was resuspended in 400 µl distilled H2O and left overnight at 4°C. Tubes were centrifuged for 20 min at 13 000 g and 300 µl of supernatant was transferred and stored at −20°C until further use.
Total RNA was isolated from frozen leaf material (ca 0.1 g fresh weight; FW) ground in liquid nitrogen using Tri-reagent (Sigma T9429), modified from Hilario and Mackay [40] with the following additional steps: 30 µl of 3 M sodium acetate and 750 µl of ice-cold absolute ethanol were added and centrifuged at 13 000 g at 4°C for 15 min and the sample was then left on dry ice for 20 min. The RNA pellets were then washed with 75% ethanol and centrifuged for 5 min. Finally, the pellets were allowed to air-dry for 10 min before re-suspension in 50 µl of purified water and were stored at −80°C. The extracted RNA was quantified via absorbance measurement at 260 nm using a NanoDrop spectrophotometer (Nanodrop Products, Wilmington, USA). cDNA was synthesized from mRNA using SuperScript first strand cDNA synthesis (Invitrogen, UK) as specified by the manufacturer.
(d). PCR and qPCR
Plants were screened for the presence of the construct from isolated DNA by PCR. Expression of the construct was determined from cDNA by qPCR and expressed relative to the gene expression of a stable reference gene for wheat (Ta2291 [41]). qPCR reactions were performed using SensiFast SYBR No-ROX mix (Bioline Reagents Ltd, London, UK) as specified by the manufacturer. Fold expression was determined according to Pfaffl [42]. Oligo sequences of primers used, with details for both PCR and qPCR are provided in the electronic supplementary material, table S1.
(e). Determination of SBPase gene copy number
For analysis of the SBPase copy number of the inserted gene construct, g-Count technology was used to estimate copy number compared to a reference gene and to predict zygosity on leaf material from T4 plants of the transformed lines. Quantitative real time PCR analysis was used to estimate the numbers of transgene copies in individual wheat plants, similar to the approach taken for barley by Bartlett et al. [43]. An amplicon from the SBPase gene (with a FAM reporter) and an amplicon from a wheat internal positive control (IPC, with a VIC reporter) were amplified together in a multiplex reaction (15 min denaturation, then 40 cycles of 15 s 95°C and 60 s 60°C) in an ABI7900 real-time PCR machine. Fluorescence from the FAM and VIC fluorochromes was measured during each 60°C step, and the Ct values obtained. The difference between the Ct values for the SBPase gene and the IPC (the ΔCt) was used to allocate the assayed samples into groups with the same gene copy number. Analysis was carried out by iDNA Genetics Ltd (Norwich, UK).
(f). Analysis of biomass and yield
Plant aboveground biomass was determined at full physiological maturity (Zadoks 9.1–9.2). For T3 plants, stems, leaves and ears (with seeds) were separated and counted, dried at 70°C (until a constant dry weight was reached) and weighed. For T4 plants, the whole plant was dried and weighed, without separation of stems and leaves. For all plants, ears were subsequently threshed and seeds were counted and weighed.
(g). Protein extraction and western blot analysis for SBPase
Leaf samples (ca 0.2 g FW) were ground in liquid nitrogen, extracted and quantified essentially as described by Harrison, et al. [26]. Equal total protein of samples was loaded onto 12% (w/v) SDS-PAGE gels, separated and transferred onto a nitrocellulose membrane. Proteins were probed using antibodies raised against SBPase. The antibodies were raised using peptide immunization in rabbit, carried out by Cambridge Research Biochemicals (Billingham, UK). Proteins were detected using horseradish peroxidase conjugated to the secondary antibody and Pierce ECL chemiluminescence detection reagent (Thermo Scientific, Rockford, IL) and visualized using a FUSION FX chemiluminescence detection system (PEQLAB Ltd, Sarisbury Green, UK).
(h). Total SBPase activity
Leaf SBPase activity was determined by phosphate release from SBP, as described by Harrison et al. [26] and Lefebvre et al. [29], with modifications. Leaf material (ca 5–6 cm2) was taken directly after measurement of photosynthesis, snap-frozen in liquid nitrogen and stored at −80°C. Leaf material was ground in liquid nitrogen to a fine powder after which 1.75 ml of extraction buffer (50 mM HEPES, pH 8.2; 5 mM MgCl2; 1 mM EDTA; 1 mM EGTA; 10% glycerol; 0.1% Triton X-100; 2 mM benzamidine; 2 mM aminocapronic acid; 0.5 mM phenylmethylsulfonylfluoride; 10 mM dithiothreitol) was added, mixed well and centrifuged for 3 min at 13 000 g at 4°C. 1 ml supernatant was purified through a pre-equilibrated desalting column (Illustra NAP-10, GE Healthcare Life Sciences, Little Chalfont, UK) and eluted with 1.5 ml of desalting buffer (extraction buffer excluding Triton X-100). The eluate was aliquoted, snap frozen in liquid nitrogen and stored at −80°C. For the assay, the reaction was started by adding 20 µl of extract to 66 µl of assay buffer (50 mM Tris, pH 8.2; 15 mM MgCl2; 1.5 mM EDTA; 10 mM dithiothreitol; 2 mM SBP) and incubated at 25°C for 10 min. The reaction was then cooled on ice and stopped by adding 50 µl of 1 M perchloric acid. Samples were centrifuged for 10 min at 13 000 g at 4°C and 30 µl of supernatant and 30 µl of phosphate standards (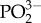 , 0.125–8 nmol) were incubated in a 96 well microtitre plate for 30 min at room temperature with 300 µl of Biomol Green (Enzo Life Sciences Ltd, Exeter, UK). Absorbance of the resulting reaction was measured at 620 nm using a microplate reader (SpectroStar Omega, BMC Labtech, Aylesbury, UK).
, 0.125–8 nmol) were incubated in a 96 well microtitre plate for 30 min at room temperature with 300 µl of Biomol Green (Enzo Life Sciences Ltd, Exeter, UK). Absorbance of the resulting reaction was measured at 620 nm using a microplate reader (SpectroStar Omega, BMC Labtech, Aylesbury, UK).
(i). A/ci photosynthetic gas exchange measurements
Photosynthetic gas exchange measurements were performed on fully emerged flag leaves for the T3 generation plants, between flag leaf sheath extension and boot (sheath) swelling (Zadoks growth stages 4.1–4.5). The response of CO2 assimilation (A) to changes in intercellular [CO2] (ci) was measured in the middle of the flag leaf with a saturating irradiance of 2000 µmol photons m−2 s−1, using an open infrared gas exchange system and 6 cm2 leaf chamber with a blue–red LED light source (LI-6400-02B; LI-COR, Lincoln, NE). Leaves were clamped in the leaf chamber and complete sealing of the gaskets around the leaf was ensured to prevent possible diffusion leakage. For plants of the T3 generation, leaf temperature was maintained at 20 ± 1°C with a vapour pressure deficit of 0.9 kPa and an ambient [CO2] (ca) of 400 µmol mol−1. Subsequently, ca was decreased to 300, 200, 100 and 50 µmol mol−1 before returning to the initial concentration. This was followed by an increase to 650, 900, 1200 and 1500 µmol mol−1. Readings were recorded when A had stabilized to the new conditions (after about 2 min). Similarly, for plants of the T4 generation, leaf temperature was maintained at 22 ± 1°C with a vapour pressure deficit of 1.3 kPa and ca of 400 µmol mol−1. Subsequently, ca was decreased to 300, 250, 150, 100 and 50 µmol mol−1 before returning to the initial concentration. This was followed by an increase to 550, 700, 900, 1100, 1300 and 1500 µmol mol−1. Readings were recorded when A had stabilized to the new conditions (after about 2 min).
(j). Model fitting of A/ci response
Model fitting for A/ci response curves was after the method of Dubois et al. [44] using the Farquhar et al. [45] model. Parameters estimated were: the maximum velocity of Rubisco for carboxylation (Vc,max) and the maximum rate of electron transport demand for RuBP regeneration (Jmax).
All calculations were done in the R environment, v. 3.2.1 [46].
3. Results
(a). Production and selection of transformants
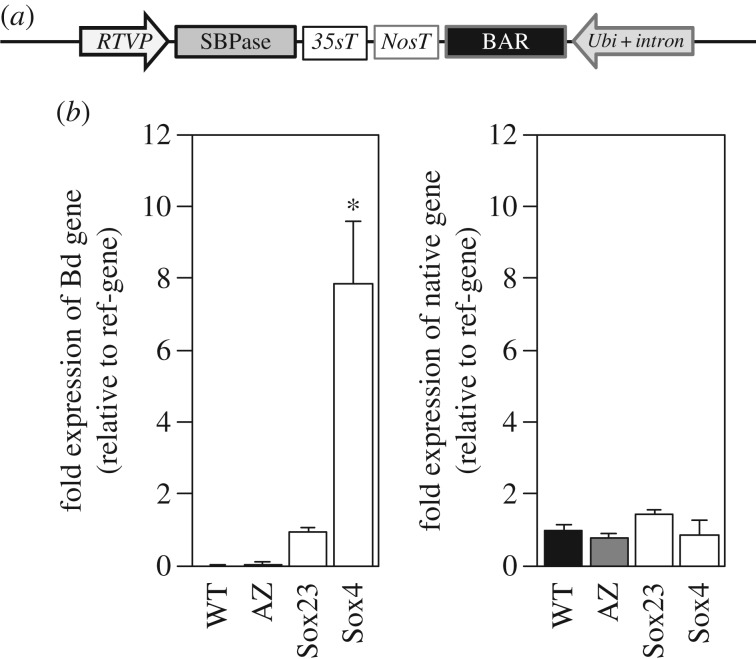
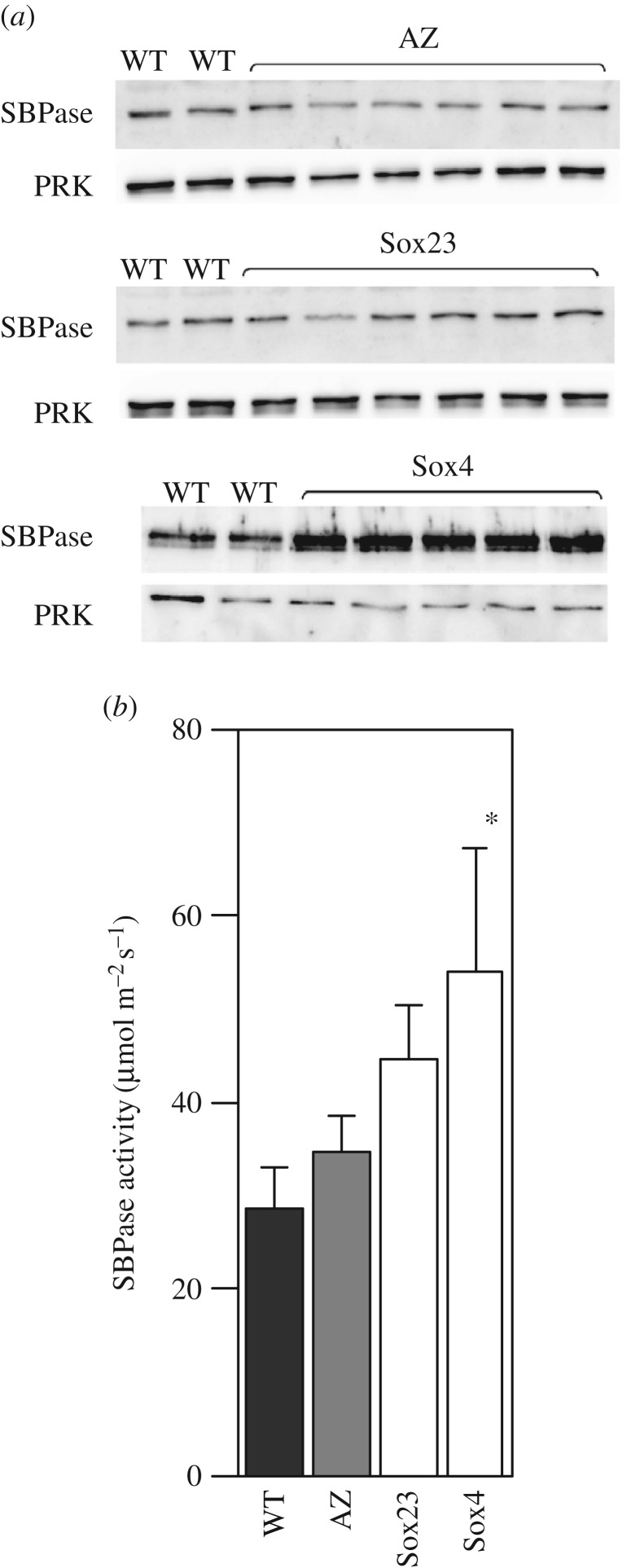
A chimeric gene construct consisting of a genomic- and cDNA SBPase hybrid sequence from Brachypodium distachyon was cloned into a modified vector (pBract302), containing an RTVP and a 35S CaMV terminator (figure 1a and electronic supplementary material, figure S1). The nptI kanamycin resistance gene in the vector was used for selection of bacteria. Although the pBract302 vector also carried a bar gene for phosphinothricin resistance, under the control of the Zea mays ubiquitin promoter plus first intron (Ubi-1)), it was known to be inefficient and consequently an additional plasmid pAHC20 [37] was used to introduce the bar gene for selection of transformed plants. The recombinant plasmids were introduced into Triticum aestivum cv. Cadenza by particle bombardment of wheat embryos, as described by Sparks and Jones [38,39]. This generated 25 independent primary transgenic plants (T0) identified by PCR analysis for both the SBPase and bar genes; these plants were allowed to self-fertilize and the resulting T1 seed collected. The subsequent T1 and T2 generation plants were grown under controlled conditions in a greenhouse and to confirm the presence of the construct, genomic DNA was isolated and screened for the gene of interest and bar gene using PCR. Expression of the introduced SBPase gene as well as the native SBPase gene in the flag leaves was determined using qPCR and, based on these results, two contrasting lines were chosen (Sox23 and Sox4) to study the effect of increased SBPase activity on wheat. The relative transcript levels of the SBPase transgene in the T3 plants of the Sox23 and Sox4 lines (compared to a reference gene Ta2991 [41]), were shown to be increased relative to wild-type and azygous plants (figure 1b), without a significant effect on the expression of the native SBPase gene (figure 1b). In flag leaves of these T3 plants, protein levels of SBPase were also found to be increased in comparison to wild-type (WT), while PRK protein levels remained constant (figure 2a). Transgene expression and SBPase protein levels verified the functionality of the introduced construct into wheat, resulting in plants differing in SBPase content. Plants of the two T3 lines with different SBPase protein levels were grown under greenhouse conditions and total SBPase activity of the flag leaf was determined. Increased SBPase activity was found in both Sox23 and Sox4 plants, with the latter having the highest activity, transcript and protein levels (figure 2b).
Figure 1.
(a) Schematic representation of the construct used for transformation (see also electronic supplementary material, figure S1). (b) Relative gene expression of the Brachypodium SBPase construct (Bd gene) and the native SBPase gene T3 lines of wild-type (WT), azygous control (AZ), lines Sox23 and Sox4. Measurements were done on fully developed flag leaves (Zadoks 4.1) of greenhouse grown plants. Means and standard error (n = 5), asterisks indicate significant difference from WT (p < 0.05).
Figure 2.
(a) Immunoblot analysis of leaf protein extracts from the Sox T3 lines (see figure 1). The same samples were used to probe for PRK. (b) SBPase activity of T3 lines. Measurements were done on fully developed flag leaves (Zadoks 4.1) of greenhouse grown plants. Means and standard error (n = 5), asterisks indicate significant difference from WT (p < 0.05).
(b). The effect of increased SBPase activity on CO2 assimilation, plant biomass and seed production
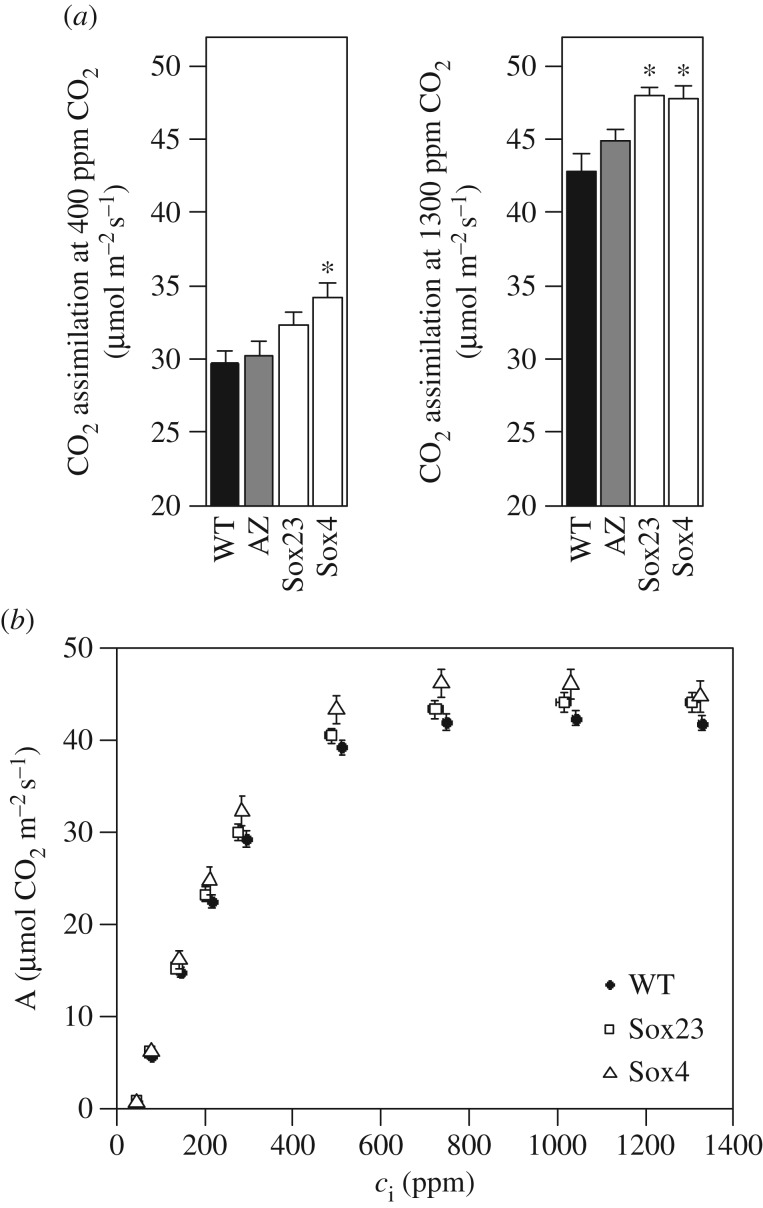
T3 SBPase transgenic and WT plants were grown in a controlled environment greenhouse (25–32°C day/18°C night) at a plant density of 25 plants m−2 from April to September, with a 16 h photoperiod of natural irradiance and supplemented with high pressure sodium lamps (minimum light level of 175 µmol m−2 s−1 PAR). At Zadoks stage 4.1, CO2 assimilation rates of the flag leaves were determined at both ambient and saturating [CO2] (400 and 1300 ppm [CO2], respectively, figure 3a). The Sox4 line, which had the higher SBPase activity, had higher rates of CO2 assimilation under both conditions, but in Sox23 CO2 assimilation was only significantly higher than WT at saturating [CO2] (figure 3a). The observed CO2 assimilation rates of the different lines had a positive, significant linear relationship with SBPase activity at both [CO2] values (electronic supplementary material, figure S2a,b). Further analysis of the photosynthetic rates of the flag leaves of these plants was carried out by determining the response of CO2 assimilation (A) to changes in intercellular CO2 concentration (ci). Both of the transgenic lines, Sox23 and Sox4, had a significantly different response of A to that of WT, particularly at high ci (figure 3b.). However, although the average values obtained for Vc,max and Jmax (table 1) were higher than for WT in both Sox 23 and Sox 4, these differences were not significant, with exception of Vc,max in the Sox4 line.
Figure 3.
(a) CO2 assimilation rates of Sox T3 lines at ambient [CO2] (400 ppm) and saturating [CO2] (1300 ppm). Means and standard error (n = 5). Asterisks indicate significant differences from WT (p < 0.05). (b) Response curves of T3 lines of CO2 assimilation rate (A) to changes in intercellular [CO2] (ci). Measurements were done on fully developed flag leaves (Zadoks 4.1) of greenhouse grown plants. Means and standard error (n = 5).
Table 1.
Vc,max and Jmax of the response of CO2 assimilation (A) to intercellular CO2 concentration for T3 plants of different lines. Parameters were fitted to the model of Farquhar et al. [45] according to Dubois et al. [44]. Means and standard error (n = 5), asterisks indicate significant difference from WT (p < 0.05). Mean and standard error (n = 4 or more).
| line | Vc,max | Jmax |
|---|---|---|
| WT | 103 (2.8) | 192 (3.7) |
| AZ | 111 (4.6) | 201 (6.5) |
| Sox23 | 109 (2.0) | 195 (4.4) |
| Sox4 | 117 (5.1)* | 210 (6.7) |
*p < 0.05.
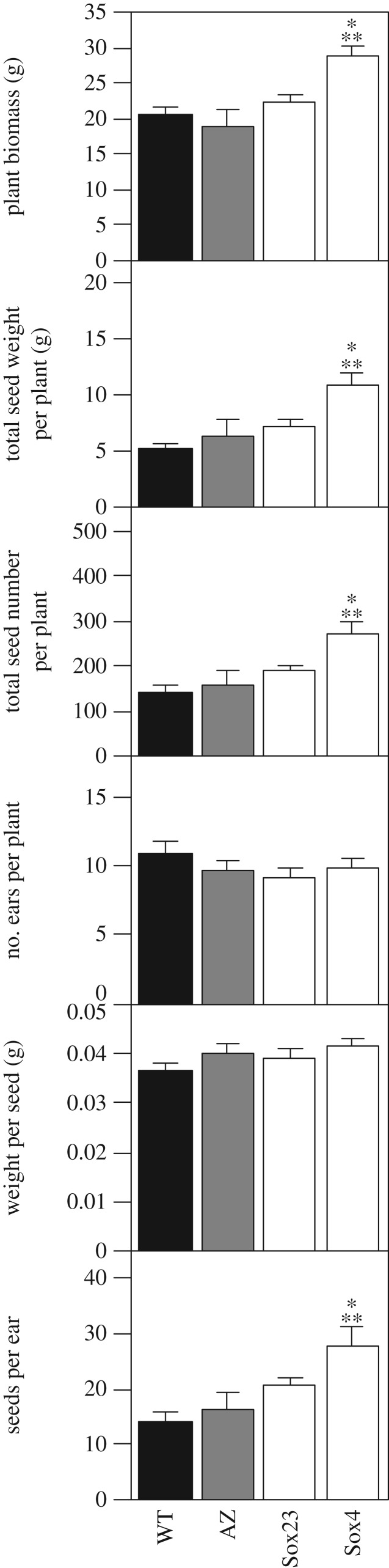
Comparison of plants at Zadoks stage 6.9 with increased SBPase activity showed that Sox4 and Sox23 were visibly taller and produced a more dense leaf canopy, compared to WT plants (figure 4). The WT plants shown are representative of the range of differences in growth between two different transformation control groups (WT), generated in separate bombardment events. The vegetative biomass and total seed weight of WT and the transgenic Sox lines (T3) were determined at full maturity (Zadoks 9.1–9.2). This analysis showed that the total biomass and the seed weight per plant at maturity was ca 40% higher in the Sox4 plants (figure 5) and the means of the different lines had a positive, significant linear relation with SBPase activity (electronic supplementary material, figure S2c,d). The allocation of biomass to leaves was not significantly different between the Sox lines and WT, but the stem fraction was lower in line Sox4 and in this line the percentage allocated to ears was increased by 10% (electronic supplementary material, figure S3). The significant increase in the total seed weight in Sox4 was the result of an increase in the total number of seeds per plant as neither the number of ears nor the weight per seed differed significantly between the Sox lines and WT (figure 5). This led to a significantly higher harvest index η = 0.38 (0.02) for Sox4 compared to η = 0.25 (0.02) for WT (mean and standard error, p = 0.006).
Figure 4.

T3 plants (Zadoks stage 6.9) from Sox lines compared to wild-type plants (WT). Plants were grown under natural light, with supplementary lighting (minimum of 175 µmol m−2 s−1 PAR), in an environmentally controlled greenhouse in the period April–July 2014. Scale bar indicates 10 cm.
Figure 5.
Biomass of T3 plants grown to full physiological maturity (Zadoks 9.1–9.2) with different SBPase activity; whole plant biomass, total dry seed weight per plant, total number of seeds per plant, number of ears per plant, average weight per seed, number of seeds per ear. Means and standard error (n = 4 or more). Asterisks (*) indicates significant difference from WT (p < 0.05), asterisks (**) indicates significant difference from AZ (p < 0.05).
(c). Increased plant biomass and seed production was consistent across generations and growing conditions
To further explore the impact of increase SBPase activity on the biomass of wheat and given variation between individual T3 plants within lines, particularly in Sox4, the next generation of plants (T4) were produced. Prior to physiological analysis of these T4 progeny, the gene copy number was determined. Sox23 plants were found consistently to have six extra copies (12 in homozygous, table 2); in contrast, Sox4 was shown to have a segregation of copy numbers. This comprised plants with one copy (two in homozygous, table 2) and two functional copies (four in homozygous, table 2). Following this analysis, in all future experiments Sox4 was divided into two groups; plants with two gene copies (Sox42) or four gene copies (Sox44). It is noteworthy that the highest SBPase activities were found in the lines with the lowest copy numbers (Sox4, or Sox42 and Sox44) and lower SBPase activity in the line with high copy numbers (Sox23).
Table 2.
Gene copy number of inserted construct for T4 plants.
| line | gene copy number |
|---|---|
| WT | 0 |
| Sox23 | 6 (12 homozygous) |
| Sox42 | 1 (2 homozygous) |
| Sox44 | 2 (4 homozygous) |
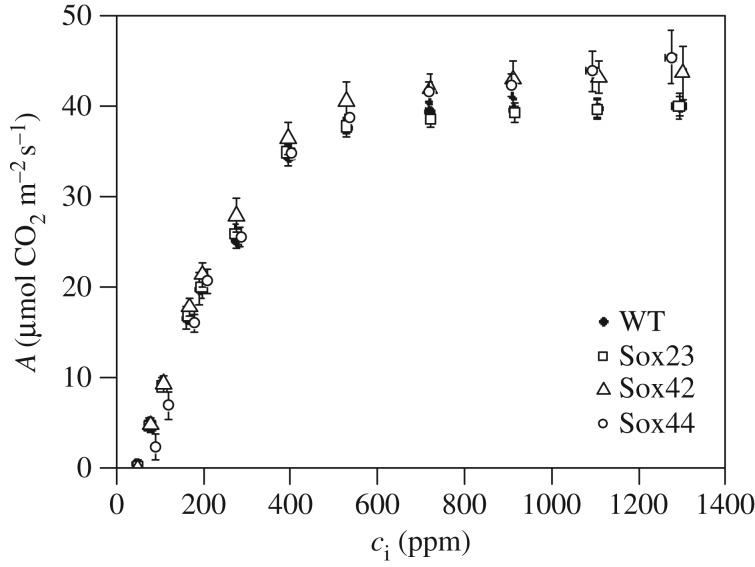
In a second experiment T4 plants were grown in a controlled environment greenhouse (25–32°C day/18°C night), with a 16 h photoperiod of natural irradiance and supplemented with high pressure sodium lamps to a minimum light level of 175 µmol m−2 s−1 PAR. In this case plants were grown at a lower density (16–18 plants m−2) to increase the chance of tillering. The A/ci response measured on flag leaves of T4 plants showed that lines Sox42 and Sox44 had a significantly different response than line Sox23 and WT (figure 6). Although no significant differences were observed in Vc,max between these lines and WT (table 3), Jmax was increased in Sox42 and was significantly higher than WT in line Sox44 (table 3).
Figure 6.
Response curves of T4 lines of CO2 assimilation rate (A) to changes in intercellular [CO2] (ci). Measurements were done on fully developed flag leaves (Zadoks 4.1) of greenhouse grown plants (May–August 2016). Means and standard error (n = 4).
Table 3.
Vc,max and Jmax of the response of CO2 assimilation (A) to intercellular CO2 concentration for T4 plants of different lines. Parameters were fitted to the model of Farquhar et al. [45] according to Dubois et al. [44]. Means and standard error (n = 4), asterisks indicate significant difference from WT (p < 0.05). Mean and standard error (n = 3 or more).
| line | Vc,max | Jmax |
|---|---|---|
| WT | 84 (2.6) | 178 (4.0) |
| Sox23 | 92 (3.6) | 184 (7.6) |
| Sox42 | 97 (8.5) | 202 (17.7) |
| Sox44 | 101 (7.1) | 222 (22.1)* |
*p < 0.05.
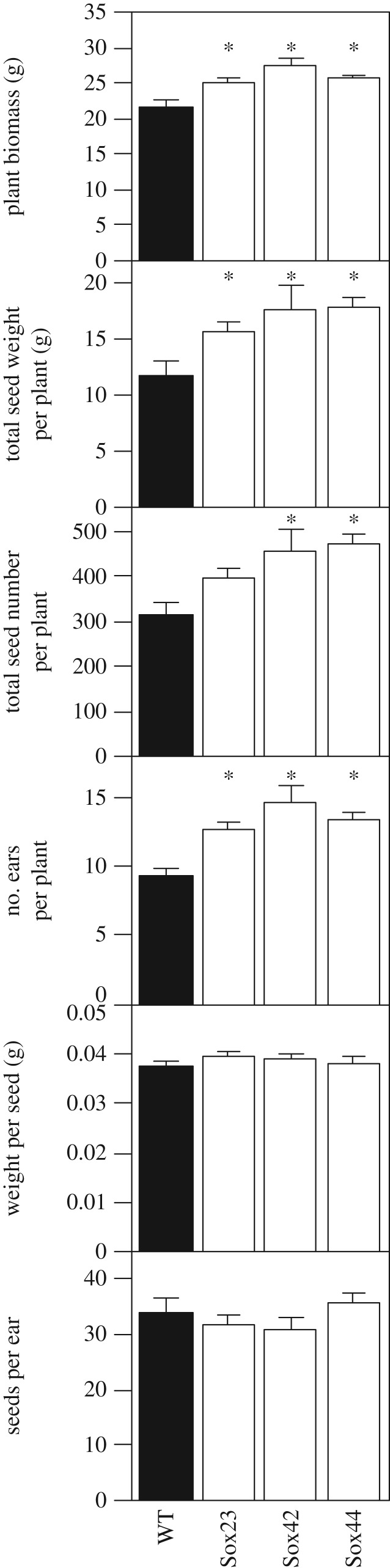
Similarly to T3 generation plants, the T4 lines with increased SBPase activity were visibly taller with more foliage (figure 7). Furthermore, the vegetative biomass and the total seed weight of Sox23, Sox42 and Sox44 plants were increased significantly when compared to WT (figure 8). For both Sox42 and Sox44, the total number of seeds was increase significantly compared to WT plants; while a small increase was also observed in Sox23, this was not significant (p = 0.13, figure 8). The number of ears was also significantly greater for the Sox23, Sox42 and Sox44 plants compared to WT (figure 8).
Figure 7.

T4 plants from lines Sox23, Sox42 and Sox44 in comparison to wild-type plants (WT). Plants were grown under natural light, with supplementary lighting (minimum of 175 µmol m−2 s−1 PAR), in an environmentally controlled greenhouse in the period May–August 2016.
Figure 8.
Biomass of T4 plants grown to full physiological maturity (Zadoks 9.1–9.2) with different SBPase activity; whole plant biomass, total dry seed weight per plant, total number of seeds per plant, number of ears per plant, average weight per seed, number of seeds per ear. Means and standard error (n = 5 or more). Asterisks (*) indicates significant difference from WT (p < 0.05).
4. Discussion
In this study we have examined the effects of increased SBPase protein levels and activity on photosynthesis and yield by expressing a Brachypodium SBPase gene-construct in wheat plants. It is shown that for an important crop such as wheat, increases in SBPase activity consistently resulted in an increase in leaf CO2 assimilation rate, particularly when measured under high [CO2]. Notably, the greatest increases in SBPase activity resulted not only in an increase in total biomass but also an increase in total seed weight (30–40% higher than WT). Two generations (T3 and T4) of transgenic SBPase overexpression wheat plants were grown under two different growth regimes: one grown in conditions which limited the chance of tillering (at high plant density) and the second in conditions that increased chances of tillering (at lower plant density). The results obtained were consistent between the two experiments. Total seed weight was found to be increased in both experiments in the plants with the highest SBPase activity. This was achieved either through a higher number of seeds being formed per ear (fewer tillers, at high plant density), or a larger number of ears being produced per plant (more tillers, at lower plant density). These results indicate that the positive effect of increased SBPase activity can be achieved at different plant densities. It also supports the contention that increasing SBPase activity in wheat has a positive effect on leaf photosynthetic capacity and, at least under controlled conditions, can lead to an increased Yp.
Higher CO2 assimilation rates were observed in the wheat plants displaying an increased SBPase activity. This is in agreement with effects of expression of either SBPase or the cyanobacterial SBPase/FBPase bifunctional enzymes that stimulated photosynthetic carbon assimilation and production of end products and/or biomass in other species [29,31,47–49]. The effects of increased SBPase activity in wheat plants on Jmax and Vc,max were relatively small when compared to those observed for tobacco [29] and were only significantly different from the WT values in lines Sox4 (T3, for Vc,max) and Sox44 (T4, for Jmax). As SBPase is involved in the regeneration of RuBP, a change in Jmax would have been expected in this study. However, the effect of increased SBPase activity on this parameter can vary depending on growth conditions [16], which may explain the observed differences in either Vc,max or Jmax between the two experiments. Nevertheless, we observed increased CO2 assimilation rates under light saturated conditions at both ambient and saturating [CO2] in plants that related positively and significantly with increased SBPase activity. Furthermore, CO2 assimilation rates for these plants were highest at saturating [CO2], which is in keeping with the role of SBPase in the regeneration of RuBP. Under future predicted atmospheric [CO2] the share of control over CO2 assimilation will move towards regeneration of RuBP and it is likely that the role of SBPase will become even more important under these conditions. Support for this comes from modelling of photosynthetic carbon metabolism by Zhu et al. [24] that identified a current underinvestment in SBPase levels. It was proposed that relatively large changes in SBPase levels should be made to achieve improved photosynthetic carbon metabolism. This suggests that there may be even more scope to increase further the biomass and seed yield of wheat and other crop species through introduction of higher levels of SBPase. Our results for wheat, in this current study, are consistent with this idea as the most significant positive effects were observed in the plants with the highest SBPase activity.
Further stimulation of photosynthesis may be obtained in wheat by the introduction of additional genes encoding photosynthesis proteins, similar to that shown recently for tobacco and Arabidopsis [48,49]. In this work further increases in biomass were obtained through the simultaneous over-expression of SBPase and fructose 1,6-bisphosphonate aldolase together with the glycine decarboxylase H subunit or the algal ictB protein [48,49]. In addition to stimulating photosynthesis a reduction of the Rubisco content may be desirable as this protein currently constitutes up to 50% of leaf N. This approach would have the potential to allow an N saving that may be reinvested in other proteins, e.g. SBPase (currently 1% of leaf N). However, this would only be useful if the reduction in Rubisco did not lead to a decrease in either photosynthesis or yield.
Previously a number of studies using model species showed that increased SBPase activity enhanced growth and photosynthesis under both controlled [29] and field conditions (tobacco) [30]. Increased SBPase activity may also be beneficial under elevated [CO2] and has also been shown to increase tolerance to salt and low and high temperatures [31–33]. This combination may prove beneficial under future climate conditions. To fully assess the potential of increased SBPase activity and improved photosynthesis for Yp in wheat under future climates, the next step would be to assess effects under ambient and elevated [CO2] and increased temperature in the field. The results of the current study provide a clear demonstration that photosynthesis can be improved by manipulation of the enzymes of the Calvin–Benson cycle and that this can be applied in relevant crops such as wheat [14,22]. This greenhouse study forms the first step in translating research on improving photosynthesis in model species to application in important crops. The next stage of this work will be to undertake studies with these transgenic SBPase wheat plants under field conditions.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Mrs Sue Corbett and Dr Philip Davey for their help with plant and greenhouse management at University of Essex. Also Amanda Riley, Angela Doherty and Melloney St Leger for wheat transformation experiments and Fiona Gilzean and Anthony Griffin for plant and greenhouse management at Rothamsted Research.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
S.M.D. designed the experiments, performed T1 and T2 line selection, acquired, analysed and interpreted data on T3 plants, analysed and interpreted data of T4 plants, drafted and revised the manuscript. A.J.S. acquired and analysed data on T4 plants and revised the manuscript. S.A. acquired data on gene expression of T3 plants. S.J.F. acquired data on SBPase activity of T3 plants. P.J.M. designed and made the gene construct. C.A.S. and H.D.J. performed gene transformation and initial line selection (T0) and revised the manuscript. T.L., M.A.J.P. and C.A.R. conceived the study, designed experiments and revised the manuscript. All authors approved the final version.
Competing interests
We declare we have no competing interests.
Funding
S.M.D., P.J.M., T.L., M.A.J.P. and C.A.R. were funded by the British Biological Sciences Research Council (BBSRC, grant no. BB/H01960X/1) awarded to C.A.R. and M.A.J.P., as part of the Crop Improvement Research Club (CIRC). P.J.M., C.A.S., H.D.J. and M.A.J.P. were funded as part of the Biological Sciences Research Council UK 20:20 Wheat Institute Strategic Programme at Rothamsted Research. A.J.S. was funded by Realising Increased Photosynthetic Efficiency to Increase Wheat Yields (IWYP, BB/N021045/1) awarded to C.A.R. S.A. and S.J.F. were funded by the Saudi Arabian Government and by the University of Essex Research Incentive Scheme to C.A.R., respectively.
References
- 1.Ray DK, Mueller ND, West PC, Foley JA. 2013. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8, e66428 ( 10.1371/journal.pone.0066428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA 108, 20 260–20 264. ( 10.1073/pnas.1116437108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Quere C, et al. 2009. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2, 831–836. ( 10.1038/Ngeo689) [DOI] [Google Scholar]
- 4.Solomon S. 2007. Climate change 2007-the physical science basis: Working group I contribution to the fourth assessment report of the IPCC. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Monteith JL. 1977. Climate and efficiency of crop production in Britain. Phil. Trans R. Soc. Lond. B 281, 277–294. ( 10.1098/rstb.1977.0140) [DOI] [Google Scholar]
- 6.Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP. 2011. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 62, 469–486. ( 10.1093/Jxb/Erq300) [DOI] [PubMed] [Google Scholar]
- 7.Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G. 2012. Achieving yield gains in wheat. Plant Cell Environ. 35, 1799–1823. ( 10.1111/j.1365-3040.2012.02588.x) [DOI] [PubMed] [Google Scholar]
- 8.Austin RB, Bingham J, Blackwell RD, Evans LT, Ford MA, Morgan CL, Taylor M. 1980. Genetic improvements in winter-wheat yields since 1900 and associated physiological-changes. J. Agric. Sci. 94, 675–689. ( 10.1017/S0021859600028665) [DOI] [Google Scholar]
- 9.Foulkes MJ, Snape JW, Shearman VJ, Reynolds MP, Gaju O, Sylvester-Bradley R. 2007. Genetic progress in yield potential in wheat: recent advances and future prospects. J. Agric. Sci. 145, 17–29. ( 10.1017/S0021859607006740) [DOI] [Google Scholar]
- 10.Shearman VJ, Sylvester-Bradley R, Scott RK, Foulkes MJ. 2005. Physiological processes associated with wheat yield progress in the UK. Crop Sci. 45, 175–185. [Google Scholar]
- 11.Horton P. 2000. Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J. Exp. Bot. 51, 475–485. ( 10.1093/jexbot/51.suppl_1.475) [DOI] [PubMed] [Google Scholar]
- 12.Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261. ( 10.1146/annurev-arplant-042809-112206) [DOI] [PubMed] [Google Scholar]
- 13.Zhu XG, Long SP, Ort DR. 2008. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 19, 153–159. ( 10.1016/j.copbio.2008.02.004) [DOI] [PubMed] [Google Scholar]
- 14.Parry MAJ, Reynolds M, Salvucci ME, Raines C, Andralojc PJ, Zhu XG, Price GD, Condon AG, Furbank RT. 2011. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 62, 453–467. ( 10.1093/Jxb/Erq304) [DOI] [PubMed] [Google Scholar]
- 15.Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29, 315–330. ( 10.1111/j.1365-3040.2005.01493.x) [DOI] [PubMed] [Google Scholar]
- 16.Raines CA. 2011. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol. 155, 36–42. ( 10.1104/pp.110.168559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol. 165, 351–371. ( 10.1111/j.1469-8137.2004.01224.x) [DOI] [PubMed] [Google Scholar]
- 18.Kruger EL, Volin JC. 2006. Reexamining the empirical relation between plant growth and leaf photosynthesis. Funct. Plant Biol. 33, 421–429. ( 10.1071/FP05310) [DOI] [PubMed] [Google Scholar]
- 19.Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Saavedra AL. 1998. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 38, 1467–1475. ( 10.2135/cropsci1998.0011183X003800060011x) [DOI] [Google Scholar]
- 20.Bernacchi CJ, et al. 2006. Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open-air field conditions. Plant Cell Environ. 29, 2077–2090. ( 10.1111/j.1365-3040.2006.01581.x) [DOI] [PubMed] [Google Scholar]
- 21.Long SP, Ainsworth EA, Leakey ADB, Nosberger J, Ort DR. 2006. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921. ( 10.1126/science.1114722) [DOI] [PubMed] [Google Scholar]
- 22.Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM. 2013. Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot. 64, 717–730. ( 10.1093/Jxb/Ers336) [DOI] [PubMed] [Google Scholar]
- 23.Poolman MG, Fell DA, Thomas S. 2000. Modelling photosynthesis and its control. J. Exp. Bot. 51, 319–328. ( 10.1093/jexbot/51.suppl_1.319) [DOI] [PubMed] [Google Scholar]
- 24.Zhu XG, de Sturler E, Long SP. 2007. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 145, 513–526. ( 10.1104/pp.107.103713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison EP, Olcer H, Lloyd JC, Long SP, Raines CA. 2001. Small decreases in SBPase cause a linear decline in the apparent RuBP regeneration rate, but do not affect Rubisco carboxylation capacity. J. Exp. Bot. 52, 1779–1784. ( 10.1093/jexbot/52.362.1779) [DOI] [PubMed] [Google Scholar]
- 26.Harrison EP, Willingham NM, Lloyd JC, Raines CA. 1998. Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta 204, 27–36. ( 10.1007/s004250050226) [DOI] [Google Scholar]
- 27.Lawson T, Bryant B, Lefebvre S, Lloyd JC, Raines CA. 2006. Decreased SBPase activity alters growth and development in transgenic tobacco plants. Plant Cell Environ. 29, 48–58. ( 10.1111/j.1365-3040.2005.01399.x) [DOI] [PubMed] [Google Scholar]
- 28.Feng LL, Li H, Jiao JM, Li D, Zhou L, Wan J, Li YS. 2009. Reduction in SBPase activity by antisense RNA in transgenic rice plants: effect on photosynthesis, growth, and biomass allocation at different nitrogen levels. J. Plant Biol. 52, 382–394. ( 10.1007/s12374-009-9049-3) [DOI] [Google Scholar]
- 29.Lefebvre S, Lawson T, Zakhleniuk OV, Lloyd JC, Raines CA, Fryer M. 2005. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 138, 451–460. ( 10.1104/pp.104.055046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal DM, Locke AM, Khozaei M, Raines CA, Long SP, Ort DR. 2011. Over-expressing the C-3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biol. 11, 123 ( 10.1186/1471-2229-11-123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding F, Wang M, Zhang S, Ai X. 2016. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 6, 32741 ( 10.1038/srep32741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng LL, Wang K, Li Y, Tan YP, Kong J, Li H, Li YS, Zhu YG. 2007. Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep. 26, 1635–1646. ( 10.1007/s00299-006-0299-y) [DOI] [PubMed] [Google Scholar]
- 33.Feng LL, Han YJ, Liu G, An BG, Yang J, Yang GH, Li YS, Zhu YG. 2007. Overexpression of sedoheptulose-1,7-bisphosphatase enhances photosynthesis and growth under salt stress in transgenic rice plants. Funct. Plant Biol. 34, 822–834. ( 10.1071/Fp07074) [DOI] [PubMed] [Google Scholar]
- 34.Braun HJ, Atlin G, Payne T. 2010. Multi-location testing as a tool to identify plant response to global climate change. In Climate change and crop production (ed. Reynolds MP.), pp. 115–138. Surrey, UK: CABI Climate Change Series. [Google Scholar]
- 35.Dixon J, Braun HJ, Kosina P, Crouch J (eds) 2009. Wheat facts and futures 2009. El Batan, Mexico: CIMMYT. [Google Scholar]
- 36.Rosegrant MW, Agcaoili M. 2010. Global food demand, supply, and price prospects to 2010. Washington, DC: International Food Policy Research Institute. [Google Scholar]
- 37.Christensen AH, Quail PH. 1996. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 5, 213–218. ( 10.1007/BF01969712) [DOI] [PubMed] [Google Scholar]
- 38.Sparks CA, Jones HD. 2009. Biolistics transformation of wheat. Methods Mol. Biol. 478, 71–92. ( 10.1007/978-1-59745-379-0_4) [DOI] [PubMed] [Google Scholar]
- 39.Sparks CA, Jones HD. 2014. Genetic transformation of wheat via particle bombardment. Methods Mol. Biol. 1099, 201–218. ( 10.1007/978-1-62703-715-0_17) [DOI] [PubMed] [Google Scholar]
- 40.Hilario E, Mackay J. 2007. Protocols for nucleic acid analysis by nonradioactive probes. Methods Mol. Biol. 353 ( 10.1385/1597452297) [DOI] [Google Scholar]
- 41.Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M. 2009. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 10, 11 ( 10.1186/1471-2199-10-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, 45e ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartlett JG, Alves SC, Smedley M, Snape JW, Harwood WA. 2008. High-throughput Agrobacterium-mediated barley transformation. Plant Methods 4, 1 ( 10.1186/1746-4811-4-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubois JJB, Fiscus EL, Booker FL, Flowers MD, Reid CD. 2007. Optimizing the statistical estimation of the parameters of the Farquhar–von Caemmerer–Berry model of photosynthesis. New Phytol. 176, 402–414. ( 10.1111/j.1469-8137.2007.02182.x) [DOI] [PubMed] [Google Scholar]
- 45.Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical-model of photosynthetic CO2 assimilation in leaves of C-3 species. Planta 149, 78–90. ( 10.1007/BF00386231) [DOI] [PubMed] [Google Scholar]
- 46.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 47.Miyagawa Y, Tamoi M, Shigeoka S. 2001. Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat. Biotechnol. 19, 965–969. ( 10.1038/Nbt1001-965) [DOI] [PubMed] [Google Scholar]
- 48.Simkin AJ, McAusland L, Headland LR, Lawson T, Raines CA. 2015. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 66, 4075–4090. ( 10.1093/jxb/erv204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simkin AJ, Lopez-Calcagno PE, Davey PA, Headland LR, Lawson T, Timm S, Bauwe H, Raines CA. 2017. Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol. 15, 805–816. ( 10.1111/pbi.12676) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.