Abstract
Background/Aims: Breast cancer (BCa) prognostication is a vital element for providing effective treatment for patients with BCa. Studies suggest that ethnicity plays a greater role in the incidence and poor prognosis of BCa in younger women than in their older counterparts. Therefore, the goal of this study was to assess the association between age and ethnicity on the overall final prognosis. Materials and Methods: Nottingham Prognostic Index (NPI) was used to analyze BCa prognosis using Howard University Cancer Center Tumor Registry and the National Cancer Institute’s Surveillance, Epidemiology, and End Results BCa datasets. Patients were grouped according to their predicted prognosis based on NPI scheme. Results: There was no correlation between the younger patients compared to their older counterparts for any of the prognostic clusters. The significance of ethnicity in poorer prognosis for younger age is not conclusive either. Conclusion: An extended prognostic tool/system needs to be evaluated for its usefulness in a clinical practice environment.
Keywords: Breast cancer, prognosis, African–American
Breast cancer is the most common cancer of females in the USA, the second most common cause of cancer death in women (1), and the main cause of death in women ages 40 to 59 years (2). In 2016, it was estimated that 249,260 new cases of breast cancer would be diagnosed, with 40,890 breast cancer deaths, and about 246,660 women and 2,600 men would be diagnosed with invasive breast cancer. Worldwide, breast cancer is the most frequently diagnosed cancer among women in 140 out of 184 countries, according to the World Cancer Research Fund International (3).
Medical prognostication is an evaluative component of medicine that encompasses the science of estimating the complication and recurrence of disease and predicted survival of patients (4). A large number of factors, including tumor grade, tumor size, and lymph node status including other aspects may influence or correlate with prognosis for patients with breast cancer.
Studies suggest that younger patients tend to have a poorer prognosis compared to their older counterparts (5-17). In addition, these studies express the suspicion that the incidence of breast cancer and prognosis in younger women differ according to ethnicity (18-20).
Existing work on breast cancer generally use the term prognosis for patients as being excellent, good, or poor based on pathological information, including tumor size, tumor grade, lymph node involvement and other prognostic factors. However, to our knowledge, there has been no report on the comparison of patient prognosis based on younger age and ethnicity with respect to the prognostic groups that the patients belonged to. Therefore, in this study we present an analysis of the prognosis for younger patients with breast cancer. We have applied the Nottingham Prognostic Index (NPI) (21) scheme to two breast cancer data sets Howard University Cancer Center (HUCC) Tumor Registry and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) (22) to group the patients based on their prognosis (i.e., excellent, good, moderate, poor). Subsequently, we were able to compare patient prognosis with respect to age (younger and older) as well as ethnicity.
Materials and Methods
NPI analysis of breast cancer prognosis in young patients. Studies suggest that prognosis tends to be more aggressive in younger women than in older women. Breast cancer risk factors, clinical outcomes, and tumor biology are somewhat different in the subgroup of women younger than 40 years, suggesting that breast cancer in young women represents a distinct entity (6,8,23,24). The definition of a ‘young woman’ in the field of breast oncology varies, with some referring to women under either age 35, 40 or 45 years as ‘young’ (25). In addition, the incidence of breast cancer and the prognosis in younger women differs according to ethnicity.
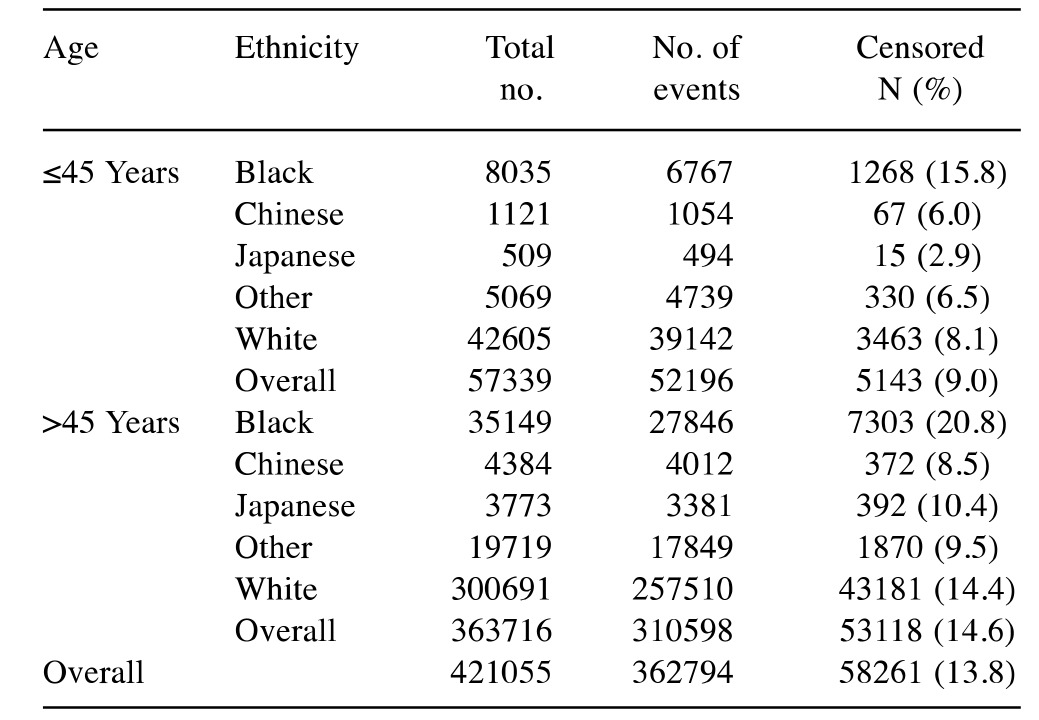
In order to better understand the association between the patients’ prognosis and the younger age and ethnicity, we used the NPI approach in grouping the patients according to their prognosis (21). Two age categories were analyzed: young group (≤45 years old) and older group (≥45 years old). Our analyses were based on the HUCC (years 1995-2013) and the SEER (1998-2013) breast cancer data sets for all patients with invasive breast cancer. By grouping the patients based on their prognosis, our goals were two-fold: i) to determine if age of younger woman has any significance on the patients’ prognosis for a prognostic group (i.e. excellent prognosis), and ii) to determine if ethnicity has any influence on patient prognosis for the younger aged patients (i.e. poor prognosis/black and white/younger and older).
The NPI is another prognostic tool used widely in Europe to determine prognosis following surgery for breast cancer. Its value is calculated using three pathological criteria: the size of the tumor, the number of involved lymph nodes, and the grade of the tumor. The index is calculated using the formula: NPI=[0.2×S] + N + G), where S is the size of the tumor in centimeters, N is the number of lymph nodes involved (scored as 0=1, 1-3=2, ≥3=3), and G is the tumor grade (scored as grade I=1, grade II=2, grade III=3). The interpretation of the NPI is as follows: Score ≥2.0 to ≤2.4, excellent prognosis, and 5 year-survival rate 93%; score ≥2.4 to ≤3.4, good prognosis, 5 year-survival rate 85%; score ≥3.4 to ≤5.4, moderate prognosis, 5 year-survival rate 70%; score ≥5.4, poor prognosis, and 5 year-survival rate 50%.
Statistical analyses. Estimates were considered statistically significant for two-tailed values of p<0.05. All analyses were carried out using the SPSS 12. 0 statistical program (SPSS Inc., Chicago, IL, USA). While the Cox model compares covariates using an overall hazard ratio, the Kaplan-Meier (KM) survival curve (26) compares the groups for single factors. It can be used to assess the statistical significance of any observed differences in a single covariate. Therefore, KM survival analyses were carried out to examine the correlation between the variables age, overall survival, and disease-free survival.
Results
Aged-based comparison of prognosis of breast cancer. We analyzed the HUCC breast cancer dataset using the NPI prognostic tool. We considered patients of age 45 years or less as young and those over 45 years as old. A total of 37.74% of young Black patients were found to have a ‘poor prognosis’ compared to 25.97% of older patients (Table I). The young patients were found to have less chance of survival than the older patients. We tested the differences using a two proportion sampling with summary hypothesis testing and the difference was statistically significant (p=0.0029). For the other prognostic categories (i.e., excellent, good, and moderate), the difference between the patients by age was not statistically significant (p≥0.05).
Table I. Nottingham Prognostic Index prognosis categories based on ethnicity and age for Howard University Cancer Center Breast Cancer Dataset for Black patients.
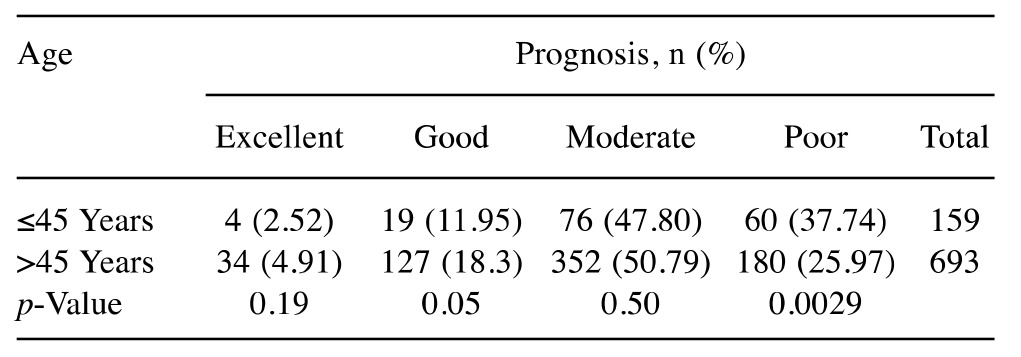
N: Total number
In order to further investigate the notion that age (young ≤45 years and old ≥45 years) is correlated with a poorer prognosis, we used the NPI tool on SEER breast cancer data set (1998-2013) with more than 400,000 records (Table II). Similar to the HUCC breast cancer dataset, data analysis showed that 30.40% of young Black patients had a poor prognosis compared to 21.49% of older patients (p<0.0001). The percentage of young Black patients with moderate prognosis was significantly higher than that of the older ones (p<0.0001). However, a significantly higher percentage of old Black patients had excellent and good prognoses than did the young patients (p<0.0001). We should point out that for prognostic categories based on the HUCC dataset (Table I), the differences between the young and old Black patients in the prognostic categories of excellent, good, and moderate were not statistically significant (p≥0.05). The percentage of those with a poor prognosis of both young Black and White patients was higher (30.40% and 22.65%, respectively; p<0.0001) than that of old patients (21.49% and 13.55%, respectively; p<0.0001). Considering the data for young White patients in Table II, similarly to young Black patients, the percentage of those with a poor prognosis (22.05%) was significantly higher than that for old White patients (13.55%) (p<0.0001). Regarding the other prognostic categories of excellent, good, and moderate, the differences between the young and old White patients were also statistically significant (p<0.0001). Overall, old White patients appear to fare better than young patients in all prognostic categories.
Table II. Nottingham Prognostic Index prognosis categories based on ethnicity and age for the National Institute Health’s Surveillance, Epidemiology, and End ResultsBreast Cancer Data set.
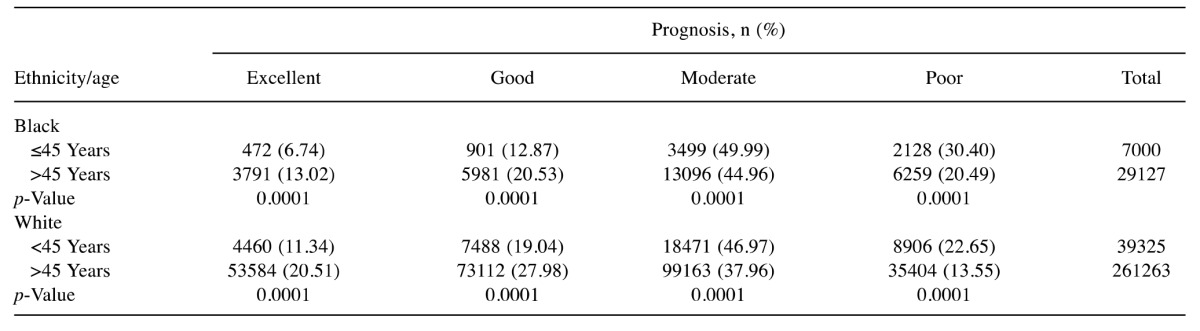
N: Total number
Ethnicity-based comparison of breast cancer prognosis. Data analysis (Table II) shows that the rate of young Black patients with a poor prognosis was higher than that for their White counterparts (30.40% vs. 22.65%; respectively; p<0.0001). Likewise, for moderate prognosis, the difference was statistically significant (p<0.0001). Similar results were also observed for the old Black and White patients for the poor and moderate prognosis categories. In the excellent and good prognosis categories, the percentage of young Black patients was significantly lower compared to young White patients (6.74% vs. 11.34%, respectively; p<0.0001).
Survival analysis for breast cancer prognosis for young patients. KM survival analyses were performed to examine the correlation between age, overall survival, and disease-free survival (Figures 1-5).
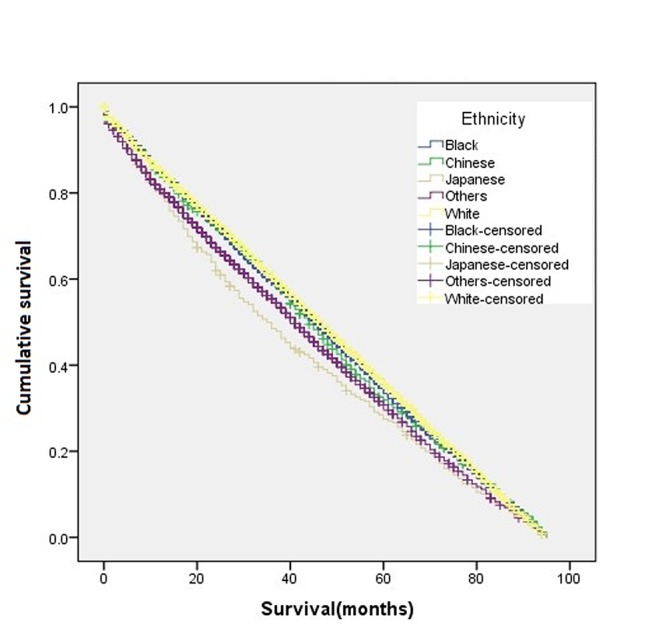
The overall disease-free survival rate of the young patients (age ≤45 years) was slightly higher than that of the old patients (age >45 years) (Figure 1). A two-sample proportion hypothesis test showed a statistically significant difference in the survival rates (p<0.0001). Table III shows the data summary of the KM survival for surviving patients by age. The curve for survival by age group for Black patients alone shows young patients fare better than old patients (Figure 2). Similar results were also observed for the Chinese, Other, and White groups. The summary of the KM survival analysis by age and ethnicity is shown in Table IV. In all ethnic groups, the differences in the survival rates between the young and old patients were statistically significant (for Chinese p=0.0057; p<0.0001 for the remaining groups). For the Japanese patients, the survival rate for the old patients was significantly higher than that of the young patients (Figure 3). Old Japanese and White patients fared better than Black, Chinese, and Other groups (Figure 4). Young White patients had the best and young Japanese patients had the worst survival rates (Figure 5).
Figure 1. Kaplan-Meier disease-free survival curve of the whole study population according to age.
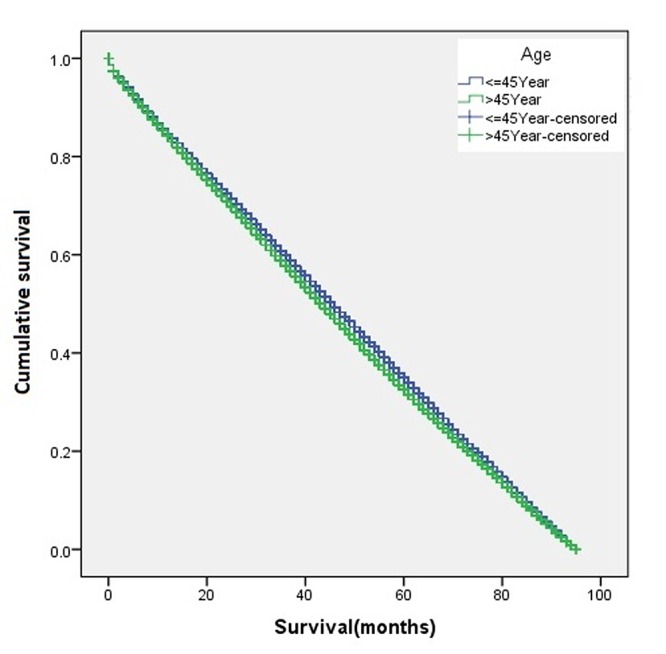
Table III. Kaplan–Meier case processing data summary for age attribute.
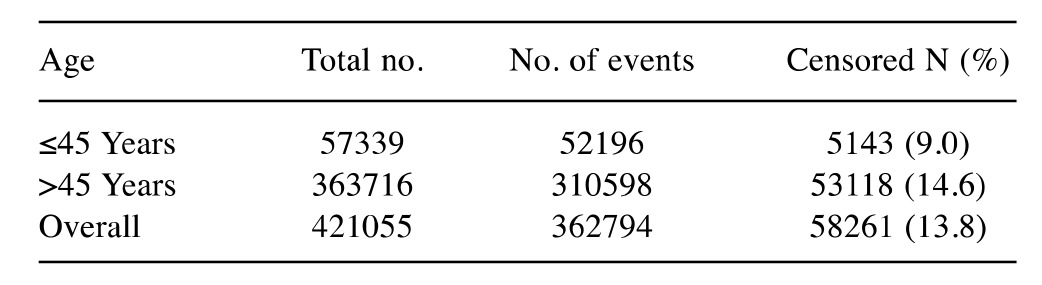
N: Total number
Figure 2. Kaplan-Meier curve for disease-free survival according to age for the Black ethnic subgroup.
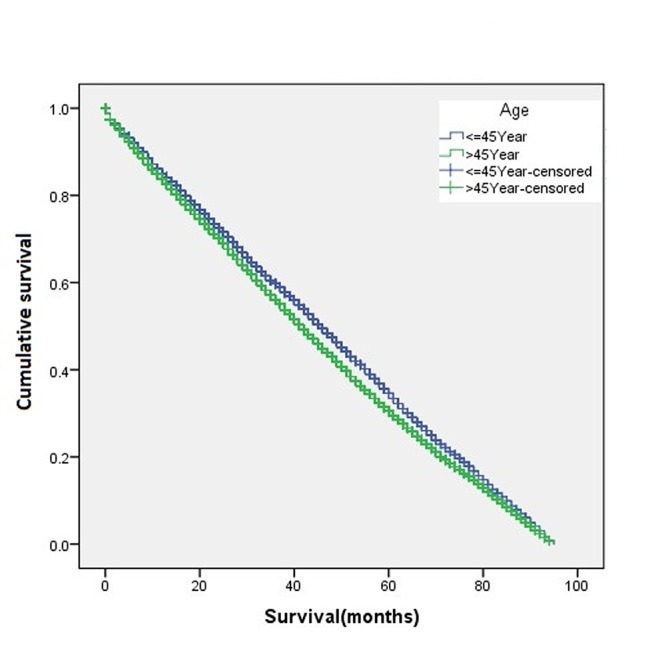
Table IV. Kaplan–Meier case processing data summary for age groups within ethnicity.
N: Total number
Figure 3. Kaplan-Meier survival curve according to age for the ethnic subgroup Japanese.
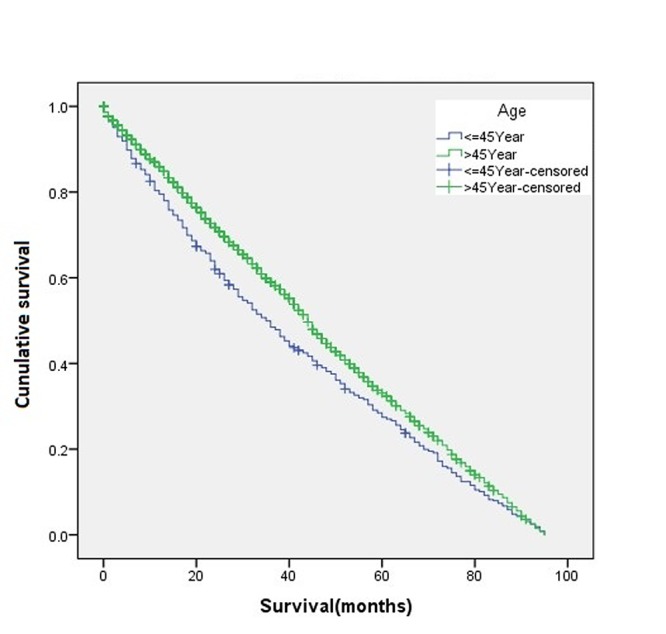
Figure 4. Kaplan-Meier disease-free survival curve for patients aged >45 years according to ethnic group.
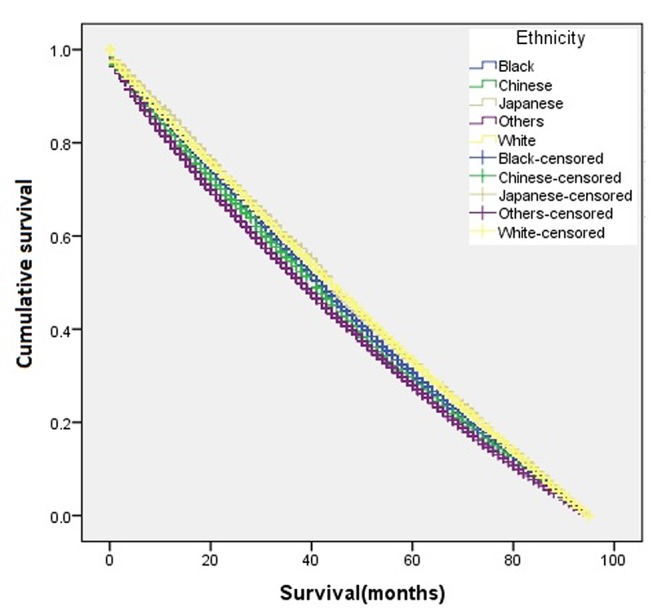
Figure 5. Kaplan-Meier disease-free survival curve for patients aged ≤45 years according to ethnic group.
Considering the disease-free survival with death from breast cancer as the event, young patients had better 5-year survival rates than the old ones (Table V). However, for those who survived more than 60 months, the old patients had better survival rates.
Table V. The summary of the Kaplan–Meier disease-free survival duration by ethnicity for the attribute cause of death=alive within age groups.
N: Total number
The KM survival-months by ethnicity and strata by age shows that younger Japanese and Chinese patients had the highest survival rates, whereas Black patients presented the lowest survival rates. Likewise, among older patients, Japanese and Chinese patients had the highest and Black patients had the lowest survival rates. The KM survival-months by age and strata by ethnicity shows that the younger Black patients had highest survival rates for survival-months ≤60 and lowest survival rates for the survival-months ≥60 compared to the old patients. Similar results have been observed for younger Chinese and White patients. For other ethnic (includes Pacific Islanders, etc.) groups, the younger patients had mostly higher survival rates. Comparably, in the Japanese, the younger patients always had higher survival rates than the older patients.
Discussion
The findings from Table I, the HUCC dataset, can be summarized as prognosis tended to be poor in young Black women than in old Black women, but the difference was statistically significant, only in the poor prognosis category. However, such differences in the other prognostic categories (i.e. moderate) were not statistically significant. This result, of course, may be the result of the small sample size. In contrast, the NIH SEER Breast Cancer Data analysis showed that old White patients fared better than the young patients in every prognostic category. Overall, young patients had a worse prognosis when compared to the old group for the poor prognosis category in both the HUCC and SEER datasets.
Medical prognosis is a field in medicine that encompasses the science of estimating the complication and recurrence of disease and to predict survival of patient (27-31). Survival analysis is a field in medical prognosis that deals with the application of various methods to estimate the survival of a particular patient suffering from a disease. Several studies have been carried out on the survivability prediction of breast cancer using naïve Bayes and classification trees, artificial neural networks and statistical techniques of regression (27-37). Delen and colleagues used data-mining algorithms artificial neural networks, decision trees, and logistic regression to develop breast cancer prediction models (28). They found that the decision tree (C5) is the best predictor, with 93.6% accuracy on the holdout sample; whereas artificial neural networks were next with 91.2% accuracy and the logistic regression models performed the worst of the three with 89.2% accuracy. Investigators have used the association rules data-mining technique (35,38,39) and data mining tool XLMiner (40) to investigate the significance of the ethnic factor on patient prognosis. The results, the association rules, suggested that ethnicity had some significance in the survivability rate of the patient.
Breast cancer incidence increases with age, with the vast majority of women being diagnosed after the age of 40 years (9,13,15-17,41-43). Breast cancer is rare in young women. Approximately 7% of women with breast cancer are diagnosed before the age of 40 years. The comparison of clinicopathological and prognostic features of breast cancer arising in younger women with those in their older counterparts has been the subject of discussion in several studies (8,9,12,14). The majority of the reports suggest that young patients with breast cancer have poorer outcomes compared to their older counterparts, which in part can be attributed to later stage disease, more aggressive tumors, and less favorable receptor status (9,12,15,44). This holds true regardless of menopausal status, as age is still a risk factor among premenopausal women. In addition, breast cancer survival rates are comparatively lower for women less than 40 years of age than for older women across all histological subtypes and stages (9). The controversy lies in the question of whether age per se is an independent risk factor for worse prognosis. Many studies have refuted this hypothesis (42,45-47); they rather propose that the effect of young age on outcome is merely a reflection of over-representation of other known prognostic pathological factors, such as higher grade of differentiation, presence of lymphovascular invasion, higher mitotic rate, lower ER/PR expression, and higher HER2 expression (7). Yet other studies have attributed the inferior outcome of those with young age to the more advanced presentation at diagnosis, including higher rates of axillary lymph node positivity and larger tumor size (42). Others have postulated that the effect of differential gene expression between different age groups might play a role (10). In any case, knowing the true impact of age on prognosis may have an effect on treatment management. If age is indeed an independent factor, then young women might benefit from more aggressive treatment than their older counterparts with the same clinical and pathological scenario (15).
Despite discrepancies in adverse prognostic features, younger age has been shown in several studies to be an independent predictor of adverse outcome (9). However, this issue is now considered controversial. Breast cancer in young women is more likely to be of a more aggressive subtype, such as triple-negative or HER2-positive breast cancer, and is more likely to present at an advanced stage, either because of its biological aggressive subtype or because of a low index of suspicion and delayed diagnosis (5). Dubsky et al. studied 885 premenopausal patients, and investigated the relationship between age, typical prognostic factors, treatment, and patient outcome (43). Young age was seen as an independent prognostic factor. According to these findings, patients diagnosed with breast cancer at ≤35 years of age had a worse prognosis compared to premenopausal women above this age.
While younger women have more aggressive disease compared with older women, it appears that ethnicity may further influence breast cancer prognosis (18,20,47). The large SEER Program (22) and National Cancer Data Base (24) analyses indicated that African American women have more advanced disease at presentation and a higher death rate compared with non-Hispanic Caucasians. Data demonstrating the relationship of age and prognosis within premenopausal cohorts are much scarcer and conflicting (43). Liu et al. looked at the ethnic disparities in breast cancer diagnoses and disease-specific survival (DSS) rates in the United States between Asians American and other ethnic groups, particularly among Asian American subgroups, in women aged 18-39 years (18). Asian patients had the least advanced disease at presentation and the lowest risk of death compared with the other groups. All of the Asian subgroups except the Hawaiian/Pacific Islander subgroup had better DSS than non-Hispanic White, black, and Hispanic-White patients. In summary, the clinical and pathological features of breast cancer at presentation differ by ethnicity in the United States, and these differences impact survival in women younger than 40 years. Walter et al. studied the influence of ethnicity on the prognosis of young patients (≤35 years) with breast cancer (20). Their study showed that Latinos suffer more aggressive disease and poorer prognosis than other young women.
Most of the existing studies reflect single-institution experiences that may not be representative of the whole population. In addition, existing reports did not attempt to compare the patients based on younger age and ethnicity as well as the patients’ prognostic group. This motivated us to look at prognosis for patients with breast cancer with respect to age and ethnicity with a different approach based on prognostic groups (namely excellent, moderate, good, poor). Our analysis of the predicted prognostic groups as defined in NPI do not show that young patients have worse prognosis in all prognostic categories. Moreover, in some prognostic categories (namely excellent, and good), the differences between the groups were not statistically significant.
Conclusion and Future Direction
This study was designed to develop a better understanding of the association between breast cancer prognosis, age and ethnicity. We used a local and national population-based cancer registry and based on NPI scheme we grouped the patients according to their predicted prognosis (excellent, good, moderate and poor). The data did not reveal any significant correlation between age as a dichotomous factor or ethnicity the different categories of prognostic groups. Similar results were found using the KM survival analysis, indicating that there is no conclusive evidence to suggest that younger patients with breast cancer have poorer prognosis than their older counterparts.
Current medical literature suggests that factors such as diet, work/occupational environment, genetics, family history, obesity, and access to healthcare could influence survival. Additional studies should be conducted based on these factors to determine their impact on prognosis in connection with age and ethnicity. The process in which existing breast cancer prognostic tools (e.g. NPI) should be further evaluated in order to accommodate age and ethnicity factors in determining the survivability rate of patients with breast cancer more accurately. Factors can be ranked based on their significance in overall survival (using sensitivity analysis, feature/factor selection, principle component analysis etc.) and weighted according to their ranking. Finally, such an extended prognostic tool/system needs to be evaluated for its usefulness in a clinical practice environment.
Conflicts of Interest
The Authors have no personal or financial conflicts of interest and have not entered into any agreement that could interfere with our access to the data on the research, or upon our ability to analyse the data independently, to prepare manuscripts, and to publish them.
References
- 1.American Cancer Society Breast Cancer Facts & Figure. American Cancer Society. 2016 https://www.cancer.org/research/cancer-facts-statistics/breastcancer-facts-figures.html. [Google Scholar]
- 2.Siegel R, Naishadham, Jemal D, Jemal A. Cancer Statistics. CA Cancer J Clin. 2012;62(10):6210–6229. [Google Scholar]
- 3.Breast Cancer Research Foundation (BCRF) 2016. https://www.bcrfcure.org/search?search_api_multi_fulltext=statistics+resources. [Google Scholar]
- 4.Ohno-Machado L. Modeling medical prognosis: survival analysis techniques. J Biomed Inform. 2001;34(6):428–239. doi: 10.1006/jbin.2002.1038. [DOI] [PubMed] [Google Scholar]
- 5.Althuis MD, Brogan DD, Coates RJ, Daling JR, Gammon MD, Malone KE, Schoenberg JB, Brinton LA. Breast cancers among very young premenopausal women (United States) Cancer Causes Control. 2003;14(2):151–160. doi: 10.1023/a:1023006000760. [DOI] [PubMed] [Google Scholar]
- 6.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. [PubMed] [Google Scholar]
- 7.Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, Perou CM. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes. J Clin Oncol. 2011;29(1):e18–20. doi: 10.1200/JCO.2010.28.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2011;29(27):3721. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 9.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36(3):237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azim HA Jr., Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, Haibe-Kains B, Piccart MJ, Sotiriou C, Loi S. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18(5):1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 11.Cox proportional-hazards regression. Cox proportional-hazards regression. http://www.medicine.ox.ac.uk/bandolier/painres/download/cox_model.pdf. [Google Scholar]
- 12.El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB, Shamseddine AI. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4(11):e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5(S1):S2–S8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Love RR, Duc NB, Dinh NV, Quy TT, Xin Y, Havighurst TC. Young age as an adverse prognostic factor in premenopausal women with operable breast cancer. Clin Breast Cancer. 2002;2(4):294–298. doi: 10.3816/cbc.2002.n.005. [DOI] [PubMed] [Google Scholar]
- 17.Maggard MA, O’Connell JB, Lane KE, Liu JH, Etzioni DA, Ko CY. Do young breast cancer patients have worse outcomes. J Surg Res. 2003;113(1):109–113. doi: 10.1016/s0022-4804(03)00179-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu P, Li X, Mittendorf EA, Li J, Du XL, He J, Ren Y, Yang J, Hunt KK, Yi1 M. Comparison of clinicopathologic features and survival in young American women aged 18-39 years in different ethnic groups with breast cancer. Br J Cancer. 2013;109(5):1302–1309. doi: 10.1038/bjc.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shavers VH, Harlan LC, Stevens J. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97:134–47. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 20.Biffl WL, Myers A, Franciose RJ, Gonzalez RJ, Darnell D. Is breast cancer in young Latinas a different disease. The American Journal of Surgery. 2001;182:596–600. doi: 10.1016/s0002-9610(01)00789-9. [DOI] [PubMed] [Google Scholar]
- 21.Galea MH, Blamey RW, Elstone CE, Ellis IO. The Nottingham Index in Primary breast cancer. Breast Cancer Res Treat. 1992;22(3):207–219. doi: 10.1007/BF01840834. [DOI] [PubMed] [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) Program: Public-Use Data (1973-2004). National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. https://seer.cancer.gov/ [Google Scholar]
- 23.Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, Luini A, Veronesi P, Intra M, Orecchia R, Catalano G, Galimberti V, Nolé F, Martinelli G, Goldhirsch A. Very young women (≤35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13(2):273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 24.Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78:1838–1843. doi: 10.1002/(sici)1097-0142(19961015)78:8<1838::aid-cncr27>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel CA, Domchek SM. Breast cancer in young women. Breast Cancer Res. 2010;12(5):212. doi: 10.1186/bcr2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costanza ME, Chen WY. Epidemiology and risk factors for breast cancer. http://cursoenarm.net/UPTODATE/ contents/mobipreview.htm?40/12/41153?source=see_link#. 2011 [Google Scholar]
- 27.Bellaachia A, Guven E. Predicting Breast Cancer Survivability Using Data Mining Techniques. http://www.siam.org/meetings/sdm06/work proceed/Scientific%20Datasets/bellaachia.pdf?q=data-mining . 2006 [Google Scholar]
- 28.Delen D, Walker G, Kadam A. Predicting breast cancer survivability: a comparison of three data mining methods. Artif Intell Med. 2005;34(2):113–27. doi: 10.1016/j.artmed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Endo A, Shibata T, Tanaka H. Comparisons of seven algorithms to predict breast cancer survival. IJBSCHS. 2008;13(2):11–16. [Google Scholar]
- 30.Gupta S, Kumar D, Sharma A. Data Mining Classification Techniques Applied For Breast Cancer Diagnosis And Prognosis. IJCSE. 2011;2 [Google Scholar]
- 31.Jonsdottir T, hvannberg ET, sigurdsson H, sigurdsson S. The feasibility of constructing a predictive outcome model for breast cancer using the tools of data mining. Expert Systems with Applications. 2008;34:108–118. [Google Scholar]
- 32.Delen D. Analysis of cancer data: a data mining approach. IJCSEIT. 2009;26(1):100–112. [Google Scholar]
- 33.Gadewadikar J, Kuljaca1 O, Agyepong K, Sarigul E, Zheng Y, Ping ZP. Exploring Bayesian networks for medical decision support in breast cancer detection. AJMCSR. 2010;3(10):225–231. [Google Scholar]
- 34.Kharya S. Using data mining techniques for diagnosis and prognosis of cancer disease. IJCSEIT. 2012;2(2):55–56. [Google Scholar]
- 35.Owrang OM, Hosseinkhah F. Association Rules Mining for Breast Cancer Survivability Prediction. Proceedings of the 28th International Conference on Computers and Their Applications (CATA-2013), March 4-6, Honolulu, Hawaii, 2013;P:159–165. [Google Scholar]
- 36.Breast Cancer Risk Assessment Tool: Gail Model. http://ww5.komen.org/BreastCancer/GailAssessmentModel.html [Google Scholar]
- 37.National Cancer Institute: Breast Cancer Risk Assessment Tool. http://www.cancer.gov/bcrisktool/ [Google Scholar]
- 38.Owrang O, Mehdi M. The Significance of the Ethnicity Factor in Breast Cancer Prognosis. The 10th International Conference on Data Mining, DMIN’14, Las Vegas, Nevada, July 21-24. 2014 [Google Scholar]
- 39.Owrang O, Mehdi M. Understanding the Significance of the Breast Cancer Prognostic Factors on Survivability”, Proceedings of the 30th International Conference on Computers and Their Applications (CATA-2015) 2015 [Google Scholar]
- 40.XLMiner On Line, User Manual. http://www.solver.com/ xlminer-data-mining [Google Scholar]
- 41.Gabriel CA, Domchek SM. Breast cancer in young women. Breast Cancer Res. 2010;12:212. doi: 10.1186/bcr2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajdos C, Tartter PI, Bleiweiss IJ, Bodian C, Brower ST. Stage 0 to stage iii breast cancer in young women. J Am Coll Surg. 2000;190(5):523–529. doi: 10.1016/s1072-7515(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 43.Dubsky PC, Gnant MF, Taucher S, Roka S, Kandioler D, Pichler-Gebhard B, Agstner I, Seifert M, Sevelda P, Jakesz R. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3(1):65–72. doi: 10.3816/CBC.2002.n.013. [DOI] [PubMed] [Google Scholar]
- 44.Bharat A, Aft RL, Gao F, Margenthaler JA. Patient and tumor characteristics associated with increased mortality in young women (≤or =40 years) with breast cancer. J Surg Oncol. 2009;100(3):248–251. doi: 10.1002/jso.21268. [DOI] [PubMed] [Google Scholar]
- 45.Jimor S, Al-Sayer H, Heys SD, Payne S, Miller I, Ah-See A, Hutcheon A, Eremin O. Breast cancer in women aged 35 and under: prognosis and survival. J R Coll Surg Edinb. 2002;47(5):693–699. [PubMed] [Google Scholar]
- 46.Kothari AS, Fentiman IS. Breast cancer in young women. Int. J. Clin Pract. 2002;56(3):184–187. [PubMed] [Google Scholar]
- 47.Baquet CR, Mishra SI, Commiskey P, Ellison GL, DeShields M. Breast cancer epidemiology in Blacks and Whites: Disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc. 2008;100(5):480–488. doi: 10.1016/s0027-9684(15)31294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


