Abstract
Background/Aim: Clinically used disinfectants are often irritating and cause skin problems. Ozone water is unique among disinfectants. It does not damage skin cells and readily decomposes to oxygen without generating harmful residues. On the other hand, it rapidly loses its sanitizing activity. Recently developed nano-bubble ozone water (NBOW) can keep its sanitizing activity much longer. This study aimed to examine the microbicidal effects of NBOW after long-term storage. Materials and Methods: The concentration of ozone in NBOW was examined by measuring the NBOW redox potential. Microbicidal activity was evaluated by colony formation assays, after incubating bacteria with NBOW for set time periods. Results: NBOW lost its microbicidal activity after 1 year of storage at 4˚C. Stocked frozen, NBOW retained appreciable microbicidal activity after 1 year of storage. Mycobacterium smegmatis, one of the most disinfectant-resistant bacteria, was killed within 15 min. NBOW was resistant to freeze-thawing. Conclusion: NBOW that had been stored frozen possessed sufficient microbicidal activity to kill bacteria even after 1 year of storage. Moreover, it was shown that NBOW is freeze-thaw resistant. NBOW possesses desirable features rendering it an attractive alternative disinfectant.
Keywords: Nano-bubble water, disinfectant, ozone
Ozone, discovered in 1839, is a triatomic molecule that is widely used in the industry because of its strong oxidizing ability. The use of ozone was first highlighted in water treatment (1). Ozone effectively inactivates microorganisms and removes organic stuff in water. Its strong sanitizing activity is also exploited in the food industry (2,3). In the clinical field, ozone is often used to sanitize soft tissues, e.g. mucosa. Ozone therapy utilizes ozone to increase the amount of oxygen in the blood and is used for treating cancer and other diseases (4-7).
Ozone exists as a pale blue gas with a pungent odour. Highly concentrated ozone is harmful to humans but ozone in aqueous solutions is less toxic (8). Moreover, ozone in aqueous solution auto-decomposes rapidly to produce oxygen and leaves no harmful residues. These features render ozone one of the best sanitizers in the food science and water industries. On the other hand, stocking ozone aqueous solutions is challenging. The half-life of ozone in aqueous solution at 20˚C is generally considered to be 20-30 min (9). The solution should be used within 5-10 min of production to assure its microbicidal activity (9). On-site generation is, therefore, required for usage.
Nano-bubble ozone water (NBOW) was developed to overcome this drawback (10). Nano-bubbles are small (less than 200 nm in diameter) and have unique properties, such as long life in solution and high solubility in solution (11). The microbicidal activity of NBOW was first reported by Hayakumo et al. and shown to be sufficient to kill periodontopathic bacteria in vitro (10). Further, NBOW is not cytotoxic to oral tissues (10).
The aim of this study was to examine the microbicidal activity of NBOW after long-term storage. The freeze-thaw sensitivity of NBOW was also tested.
Materials and Methods
Bacterial strains, sample preparation and culture conditions. Escherichia coli W3110 was cultured in LB broth at 37˚C for full growth. For the colony formation assay, E. coli W3110 was grown on LB agar plates. Mycobacterium smegmatis was grown in Middlebrook 7H9 broth (BD Biosciences, San Jose, CA, USA) at 37˚C. Middlebrook agar 7H10 (BD Biosciences, San Jose, USA) plates were used in the colony-forming assay.
NBOW and ozone concentration measurements. Commercially available NBOW, (NAnO3; OPT CREATION INC., Yokohama, Japan), was used. NAnO3 was delivered frozen and was once thawed and dispensed. The dispensed NAnO3 was frozen and stored at –20˚C. The ozone concentration was examined indirectly by measuring its redox potential. Redox potential was measured using the 4-aminoantipyrine visual colorimetric method (KYORITSU CHEMICAL-CHECK Lab., Tokyo, Japan).
Freeze-thaw experiments. The dispensed NAnO3 was thawed at room temperature (25˚C) just before use and the ozone concentration was measured. Immediately after measurement, NAnO3 was frozen and stored at -20˚C.
Colony formation assays. E. coli W3110 was cultured in LB broth at 37˚C for full growth (107 colony-forming units (CFU)/ml). M. smegmatis was cultured in Middlebrook 7H9 broth at 37˚C for full growth (108 CFU/ml). For the colony formation assays, 100 μl of the bacterial suspension was mixed with 900 μl of NAnO3. A portion of the mixture was spread on agar plates at set time points. Colonies were counted after incubation at 37˚C. Unless mentioned otherwise, the experiments were performed in triplicate.
Results
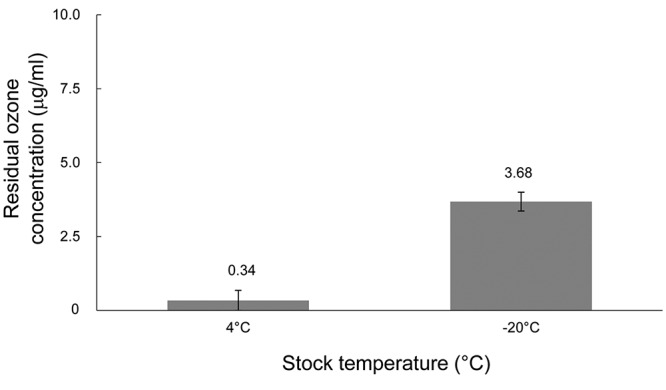
Sustainability of NBOW. The sustainability of NBOW was tested at 25˚C and 4˚C. NBOW lost 90% of ozone after a week of storage at 25˚C (Figure 1A). NBOW retained more than 90% of ozone after a week of storage at 4˚C; 65% of ozone was retained after a month of storage at this temperature (Figure 1B). After 1 year of storage at 4˚C, almost all NBOW ozone had gone. The measured residual ozone concentration was 0.34 μg/ml (Figure 2). By contrast, stored frozen NBOW retained a high ozone concentration; the actual residual ozone concentration was 3.68 μg/ml (Figure 2).
Figure 1. Residual ozone concentrations in nano-bubble ozone water (NBOW) during storage. A: Residual ozone concentration in NBOW stored at 25˚C for 1 week. B: Residual ozone concentration in NBOW stored at 4˚C for 1 month. The experiments were repeated three (A) or five (B) times. The values are presented as the means±SD.

Figure 2. Residual ozone concentrations in nano-bubble ozone water (NBOW) stored for extended periods of time. The residual ozone concentrations in NBOW stored for 1 year at different temperatures (4°C and –20°C) are shown. The experiments were performed in triplicate and the values are presented as the means±SD. The actual mean ozone concentrations are denoted above the bars.
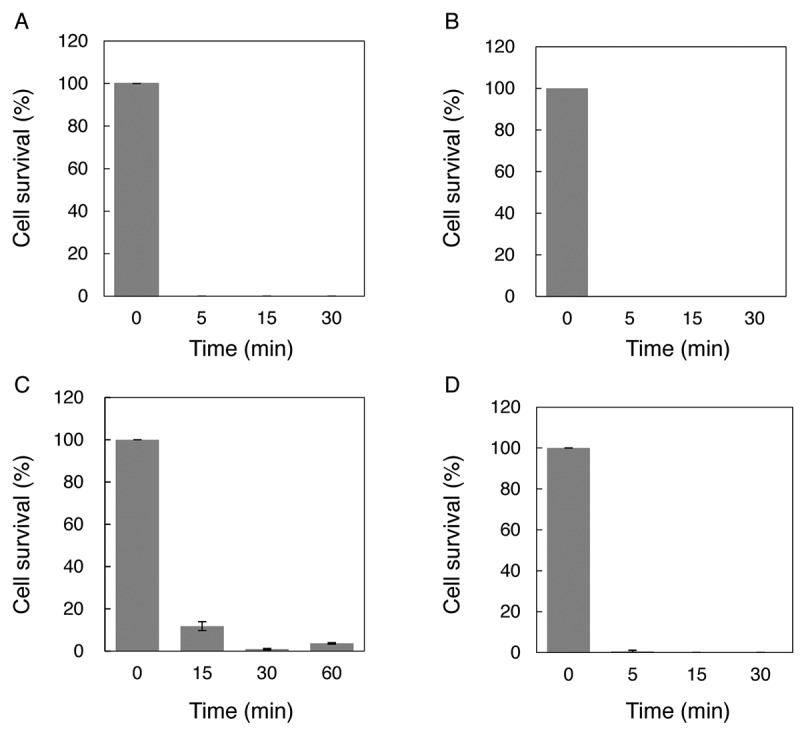
Microbicidal activity of NBOW after long-term storage. To evaluate the microbicidal activity of NBOW after long-term storage, colony formation assays were performed. The microbicidal activity of NBOW was tested against a Gram-negative bacterium, E. coli W3110. Most cells survived a 15-min exposure to NBOW that had been stored at 4˚C, with ca. 60% of the cells surviving a 60-min exposure (Figure 3). NBOW that had been stored frozen was then tested. NBOW stored frozen for 1 month retained sufficient microbicidal activity to kill E. coli W3110 within 5 min (Figure 4A); NBOW stored frozen for 3 months killed E. coli W3110 within 15 min (Figure 4B); NBOW stored frozen for 1 year killed >95% of E. coli W3110 within 60 min (Figure 4C). M. smegmatis, one of the most disinfectant-resistant bacteria, was then tested. Similarly to its effect on E. coli, NBOW stored frozen for 1 year also killed all M. smegmatis cells within 15 min.
Figure 3. Microbicidal activity of nano-bubble ozone water (NBOW) stored for 1 year at 4˚C. The microbicidal activity of NBOW was tested using E. coli W3110. The colony formation assay is described in Materials and Methods. The experiments were performed in triplicate and the values are presented as the means±SD.
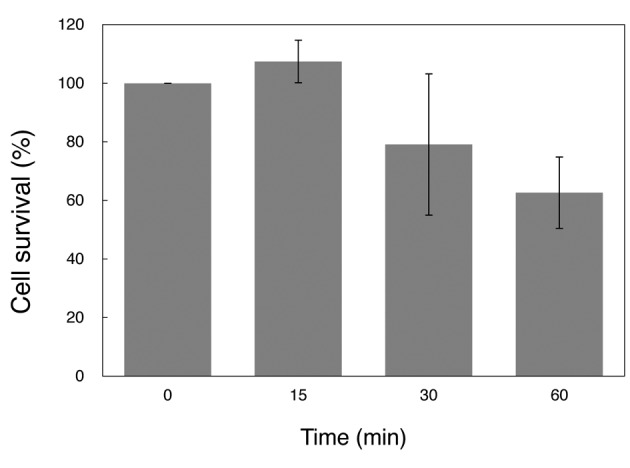
Figure 4. Microbicidal activity of nano-bubble ozone water (NBOW) that had been stored frozen for up to 1 year. The microbicidal activity of NBOW was first tested with E. coli W3110: A: Microbicidal activity of NBOW stored frozen for 1 month; B: Microbicidal activity of NBOW stored frozen for 3 months; C: Microbicidal activity of NBOW stored frozen for 1 year. The microbicidal activity of NBOW stored frozen for 1 year was also tested with M. smegmatis (D). The colony formation assay is described in Materials and Methods. The experiments were performed in triplicate and the values are presented as the means±SD.
Freeze-thaw sensitivity testing. The initial ozone concentration in the dispensed frozen NBOW was ca. 30 mg/l. The ozone concentration did not change after the first freeze-thaw cycle. It was slightly reduced after four cycles of freeze-thawing and ca. 90% of ozone was retained (Figure 5).
Figure 5. Sensitivity of nano-bubble ozone water (NBOW) to freezethawing. Frozen NBOW was thawed at 25˚C and the redox activity of the solution was measured. NBOW was then frozen in a freezer (–20˚C). The experiments were repeated four times and the values are presented as the means±SD.

Discussion
Infectious diseases are still a serious problem for society. We have partially succeeded in controlling infectious diseases by developing antibiotics and antivirals; however, changes in society led to new problems, namely, the acquisition of microbial resistance to these drugs.
The use of disinfectants is a fundamental method of hygiene management. It has been used for a long time as a cost-effective method of controlling infectious diseases. In fact, most bacteria and viruses do not acquire resistance to disinfectants.
Strong disinfectants, such as chlorine and alcohol, have been most widely used in the clinical field. These disinfectants are irritating compounds and cannot be applied to soft tissues, such as mucosa. Instead, povidone iodine is used to sanitize the oral mucosa and eyes; however, residual povidone iodine is problematic because of its cytotoxicity. The development of an alternative disinfectant is, hence, of utmost importance. In this regard, ozone water is an interesting alternative. Unlike other strong disinfectants, it does not damage the skin cells and repeated sanitizing does not result in skin roughness. Moreover, ozone rapidly decomposes to oxygen and leaves no harmful residues after use. On the other hand, ozone water rapidly loses its sanitizing activity, necessitating on-site generation. This is a marked drawback and prevents ozone water from being widely used. In the current study, we showed that frozen NBOW retains enough microbicidal activity after 1 year of storage. In addition, multiple freezing and thawing cycles did not reduce the ozone concentration in NBOW. These features of NBOW obviate the need for on-site generation of ozone water.
Although NBOW can be easily handled by freezing and convenience of usage is expected, limitations for clinical applications still exist. Hayakumo et al. (10) reported its application in eradicating periodontopathic bacteria. The authors suggested that clinical usage of NBOW is not straightforward because saliva and bacterial biofilm may reduce its microbicidal activity. In this regard, a pathogenic mycobacterium, M. avium, also forms biofilms by oxidative stress, similarly to the periodontopathic bacteria mentioned above(12). In fact, M. avium is resistant to most strong disinfectants, including ozone water (12). The ozone sensitivity of Mycobacterium might vary by species and all pathogenic mycobacteria, including M. tuberculosis and M. leprae, should be tested.
Ozone can be easily consumed by oxidizable organic stuff and reductants present in its surroundings (13). Notably, in our in vitro experiments, NBOW that had been stored frozen for 1 year killed one of the most resistant bacteria, M. smegmatis, within 15 min; however, E. coli W3110 was not entirely killed even after a 60-min exposure. It is probable that oxidizable organic substances and reductants in the media may have consumed the ozone. Taken together, further examination is necessary before practical usage of NBOW.
NBOW is characterized by remarkable usability. We have not yet tested NBW3, another NBOW described by Hayakumo et al., and, therefore, we cannot expand the findings described herein to all the available NBOW. Nevertheless, the sustainability of NAnO3 is not affected by freeze-thawing and NAnO3 exhibited enough microbicidal activity even after 1 year of frozen storage. Considering the above, NBOW succeeded in overcoming the pronounced practical drawbacks associated with the use of ozone water and its wide usage is expected.
Acknowledgements
This work was not financially supported. The Authors have no financial conflicts of interest.
References
- 1.Joss A, Siegrist H, Ternes TA. Are we about to upgrade wastewater treatment for removing organic micropollutants. Water Sci Technol. 2008;57:251–255. doi: 10.2166/wst.2008.825. [DOI] [PubMed] [Google Scholar]
- 2.Moore G, Griffith C, Peters A. Bactericidal properties of ozone and its potential application as a terminal disinfectant. J Food Prot. 2000;63:1100–1106. doi: 10.4315/0362-028x-63.8.1100. [DOI] [PubMed] [Google Scholar]
- 3.Kim JG, Yousef AE, Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J Food Prot. 1999;62:1071–1087. doi: 10.4315/0362-028x-62.9.1071. [DOI] [PubMed] [Google Scholar]
- 4.Morishima A, Inagawa H. Clinical effects of orally administered lipopolysaccharide derived from pantoea agglomerans on malignant tumors. Anticancer Res. 2016;36:3747–3751. [PubMed] [Google Scholar]
- 5.Inui T, Amitani H, Kubo K, Kuchiike D, Uto Y, Nishikata T, Mette M. Case report: A non-small cell lung cancer patient treated with GcMAF, sonodynamic therapy and tumor treating fields. Anticancer Res. 2016;36:3767–3770. [PubMed] [Google Scholar]
- 6.Schulz S, Häussler U, Mandic R, Heverhagen JT, Neubauer A, Dünne AA, Werner JA, Weihe E, Bette M. Treatment with ozone/oxygen-pneumoperitoneum results in complete remission of rabbit squamous cell carcinomas. Int J Cancer. 2008;122:2360–2367. doi: 10.1002/ijc.23382. [DOI] [PubMed] [Google Scholar]
- 7.Luongo M, Brigida AL, Mascolo L, Gaudino G. Possible therapeutic effects of ozone mixture on hypoxia in tumor development. Anticancer Res. 2017;37:425–435. doi: 10.21873/anticanres.11334. [DOI] [PubMed] [Google Scholar]
- 8.Stokinger HE. Evaluation of the hazards of ozone and oxides of nitrogen; factors modifying toxicity. AMA Arch Ind Health. 1957;15:181–190. [PubMed] [Google Scholar]
- 9.Tomiyasu H, Fukutomi H, Gordon G. Kinetics and mechanism of ozone decomposition in basic aqueous solution. Inorganic Chemistry. 1985;24:2962–2966. [Google Scholar]
- 10.Hayakumo S, Arakawa S, Takahashi M, Kondo K, Mano Y, Izumi Y. Effects of ozone nano-bubble water on periodontopathic bacteria and oral cells – in vitro studies. Sci Technol Adv Mater. 2014;15:055003. doi: 10.1088/1468-6996/15/5/055003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Ng WJ, Liu Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere. 2011;84:1175–1180. doi: 10.1016/j.chemosphere.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Geier H, Mostowy S, Cangelosi GA, Behr MA, Ford TE. Autoinducer-2 triggers the oxidative stress response in Mycobacterium avium, leading to biofilm formation. Appl Environ Microbiol. 2008;74:1798–1804. doi: 10.1128/AEM.02066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgassi S, Zanardi I, Travagli V, Montomoli E, Bocci V. How much ozone bactericidal activity is compromised by plasma components. J Appl Microbiol. 2009;106:1715–1721. doi: 10.1111/j.1365-2672.2008.04141.x. [DOI] [PubMed] [Google Scholar]


