Abstract
Background/Aim: Hemorrhoidectomy is often associated with significant postoperative complications that may result in slow wound healing. The traditional Chinese medicine (TCM) compound Sophora flavescens (CSF) has shown efficacy on many inflammatory disorders. The aim of the present study was to examine the efficacy of CSF on wound healing in a rat model of perianal ulceration. Materials and Methods: A rat model of perianal ulceration was induced by subcutaneous injection of 75% glacial acetic acid. The animals with induced perianal ulcer received topical treatment of low, medium, and high doses of CFS twice daily. Potassium permanganate (PP); 0.02%) was given to the animals for comparison. Macroscopic and histological assessments of the ulcerated area were performed after treatment. The expression of pro-inflammatory cytokines prostaglandin E2 (PGE2) and interleukin-8 (IL-8) was detected by immunohistochemical analysis. Results: Topical administration of medium- and high-dose CSF significantly enhanced perianal ulcer healing as compared to the untreated control (p<0.05). The macroscopic ulceration score was significantly reduced only in the high-dose CSF-treated group as compared to the control (p<0.01). All doses of CSF and PP ameliorated histological damages in the rats with induced perianal ulceration. High-dose CSF or PP significantly reduced the expression of PGE2 and IL-8 as compared to the control (p<0.01). No treatment-related toxicity was found in either the CSF- or the PP-treated mice. Conclusion: CSF enhances wound healing in a rat model of perianal ulceration. The inhibitory effect of CSF on pro-inflammatory cytokines PGE2 and IL-8 may be involved in the mechanism of enhanced wound-healing.
Keywords: Compound Sophora flavescens, topical treatment, cytokine, perianal ulceration
Hemorrhoidectomy is often associated with significant postoperative complications, including edema, pain, bleeding and ulceration, resulting in a protracted period of wound healing and recovery (1-9). Perianal tissue inflammation around the open wound can occur after hemorrhoidectomy (10,11). Various types of medication, including metronidazole, glyceryl trinitrate (0.2%), steroids, local anesthetics (bupivacaine), anti-inflammatory drugs, hemorrhoid creams, are being used for treatment of complications of hemorrhoidectomy with variable outcome (12-14).
Traditional Chinese medicine (TCM) has a long history of human use in China and other Asian countries for treating and preventing diseases. Compound Sophora flavescens (CSF) is a TCM formula consisting of a combination of 10 herbs, including Sophora flavescens, Phellodendron amurense, Radix sanguisorbae, Scutellaria baicalensis, white peony, Gardenia florida, Betel nut, Rhubarb, Mint and Licorice. Sophora flavescens, as a main component, is widely used in traditional herbal medicine for the treatment of fever, inflammatory disorders, ulcers, and skin burns (15). The components in the CSF formula have various effects, including anti-inflammation, antibacterial activity, blood nourishment, pain relief, hemostasis, etc. (16-24). CSF has demonstrated improvement in local microcirculation, control of local inflammation, pain relief, and promotion of wound healing. However, CSF has never been tested for therapy of complications of anal diseases.
In the present study, we established a rat model of perianal ulceration to mimic clinical postoperative complications of anal hemorrhoidectomy and evaluated the efficacy of CSF on wound healing.
Materials and Methods
Animals. Male Sprague-Dawley (SD) rats (170-210 g) were purchased from the Animal Department of the College of Medicine, Yangzhou University, Yangzhou, P.R China (certificate No. SYXK(Su) 20140012)). Rats were maintained in a high-efficiency particulate arrestance (HEPA)-filtered environment at 24-25˚C with humidity maintained at 50-60%. Rats were fed with autoclaved laboratory rodent diet. All animal experiments were approved by the Animal Committee of Nanjing Origin Biosciences, Nanjing, China (OB1409).
Perianal ulceration model. Rats were anaesthetized with 2% sodium pentobarbital (0.3 ml/100 g) via intraperitoneal injection. The rats were then fixed in a ventral decubitus position. The perianal skin was sterilized with 75% alcohol. Glacial acetic acid (40 μl, 75%) was subcutaneously injected into 4 symmetrical points in the perianal area. The rats were allowed to recover from anesthesia. After 24 hours, the perianal skin developed ulceration with appearance of redness, swelling, and inflammatory exudation.
Treatment. Compound Sophora flavescens lotion (CSF) used in the study was supplied by the Yuhang District First People’s Hospital (Hangzhou, Zhejiang Province, P.R. China). Undiluted CSF was used as the high dose. CSF was diluted 2 times with distilled water and used as the medium dose and 3 times diluted as the low dose. All animals with a similar-sized perianal ulceration were randomly assigned to 5 groups of 8 mice each 24 hours after the injection of glacial acetic acid. Group 1 was treated with distilled water twice daily as the negative control; Group 2 was treated with PP solution (0.02%) twice daily; Group 3 received low-dose CFS twice daily; Group 4 received medium-dose CFS twice daily; Group 5 received high-dose CFS twice daily. All treatments were carried out via topical application on the ulcer surface and continued daily for 7 days. Animal body weights and clinical signs were recorded daily over the course of the experiments.
Macroscopic assessment. The ulcer was measured with calipers on days 1, 4, and 8 after initial treatment. The ulcerated area was calculated using the formula (area=a×b), where a and b represent the perpendicular minor dimension and major dimension, respectively. Macroscopic perianal ulceration was assessed using a magnifying glass by an independent observer and scored. The scale for macroscopic damage ranged from 0-6 based on redness and swelling, severity of inflammatory filtration, and ulcerated area (25). All animals were sacrificed 24 h after the last treatment. The perianal ulcerated tissues were collected and fixed in 10% formalin for histological and immunohistochemical analyses.
Histological assessment. For histological examination, the perianal ulcerated tissue was fixed in 10% formalin, dehydrated, paraffin-embedded, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E). Histological changes were evaluated by a pathologist who was blinded to the experimental groups.
Immunohistochemical analysis. Paraffin-embedded perianal ulcerated tissue was sectioned at 4 μm thickness. All the sections were de-paraffinized and dehydrated through graded ethanol. The sections were washed three times with PBS for 5 min each, blot dried, and then treated with 3% hydrogen peroxide for 30 min at room temperature to block endogenous peroxidase activity. The sections were immersed in antigen-retrieval solution (citrate buffer, pH 6.0) for 10 min, followed by rinsing with PBS. After blocking with normal goat serum for 30 min at 37˚C, sections were co-incubated with primary anti-prostaglandin E2 (PGE2) and interleukin-8 (IL-8) antibodies (Biosynthesis Biotechnology Co., Ltd., Beijing, P.R. China) (1:100 dilution in PBS) overnight, and then with a peroxidase-conjugated anti-rabbit IgG secondary antibody (Biosynthesis Biotechnology Co., Ltd.,) for 1 h at room temperature. Subsequently, the sections were incubated with 3,3-diaminobenzidine (DAB) reagent (ZSGB-BIO, Beijing, P.R.China) for 10 minutes, counterstained with hematoxylin, dehydrated and mounted for microscopy. The slides were viewed at 400× magnification with positive cells identified by the appearance of brown-stained cells. Expression levels were quantified by the average optical density (AOD) of the positive cells in 5 fields/sample with Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis. Statistical analysis was performed using SPSS16.0 software (SPSS Inc., Chicago, IL, USA). All results are expressed as mean±standard deviation (SD). Comparisons between two or multiple groups were made with the Student’s t-test or ANOVA. p<0.05 was considered significant.
Results and Discussion
CFS enhances perianal ulcer healing. The rat model of perianal ulceration was developed 24 hours after subcutaneous injection of glacial acetic acid. The rats displayed severe perianal ulceration with redness, swelling, necrosis and inflammation. The efficacy of CSF and PP treatment on the perianal ulcer was evaluated by the measurement of the ulcerated area and macroscopic ulceration score. Representative images and mean ulcerated areas in each treated group are shown in Figures 1A, 1B and 1C. Ulcer healing in the groups treated with medium- and high-dose CSF were enhanced compared with the untreated control group (p<0.05). However, the macroscopic ulceration score was only significantly reduced by high-dose CSF compared with the control (p<0.01) (Figure 1D). PP did not significantly reduce the ulcerated area and macroscopic ulceration score as compared with the control (p>0.05). We used PP for comparison, as it is commonly used for postoperative treatment of many perianal diseases.
Figure 1. CFS promotes wound healing in the rat model of perianal ulceration. A: Representative images of perianal ulcers in each group (G) before and after treatment. The ulcerated area is outlined. B: Perianal ulceration changes during the course of treatment. C: Mean ulcerated area at the end of treatment. D: Macroscopic pathological scores. Data are represented as mean±SD of 8 animals of each group. *p<0.05, **p<0.01 when compared to the control group. CFS, Compound Sophora flavescens; PP, potassium permanganate.
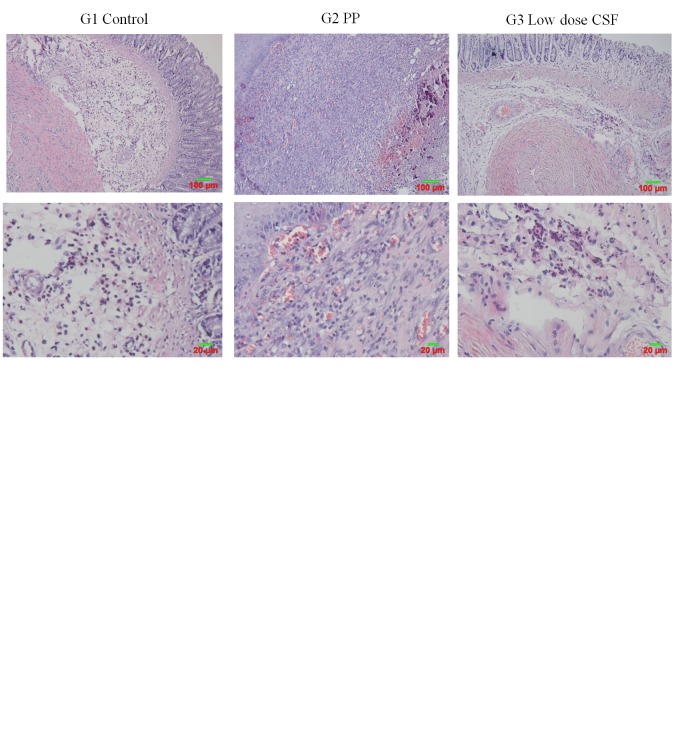
CFS improves histopathological changes in perianal ulceration. As shown in Figure 2, histologic evaluation of the rats with perianal ulceration showed obvious tissue edema, large squamous epithelium necrosis, bleeding, extravasated blood in mucoderm, numerous neutrophils, and lymphocyte infiltration. These histologic signs were improved in the rats treated with PP and CSF. The high-dose CSF-treated group showed significant reduction of tissue necrosis and inflammatory infiltration as compared with the untreated control group.
Figure 2. Efficacy of compound Sophora flavescens (CSF) on histological damage in the rat model of perianal ulceration. Representative histological images of hematoxylin and eosin (H&E) staining of perianal ulcerated tissues in each group (G) (magnification: upper panel ×100; lower panel ×200).
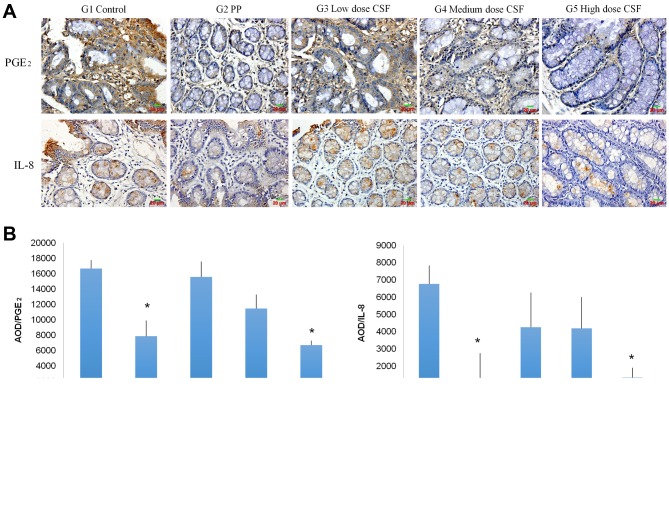
CFS inhibits expression of pro-inflammatory cytokines. Pro-inflammatory cytokines play a very important role in wound healing. The efficacy of CSF on the expression of the pro-inflammatroy cytokines PGE2 and IL-8 was detected using immunohistochemical analysis. As shown in Figure 3, topical treatment of the perianal ulcer with PP and high-dose CSF resulted in a significant decrease in PGE2 and IL-8 expression in the perianal ulcerated tissue compared to the untreated control group (p<0.01). Our results suggest the reduction of pro-inflammatory cytokines PGE2 and IL-8 is involved in the mechanism of the wound-healing efficacy of CSF.
Figure 3. CFS inhibits expression of pro-inflammatory cytokines in the rat model of perianal ulceration. A: Representative images of immunostaining of perianal ulcerated tissues for PGE2 and IL-8 (magnification, 400×). B: AOD of immunostained perianal ulcerated tissues for PGE2 and IL-8. Data are mean (±SD) of 8 animals of each group (G). *p<0.01 when compared to the control group. CFS, Compound Sophora flavescens; PP, potassium permanganate; AOD, average optical density; PGE2, prostaglandin E2; IL-8, interleukin-8.
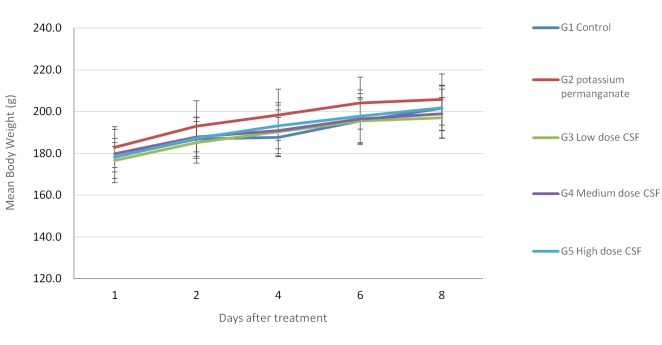
Efficacy of CFS on body weight and toxicity. Clinical observation and body-weight measurement of animals during the study were performed to assess the toxicity of CFS treatment. No physical or behavioral signs that indicated adverse effects were observed in any treatment group. As shown in Figure 4, a stable body-weight in all treated groups indicated no obvious toxicity.
Figure 4. Effect of compound Sophora flavescens (CFS) on body weight in a rat model of perianal ulceration. No significant body weight loss was found in any group of treated rats.
In conclusion, the present study demonstrates that CSF facilitates wound healing in a rat model of perianal ulceration. The inhibitory effect of CSF on pro-inflammatory cytokines PGE2 and IL-8 suggests these cytokines may be involved in its wound-healing efficacy mechanism. The present results suggest the potential of the TCM formulation CFS as an effective topical treatment for postoperative complication of hemorrhoidectomy.
Conflicts of Interest
None of the Authors have a conflict of interest with regard to this study.
Acknowledgements
This work was supported by the Hangzhou Health Science and Technology Fund No. 201553344
References
- 1.Solorio-López S, Palomares-Chacón UR, Guerrero-Tarín JE, González-Ojeda A, Cortés-Lares JA, Rendón-Félix J, García-Rentería J, Chávez-Tostado M, Cuesta-Márquez LA, Salazar-Parra M, Fuentes Orozco C. Efficacy of metronidazole versus placebo in pain control after hemorrhoidectomy. Results of a controlled clinical trial. Rev Esp Enferm Dig. 2015;107:681–685. doi: 10.17235/reed.2015.3926/2015. [DOI] [PubMed] [Google Scholar]
- 2.Shafik A. ‘Somatoanal’ reflex or ‘thermosphincteric’ reflex. Dis Colon Rectum. 2000;43:726–728. doi: 10.1007/BF02235602. [DOI] [PubMed] [Google Scholar]
- 3.Riss S, Weiser FA, Schwameis K, Riss T, Mittlböck M, Steiner G, Stift A. The prevalence of hemorrhoids in adults. Int J Colorectal Dis. 2012;27:215–220. doi: 10.1007/s00384-011-1316-3. [DOI] [PubMed] [Google Scholar]
- 4.Acheson AG, Scholefield JH. Management of haemorrhoids. BMJ. 2008;336:380–383. doi: 10.1136/bmj.39465.674745.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox A, Tietze PH, Ramakrishnan K. Anorectal conditions: Hemorrhoids. FP Essent. 2014;419:11–19. [PubMed] [Google Scholar]
- 6.Rivadeneira DE, Steele SR, Ternent C, Chalasani S, Buie WD, Rafferty JL. Standards Practice Task Force of The American Society of Colon and Rectal Surgeons: Practice parameters for the management of hemorrhoids (revised 2010). Dis Colon Rectum. 2011;54:1059–1064. doi: 10.1097/DCR.0b013e318225513d. [DOI] [PubMed] [Google Scholar]
- 7.Wang JY, Lu CY, Tsai HL, Chen FM, Huang CJ, Huang YS, Huang TJ, Hsieh JS. Randomized controlled trial of LigaSure with submucosal dissection versus Ferguson hemorrhoidectomy for prolapsed hemorrhoids. World J Surg. 2006;30:462–466. doi: 10.1007/s00268-005-0297-1. [DOI] [PubMed] [Google Scholar]
- 8.Chik B, Law WL, Choi HK. Urinary retention after haemorrhoidectomy: Impact of stapled haemorrhoidectomy. Asian J Surg. 2006;29:233–237. doi: 10.1016/S1015-9584(09)60094-4. [DOI] [PubMed] [Google Scholar]
- 9.Pescatori M. Closed vs. open hemorrhoidectomy: Associated sphincterotomy and postoperative bleeding. Dis Colon Rectum. 2000;43:1174–1175. doi: 10.1007/BF02236571. [DOI] [PubMed] [Google Scholar]
- 10.Pattana-Arun J, Sooriprasoet N, Sahakijrungruang C, Tantiphlachiva K, Rojanasakul A. Closed vs ligasure hemorrhoidectomy: A prospective, randomized clinical trial. J Med Assoc Thai. 2006;89:453–458. [PubMed] [Google Scholar]
- 11.Sugimoto T, Tsunoda A, Kano N, Kashiwagura Y, Hirose K, Sasaki T. A randomized, prospective, double-blind, placebo-controlled trial of the effect of diltiazem gel on pain after hemorrhoidectomy. World J Surg. 2013;37:2454–2457. doi: 10.1007/s00268-013-2124-4. [DOI] [PubMed] [Google Scholar]
- 12.Chung CC, Cheung HY, Chan ES, Kwok SY, Li MK. Stapled hemorrhoidopexy vs. harmonic scalpel hemorrhoidectomy: A randomized trial. Dis Colon Rectum. 2005;48:1213–1219. doi: 10.1007/s10350-004-0918-z. [DOI] [PubMed] [Google Scholar]
- 13.Cheetham MJ, Phillips RK. Evidence-based practice in haemorrhoidectomy. Colorectal Dis. 2001;3:126–134. doi: 10.1046/j.1463-1318.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 14.Carapeti EA, Kamm MA, McDonald PJ, Chadwick SJ, Phillips RK. Randomized trial of open versus closed day-case haemorrhoidectomy. Br J Surg. 1999;86:612–613. doi: 10.1046/j.1365-2168.1999.01127.x. [DOI] [PubMed] [Google Scholar]
- 15.Bae K. Medicinal Plants of Korea. Kyo-Hak Pub, Seoul. 2000:p. 261. [Google Scholar]
- 16.Piao XL, Piao XS, Kim SW, Park JH, Kim HY, Cai SQ. Identification and characterization of antioxidants from Sophora flavescens. Biol Pharm Bull. 2006;29:1911–1915. doi: 10.1248/bpb.29.1911. [DOI] [PubMed] [Google Scholar]
- 17.Kuroyanagi M, Arakawa T, Hirayama Y, Hayashi T. Antibacterial and antiandrogen flavonoids from Sophora flavescens. J Natural Products. 1999;62:595–1599. doi: 10.1021/np990051d. [DOI] [PubMed] [Google Scholar]
- 18.Yen CH. The pharmacology of Chinese herbs. 1st ed. Taipei. Chin-Yin Publishing. 1999:376–377. [Google Scholar]
- 19.Yang JH, Hwang YH, Gu MJ, Cho WK, Ma JY. Ethanol extracts of Sanguisorba officinalis L. suppress TNF-α/IFN-γ-induced pro-inflammatory chemokine production in HaCaT cells. Phytomedicine. 2015;22:1262. doi: 10.1016/j.phymed.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Dong YC, Shang YZ. Advances of Scutellaria Barbata in pharmacology. J Chengde Med Coll. 2009;26:98–100. [Google Scholar]
- 21.Pharmacopoeia of People’s Republic of China (2010) [S]. Beijing. China Medical Science Press. 2010:96–97. [Google Scholar]
- 22.Chu NS. Cardiovascular responses to betel chewing. J Formos Med Assoc. 1993;92(9):835– 837. [PubMed] [Google Scholar]
- 23.Lu K, Zhang C, Wu W, Zhou M, Tang Y, Peng Y. Rhubarb extract has a protective role against radiation-induced brain injury and neuronal cell apoptosis. Mol Med Rep. 2015;2:2689–2694. doi: 10.3892/mmr.2015.3693. [DOI] [PubMed] [Google Scholar]
- 24.Wang JJ, Chen XQ, Wang W, Zhang Y, Yang ZY, Jin Y, Ge HM, Li E, Yang G. Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease. J Ethnopharmacol. 2013;147:114–121. doi: 10.1016/j.jep.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu QB, Hong S, Zheng Xl. Progress in preparation methodology of animal models of hemorrhoids. Chinese J Comp Med. 2007;17:119–122. [Google Scholar]