Abstract
Background.
Neuroblastoma (NB) is the most common extracranial solid tumour in children. This is a very rare disease with heterogeneous biology varying from complete spontaneous regression to a highly aggressive tumour responsible for 15% of malignancy-related death in early childhood. Analyses of survival rates in Europe have shown a considerable difference between Northern/Western and Eastern European countries. Treatment results of NB in Lithuania have never been analyzed.
Aim.
To assess the survival rate of children with NB according to initial spread of the disease, age at diagnosis, the MYCN amplification, risk group, and treatment period.
Patients and methods.
A retrospective single-centre analysis of patients’ records was performed. Children diagnosed and treated for NB between 2000 and 2015 at the Centre of Paediatric Oncology and Haematology of the Children’s Hospital, Affiliate of Vilnius University Hospital Santaros Klinikos were included. The patients were divided into three groups according to the spread of the disease: group 1 – patients with local NB older than 12 years of age; group 2 – stage IV patients, also called the M stage; group 3 – infants with stages 4S and MS. The patients were stratified into three risk groups – low, intermediate and high risk. Estimates of five-year overall survival (OS5y) were calculated using the Kaplan-Meier method comparing survival probability according to spread of the disease, age at diagnosis, the MYCN amplification, risk group and treatment period (2000–2007 vs 2008–2015).
Results.
Overall 60 children (31 girls and 29 boys) with NB were included. The median age at diagnosis was 1.87 years (ranged from 4 days to 15 years). Seventy-eight percent of cases were found to be differentiated or undifferentiated NB, 22% – ganglioneuroblastoma. The local form of the disease was predominant: 57% (34/60) of patients were allocated to the group 1, 37% (22/60) with initial metastatic disease were assigned to group 2, and infants with 4S or MS stage comprising 7% (4/60) allocated to group 3, respectively. The probability of OS5y for the entire cohort was 71% with the median follow-up of 8.8 ± 4.8 years. The probability of OS5y for local disease (group 1) was significantly higher compared to metastatic disease (group 2) (94% vs. 34%, p = 0.001, respectively) as well as for infants compared to children older than 12 months at the time of diagnosis (90% vs 60%, p = 0.009, respectively). The MYCN gene amplification had a negative influence on OS5y, with 78% of MYCN-negative patients surviving in comparison to 40% of MYCN-positive patients who did not survive (p = 0.153). The high-risk patients had significantly worse OS5y than children with intermediated or low risk (35% vs. 82% vs. 100%, respectively, p = 0.001). Comparison of OS5y between two treatment periods in the entire patient population revealed a non-significant increase in survival from 66% in the 2000–2007 period to 82% in the 2008–2015 period (p = 0.291), mostly due to a dramatic improvement achieved for high-risk patients whose survival rate increased from 9% in the 2000–2007 period to 70% in the 2008–2015 period (p = 0.009).
Conclusions.
There was a slight predominance of low-risk patients, probably due to a higher number of infants. A better probability of OS5y was confirmed in infants with local disease and in MYCN-negative patients. The OS5y for children treated for NB at our institution over 16 years increased from 66% in the 2000–2007 period to 82% in the 2008–2015 period with the most significant improvement achieved for high risk patients. The current survival rate of children treated for NB at our institution is in line with the reported numbers in Northern and Western European countries.
Keywords: neuroblastoma, overall survival, risk groups, MYCN amplification
Abstract
NEUROBLASTOMOS GYDYMO REZULTATAI LIETUVOJE: VIENO CENTRO PATIRTIS
Santrauka
Neuroblastoma (NB) – dažniausias vaikų ekstrakranijinis solidinis navikas, embriogenezės metu kilęs iš simpatinės nervų sistemos, randamas antinksčių šerdinėje dalyje, simpatiniuose ganglijuose ir paraganglijuose. NB yra reta mažų vaikų onkologinė liga, ypatinga savo heterogenine klinikine išraiška: nuo visiškos naviko spontaninės regresijos iki labai agresyvių ir gydymui atsparių formų.
Tyrimo tikslas. Įvertinti NB ligos atvejų 2000–2015 m. Vaikų ligoninės, Vilniaus universiteto ligoninės Santaros klinikų filialo Vaikų onkohematologijos centre gydymo rezultatus. Siekėme išsiaiškinti ligonių pasiskirstymą pagal amžių, lytį, ligos stadiją, nustatyti penkerių metų bendrą išgyvenamumą (BI5m) priklausomai nuo amžiaus, ligos išplitimo (stadijos), MYCN geno amplifikacijos, rizikos grupės ir gydymo laikotarpio.
Tyrimo metodika. Atlikta retrospektyvinė vaikų, kuriems diagnozuota NB 2000–2015 m., ligos analizė. Pagal ligos išplitimą ligoniai suskirstyti į tris grupes: I grupė – pacientai su lokalia NB vyresni nei 12 mėn., II grupė – pacientai su metastazine liga ir III grupė – kūdikiai su 4S ir MS. Priklausomai nuo amžiaus, ligos stadijos ir MYCN geno amplifikacijos pacientai taip pat suskirstyti į mažos, vidutinės ir didelės rizikos grupes.
Rezultatai. Analizuoti 60 pacientų (31 mergaitė ir 29 berniukai) duomenys. Vidutinis amžius diagnozės nustatymo metu buvo 1,87 metai (svyravo nuo 4 dienų iki 15 metų). Pasiskirstymas pagal histologinį tipą: 47 iš 60 pacientų (78 %) diagnozuota blogai diferencijuota arba nediferencijuota NB, 13 (22 %) vaikų – ganglioneuroblastoma, 34 iš 60 pacientų (57 %) diagnozuota lokali liga (I grupė), 37 % – metastazinė liga (II grupė), 7 % – kūdikiai su metastazine liga (III grupė). Visų tiriamųjų BI5m sudarė 71 %. Esant lokaliai formai BI5m siekė 94 %, palyginti su 34 % metastazinės ligos atveju (p = 0,001). Ligą diagnozavus kūdikiams, BI5mBI5m buvo patikimai didesnis (90 %) nei vyresnių vaikų – 60 % (p = 0,009). MYCN geno amplifikacija turėjo neigiamą įtaką bendram išgyvenamumui – išgyveno 78 % pacientų, neturėjusių MYCN geno amplifikacijos, palyginus su 40 % pacientų, kuriems MYCN geno amplifikacija buvo nustatyta (p = 0,153). BI5m statistiškai patikimai skyrėsi priklausomai nuo rizikos grupės ir sudarė 35, 82 ir 100 % (p = 0,001) atitinkamai didelės, vidutinės ir mažos rizikos grupių pacientų. Lyginant skirtingus gydymo laikotarpius visos tirtos kohortos BI5m padidėjo nuo 66 % 2000–2007 m. iki 82 % 2008–2015 m. (p = 0,291), daugiausia dėl didelės rizikos pacientų, kurių BI5m reikšmingai padidėjo nuo 9 % 2000–2007 m. iki 70 % 2008–2015 m. (p = 0,009).
Išvados. Dominavo lokali ligos forma. Didžiausią tikimybę išgyventi penkerius metus turėjo kūdikiai su lokalia ligos forma bei neigiama MYCN amplifikacija. Pacientų, gydytų dėl NB mūsų centre 2000–2015 m., BI5m padidėjo nuo 66 iki 82 %, daugiausia dėl reikšmingo pagerėjimo gydant didelės rizikos grupės pacientus. Mūsų onkohematologijos centro NB gydymo rezultatai atitinka Šiaurės ir Vakarų Europos klinikų rezultatus.
Raktažodžiai: neuroblastoma, išgyvenamumas, rizikos grupės, MYCN amplifikacija
INTRODUCTION
Neuroblastoma (NB) is the most common extracranial solid tumour in children that develops from the sympathetic nervous system during embryogenesis and is found in the medulla of adrenal glands, sympathetic ganglia and paraganglia (1). NB represents 7% of malignant tumours diagnosed between birth and 14 years of age. It is a very rare disease that develops in one of 70,000 children in the above-mentioned age group (www.opha.net). In Lithuania, six to eight new cases are diagnosed each year.
An international multi-centre study that analysed the survival in the cases of tumours in children has shown that the survival in Eastern Europe is lower than that in Northern, Western, and Central European countries (2). For example, the 5-year overall survival (OS5y) of children with NB treated in Northern European countries from 2005 to 2007 was 79.6%, meanwhile, in Eastern Europe the percentage was 61.6%. Results of NB treatment in Lithuania have not been analysed before.
Almost 15% of children’s deaths from progressing cancer are related to NB (3). NB is characterised by a great variety of prognostic markers, which results in a heterogeneous expression of the disease varying from complete spontaneous regression to highly aggressive forms unresponsive to treatment. Due to its heterogeneity, the disease is divided into several risk groups with different course of the disease, different therapy requirements, and prognosis.
Taking into account the differentiation level of tumour cells and the ratio of neuroblasts and tumour stroma, NB is classified into undifferentiated, poorly differentiated, and differentiated NB.
When NB is diagnosed, the MYCN gene has the decisive influence on prognosis. The significance of the MYCN gene amplification in the pathogenesis of NB has been first described in 1980 and has been associated with high-risk tumours and low survival of patients. The MYCN gene is located in the 2nd chromosome and its hyperexpression is mostly associated with the advanced stage, rapid progression of disease, and poor prognosis even with localised tumours. Poor prognosis of the disease is also associated with various aberrations of other chromosomes: deletion of 1p, 3p, 14q and 11q, duplication of 1q, 11p and 17q, and others. Meanwhile, the changes in the number of chromosomes (hyperploidy) are associated with a good prognosis (4).
Approximately 1–2% of patients with NB are diagnosed with a familial form. It is associated with the hyperexpression of ALK and PHOX2B genes (5).
In many European countries including Lithuania, the INSS (International Neuroblastoma Staging System) postoperative staging system based on the anatomical location and resectability of the tumour was used for a long time. In 2009, a new stage classification system – INRGSS (International Neuroblastoma Risk Group Staging System) – was introduced, which has four stages of the disease. Based on this system, a localised tumour can have stages L1 and L2 depending on risk factors (the position of the tumour in relation to nearby structures such as large vessels, nerve plexuses, and adjacent organs) identified by imaging during diagnosing (6). A metastatic disease also has two stages (M and MS) that correspond to stages 4 and 4S of the INSS system (Table 1).
Table 1.
International Neuroblastoma Risk Group Staging System (INRGSS) (6)
| Stage | Description |
|---|---|
| L1 | Localized tumour not involving vital structures as defined by the list of image-defined risk factors and confined to one body compartment |
| L2 | Locoregional tumour with presence of one or more image defined risk factors |
| M | Distant metastatic disease (except stage MS) |
| MS | Metastatic disease in children younger than 18 months with metastases confined to skin, liver, and/or bone marrow |
The treatment strategy and prognosis of various NB forms differ substantially depending on the patient’s age, the disease stage, the histological type, and molecular genetic markers. Based on the entirety of the above-listed indicators, as much as 16 different therapeutic groups are identified. For instance, patients with stage L1 require only surgical treatment but do not need chemotherapy or radiotherapy. Infants with stage MS usually do not undergo any treatment because the tumour regresses spontaneously. Meanwhile, patients with stage M need a very complicated combined treatment which is often not limited to first-line chemotherapy but also includes second-line or even third-line chemotherapy as well as surgical treatment, high-dose chemotherapy with autologous hematopoietic stem cell transplantation, and immunotherapy. This treatment requires a well-coordinates and experienced multidisciplinary team who carefully coordinated each phase of treatment. Each step in this treatment (which sometimes may last for more than one year) is very important since the prognosis for patients with the metastatic form and unfavourable genetic markers is poor. At the Centre of Paediatric Oncology and Haematology (CPOH), patients with NB are treated according to the SIOPEN (International Society of Paediatric Oncology Europe Neuroblastoma) treatment protocols.
The summary of our 16-year experience in the treatment of this rare heterogeneous tumour is presented below. The aim of our research was the analysis of forms and treatment results in patients diagnosed with NB treated at the CPOH. We intended to ascertain the distribution of patients by age, gender, and stage of disease and to determine the overall survival in relation to age, spread of disease (stage), mutation of the MYCN gene, risk group, and treatment period.
MATERIALS AND METHODS
A retrospective single-centre study based on the data accumulated in the CPOH patient database and medical documentation was performed. We analysed the medical records of the children diagnosed with NB (undifferentiated, poorly differentiated, or ganglioneuroblastoma) between 2000 and 2015. Patients diagnosed with ganglioneuroma, i.e., a benign form of NB not requiring combined treatment, were excluded.
Before 2013, the disease stages were determined on the basis of the INSS staging system (stages 1, 2, 3, 4 and 4S). Since 2013, the CPOH has been using the INRGSS staging system (stages L1, L2, M and MS).
Based on the spread of disease, the patients were divided into three groups: group 1 of localised NB (INSS stages 1–3 and INRGSS stages L1 and L2), group 2 of metastatic disease (INSS stage 4 and INRGSS stage M), and group 3 consisting of children under 12 months of age with a metastatic disease (INSS stage 4S and INRGSS stage MS).
Based on their age at diagnosis, children were divided into two groups: under 12 months of age (35%) and 12 months and older (65%). The subjects were divided into three risk groups. The low-risk group included all patients under 18 months of age at the time of diagnosis with a localised form and all patients under 12 months of age with stage 4S or MS. The intermediate-risk group included patients older than 18 months with a localised form and infants under 12 months of age with stage 4 or M. The highrisk patients were children older than 12 months with a metastatic disease and all patients with a detected MYCN gene amplification.
Overall survival was assessed taking into account the stage, age at the time of diagnosis, the risk group, the MYCN amplification and treatment period (2000–2007 and 2008–2015). Survival analysis was carried out using the Kaplan-Meier estimation method. The significance of differences in the distribution of survival data was assessed using a log-rank test. The selected level of statistic significance was 0.05.
RESULTS
From 2000 to 2015, 65 patients with the diagnosis of NB were identified in the CPOH database. One child left Lithuania to live and be treated abroad and four patients were diagnosed with benign form (ganglioneuroma), so we did not include these patients into the final analysis. Therefore, we reviewed the data of 60 children.
The analysed characteristics of the patients are summarised in Table 2. Based on the histological type, the majority of children (47 of 60, 78%) had a poorly differentiated or undifferentiated NB, and 13 children (22%) were diagnosed with ganglioneuroblastoma. The study cohort consisted of 31 girls (52%) and 29 boys (48%). The median age at the time of diagnosis was 1.87 years (ranging from 4 days to 15 years).
Table 2.
Patients’ characteristics (n = 60)
| Patients’ characteristics | N (%) | |
|---|---|---|
| Histologic type | Neuroblastoma | 47 (78) |
| Ganglioneuroblastoma | 13 (21) | |
| Sex | Girls | 31 (52) |
| Boys | 29 (48) | |
| Age at diagnosis | <12 months | 22 (37) |
| ≥12 months | 38 (63) | |
| INSS stage 2000–2012 (n = 46) | 1 | 3 (7) |
| 2 | 13 (28) | |
| 3 | 8 (17) | |
| 4 | 19 (41) | |
| 4S | 3 (7) | |
| INRGSS stage 2013–2015 (n = 14) | L1 | 3 (21) |
| L2 | 7 (50) | |
| M | 3 (21) | |
| MS | 1 (7) | |
| Group of stage | Group 1 – localised neuroblastoma (stages I–III according to INSS and L1, L2 according to INRGSS) | 34 (57) |
| Group 2 – metastatic disease (4 stage according to INSS and M stage according to INRGSS) | 22 (37) | |
| Group 3 – infants with 4S and MS stages | 4 (7) | |
| Risk groups | Low | 27 (45) |
| Intermediate | 12 (20) | |
| High | 21 (35) | |
In terms of the disease stage according to INSS system from 2000 to 2012 (inclusive), the majority of 46 patients (24/46, 52%) were diagnosed with a localised form, i.e., stages 1–3, while a metastatic disease, i.e., stage 4, was diagnosed in 19 out of 46 children (41%). Localised forms were also prevailing in the period from 2013 to 2015 when the disease stage was determined according to the INRGSS system: a localised NB (stages L1 and L2) was diagnosed in 10 out of 14 (71%) patients and metastatic form was diagnosed in 3 out of 14 (21%) patients. Infants diagnosed with stage 4S or MS according to the previous and current staging systems represented 7%, respectively.
Having distributed the patients into the abovementioned groups based on the initial spread of the disease, all 60 subjects were distributed as follows: group 1: 57% (34 children), group 2: 37% (22 children), and group 3: 6% (4 infants). Hence, the localised stage of the disease was the most common form, while stages 4S or MS were the least common. The characteristics of patients are summarised in Table 2.
Infants under 12 months of age were mostly diagnosed with a localised NB and older children were mostly diagnosed with a metastatic disease. Out of 22 infants, 15 (68%) patients were diagnosed with a localised form of disease and 7 (32%) patients were diagnosed with a metastatic disease, of which 3 patients had stage 4 or M, and 4 patients had stage 4S or MS. Out of 38 children aged 12 months or older, 19 (50%) patients were diagnosed with a localised NB and the remaining 19 (50%) were diagnosed with a metastatic disease (stage 4 or M).
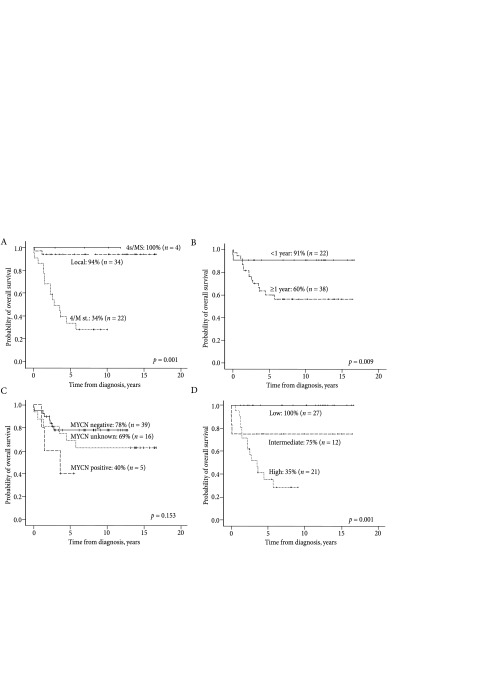
The OS5y of all patients was 71 ± 6% with the average follow-up time of 8.8 ± 4.8 years. Based on the spread of disease, the OS5y of patients older than 12 months with a metastatic disease was significantly lower (Fig. 1A): in the case of a localised NB, OS5y was 94 ± 4% in comparison to 34 ± 11% (p = 0.001) in the case of a metastatic NB (stage 4 or M). The exception was patients from Group 3 (younger than 12 months of age with stage 4S or MS), who had a particularly good OS5y (100%). There was a substantial difference in OS5y depending on the age at diagnosis regardless of the spread of disease (Fig. 1B): when the disease was diagnosed in infants under 12 months, OS5y was 91 ± 6%, and when it was diagnosed in children aged 12 months or older it was only 60 ± 9% (p = 0.009).
A very important prognostic marker is the MYCN gene amplification. Before 2004, the MYCN gene amplification test was carried out for individual patients at various foreign clinics; from 2004 to 2011, this test was performed for all children at the Children’s Cancer Research Institute in Vienna, and since 2011, the MYCN gene amplification has been routinely tested at the Lithuanian National Centre of Pathology. A positive MYCN gene amplification was found in five patients. It had a negative influence on OS5y with 78 ± 7% of MYCN-negative patients surviving in comparison to 40 ± 22% of MYCN-positive survivors (p = 0.153) (Fig. 1C). The OS5y of children with an untested MYCN amplification was 63 ± 12%.
In terms of risk groups, the low-risk group consisted of 27 patients (45%), the intermediate-risk group had 12 patients (20%), and the high-risk group consisted of 21 patients (35%). It has been found that there were no deaths in the low-risk group (100% survival), but the OS5y of the subjects from the intermediate-risk group was 75 ± 12% (Fig. 1D), and the prognosis of the high-risk patients was even worse: only 35 ± 11% of children had the probability of 5-year survival (p = 0.001). It must be noted that two patients from the intermediate-risk group died of major haemorrhage before the initiation of chemotherapy: one child died after biopsy and the second one died of acute haemorrhage from cystic NB before biopsy. The main reasons of death of all other non-surviving patients were NB relapse or progression. No children died from complications caused by chemotherapy. At the moment of analysis, none of the patients had a secondary tumour with the average fpllow-up time being 8.8 ± 4.8 years.
Fig. 1.
The overall survival rate according to the group of stages (A), years at diagnosis (B), the MYCN amplification (C), and risk groups (D)
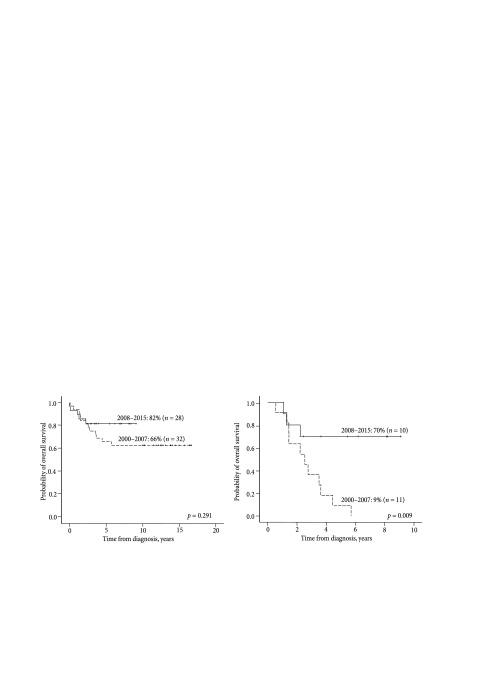
The analysis of overall survival based on treatment periods (2000–2007 and 2008–2015) has shown that treatment results have improved: OS5y was 66 ± 8% during the first period and 82 ± 6% during the second period (Fig. 2A), although the difference is not statistically significant (p = 0.291). The overall survival of the low-risk group was 100% in both periods. There were no significant differences in the survival rate of the intermediate-risk patients between the compared treatment periods, although OS5y decreased from 83% in 2000–2007 to 67% in 2008– 2015 (p = 0.529). However, in the high-risk patient group OS5m significantly increased from 9 ± 9% to 70 ± 15% (p = 0.009) in 2000–2007 and 2008–2015, respectively (Fig. 2B).
Fig. 2.
The overall survival rate according to the treatment period: (A) all patients (n = 60) and (B) patients of the high risk group (n = 22)
DISCUSSION
Neuroblastoma (NB) is the most common extracranial solid tumour in children representing 7% of all cancer cases in children and almost 15% of children’s deaths from oncology diseases (3). It is an embryonic tumour of the sympathetic nervous system and, together with ganglioneuroblastoma and benign ganglioneuroma, it belongs to the group of neuroblastic tumours. This tumour is most often located in adrenal glands, although it can also develop along the sympathetic trunk in the neck, chest, or abdomen. Based on literature (7), girls are more likely to suffer from NB than boys (1.3 : 1); in our research, there were slightly more girls than boys as well (1.1 : 1).
In the majority of cases, NB is not hereditary: familial neuroblastomas represent only about 1% of all cases (8). According to the study by Rios et al., congenital malformations (mostly in skeletal and urogenital systems) and a foetus being too small or too large for its gestational age are related to an increased NB risk (1). And, on the contrary, enriching mother’s diet with folic acid, vitamins or minerals for three months before conception and breastfeeding were related to a lower risk of NB development.
Based on literature, the average age of patients at diagnosis is two years (9), with 40% of cases diagnosed in infants younger than one year and 85% diagnosed in children under five years of age (10). In our research, children under two years of age also dominant: the median age at diagnosis was 1.87 years with 37% of cases diagnosed in children under 12 months of age and 82% of cases diagnosed in patients under five years. The clinical expression of NB is highly varied: in some patients, the tumour may regress spontaneously while in others it progresses despite intense multimodal therapy (5). The metastatic disease in infants, the so-called stage MS (4S according to the INSS), represents 7–10% of all NB cases (11). It is a unique, neuroblastoma-specific stage, which, despite its massive metastasising to the liver, skin, and bone marrow, is usually characterised by a low risk and a good prognosis. This stage of NB has the highest spontaneous regression frequency among all malignant tumours although in some cases, particularly in infants younger than two months of age, the disease can rapidly progress with mortality rate being 10–20% (12). The most common reason of death in such cases is hypoalbuminaemia caused by massive liver metastases, coagulopathy, and mechanically induced inferior vena cava syndrome (13).
Since spontaneous regression is typical of NB in infants, the tactics of waiting and monitoring is first offered to patients with the MS stage and to infants with a localised adrenal gland NB that was accidentally discovered during an ultrasonic scan (14). Treatment is only offered when the disease progresses or life-threatening symptoms are present.
Based on published data (15), a localised disease is diagnosed in about 40% of cases, a metastatic disease represents about a half of all cases, and stage 4S represents about 5% of cases (3). Our results were a little different: localised neuroblastoma was the most common form (57%), the metastatic disease was diagnosed in 35% of cases, and stage 4S/MS represented 8% of cases. This disproportion in children’s distribution might have been caused by a comparatively small number of subjects. Also, we cannot exclude the possibility that the results might have been affected by the fact that some patients did not have the MYCN test and, therefore, it is possible that some of the patients with a localised form had the MYCN amplification and should have been included in the high-risk group.
Patients were divided into low-, intermediateand high-risk groups based on their age at the time of diagnosis, the spread of the tumour, and detection of the MYCN gene amplification. In the cases of low- and intermediate-risk groups, NB prognosis was usually good, particularly in patients under 12 months of age at diagnosis, with 5-year overall survival rate being more than 90%. The data of our research are similar to the multi-centre study (15) that analysed NB cases in the USA, Japan, Israel, and in six European clinics. The said study ascertained that low-risk patients had an 80% 5-year event-free survival and 98% overall survival. The overall survival of our low-risk patients was 100%.
The overall survival of the intermediate-risk group patients was 83% to 95% according to various studies (15–17), while in our study the overall survival of this group was lower (75%). With time, the overall survival of the intermediate-risk group patients decreased insignificantly from 83% in the period of 2000 to 2007 to 67% from 2008 to 2015 (p = 0.529). This can be associated with a small number of patients: we only had six children in each of these periods. During the second period, both non-surviving children died from acute haemorrhage before the start of chemotherapy (when biopsy was performed and tumour cysts ruptured) but not from disease progression or the complications of chemotherapy. Therefore, with these patients excluded from the assessment, the survival of the intermediate-risk group patients becomes even higher. During the first period, only one child out of six died from disease progression.
Contrary to these groups, in the case of high risk neuroblastomas (a metastatic disease in patients older than 12 months and patients of any age with the MYCN gene amplification), 5-year survival rate is only 40–50% despite very intense treatment consisting of chemotherapy, surgical treatment, radiotherapy, high-dose chemotherapy with the transplantation of hematopoietic stem cells, and immunotherapy (18, 19). After immunotherapy was introduced, 2-year survival of high-risk patients increased by approximately 20% (20) but so far no published data about a long-term survival are available. The survival of high-risk patients whom we analysed as a cohort is low, i.e., 35%. However, when comparing the survival of high-risk patients in different periods, there is a significant improvement in treatment results: from 9% in the 2000–2007 period to 70% in the 2008–2015 period (p = 0.009). This can be associated with improved diagnostics, particularly with genetic tests that are performed for each patient in recent years, as well as with improving treatment protocols and increasing experience of the whole medical team (paediatric haematologists, paediatric surgeons, radiologists, and radiotherapists). Since the end of 2014, neuroblastoma molecular karyotyping test has been available in Lithuania which is important for the stratification of neuroblastomas in infants under 12 months of age into smaller subgroups determining which patients should be administered treatment and which do not require treatment and should only be monitored.
The MYCN amplification is a genetic aberration found in 10% to 19% (21, 22) of primary tumours and is one of the most significant independent prognostic factors. Among the patients analysed, the MYCN amplification was found in five out of 44 (11%). This is because in Lithuania this test has been used in clinical practice only since 2004, while the published studies (21, 22) analysed considerably larger numbers of tested patients (n = 357–1185). Based on the data of Schmidt et al., 3-year eventfree survival of infants with stage 4 NB was 93% without the MYCN amplification while event-free survival in the case of the MYCN amplification was 10% (p < 0.0001) (23). A later study stated that four out of five infants with the MYCN amplification were alive with an average monitoring period of 47 months (24). The results of this research suggest that the prognosis for infants, even if they have the MYCN amplification, is better than for children aged 1 year or older with the MYCN amplification. Among the patients analysed by us, the survival rate was 78% for patients with no MYCN detected and only 40% for patients with the MYCN amplification.
It used to be considered that patients older than 18 months have a poorer prognosis than younger children (25). On the other hand, almost one-third of NB cases are diagnosed in children younger than one year, and half of them are diagnosed with a localised form (26). Based on another study, the prognosis for infants is better than for older children, in particular when they have an advanced stage (27). The OS5y of children younger than one year is 90% and the OS5y of children older than one year is: 68% for children aged 1 to 4 years, and 52% for children aged 5 to 9 years. Hero et al published data showing that 3-year event-free (no relapse or progression of disease) survival of infants with a localised NB was 77%, and the overall 3-year survival was 98% (28). Also, the influence of age on the prognosis is still observed after one year which shows that children between 12 and 18 months of age have a better prognosis than older patients. Schmidt et al. described neuroblastoma cases with favourable prognosis in children between 12 and 18 months of age even when they had stage 4 without the MYCN amplification (23). The event-free survival rate was 74% in the age group of 12–18 months compared to 31% survival in the age group of 18 to 24 months.
The results of our research do not contradict the afore-discussed studies with similar survival trends in all studied groups. The 82% OS5y achieved in the 2008–2015 period corresponds to the data of international analysis where the best OS5y of children treated for NB in the 2005–2007 period was achieved in the countries of Northern Europe and was 79.6% (2). The improvement of overall survival in the entire studied population and, in particular, among the patients of the high-risk group shows that the CPOH has developed full infrastructure for modern diagnostics and advanced treatment of this rare heterogeneous tumour. This is also confirmed by our analyses of the treatment of childhood acute lymphoblastic (29) and myeloblastic leukaemia (Kairienė et al., forthcoming) where the numbers of recovered patients match the clinical practice in other developed countries.
CONCLUSIONS
A complex assessment of the parameters of all patients treated at the CPOH during a period of sixteen years (age, stage of disease, the MYCN amplification) suggests that the survival rates were very good for low- and intermediate-risk patients but unsatisfactory for high-risk patients. However, the results of patients treated in recent years (2008–2015) show a significant improvement with overall survival of 82% and overall survival of high-risk group patients as high as 70%. Therefore, current results of NB treatment at the CPOH are consistent with the data published by advanced European clinics. The experience of the multidisciplinary team at the CPOH and close cooperation of its members, as well as the concentration of patients in the children’s oncology centre compliant with European quality standards are very important for the treatment of this rare and highly heterogeneous tumour. Further optimisation of treatment can be achieved by increasing the availability of immunotherapy and enrolling the patients into international academic clinical trials as part of their treatment.
Austėja Juškaitė, Indrė Tamulienė, Jelena Rascon
References
- Rios P, Bailey HD, Orsi L, Lacour B, Valteau-Couanet D, Levy Det al. Risk of neuroblastoma, birth-related characteristics, congenital malformations and perinatal exposures: a pooled analysis of the ESCALE and ESTELLE French studies (SFCE). Int J Cancer. 2016; 139(9): 1936–48. [DOI] [PubMed] [Google Scholar]
- Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel Jet al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5 – a population- based study. Lancet Oncol. 2014; 15(1): 35–47. [DOI] [PubMed] [Google Scholar]
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007; 369(9579): 2106–20. [DOI] [PubMed] [Google Scholar]
- Domingo-Fernandez R, Watters K, Piskareva O, Stallings RL, Bray I. The role of genetic and epigenetic alterations in neuroblastoma disease pathogenesis. Pediatr Surg Int. 2013; 29(2): 101–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM, Bagatell R. Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol. 2014; 11(12): 704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes Ket al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009; 27(2): 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydn GB, Kutluk MT, Yalcn B, Buyukpamukcu M, Kale G, Varan Aet al. Neuroblastoma in Turkish children: experience of a single center. J Pediatr Hematol Oncol. 2009; 31(7): 471–80. [DOI] [PubMed] [Google Scholar]
- Schleiermacher G, Janoueix-Lerosey I, Delattre O. Recent insights into the biology of neuroblastoma. Int J Cancer. 2014; 135(10): 2249–61. [DOI] [PubMed] [Google Scholar]
- Papaioannou G, McHugh K. Neuroblastoma in childhood: review and radiological findings. Cancer Imaging. 2005; 5: 116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour B, Guyot-Goubin A, Guissou S, Bellec S, Desandes E, Clavel J. Incidence of childhood cancer in France: National Children Cancer Registries, 2000–2004. Eur J Cancer Prev. 2010; 19(3): 173–81. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang D, Zhang W, Tang S, Han T, Zhu Xet al. Clinical characteristics of infant neuroblastoma and a summary of treatment outcome. Oncol Lett. 2016; 12(6): 5356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Mala TA, Mathur P, Paul R, Mala SA. Stage 4S bilateral adrenal neuroblastoma in a newborn. APSP J Case Rep. 2014; 5(1): 9. [PMC free article] [PubMed] [Google Scholar]
- Weintraub M, Waldman E, Koplewitz B, Bloom AI, Gross E, Freeman AIet al. A sequential treatment algorithm for infants with stage 4s neuroblastoma and massive hepatomegaly. Pediatr Blood Cancer. 2012; 59(1): 182–4. [DOI] [PubMed] [Google Scholar]
- Cozzi DA, Mele E, Ceccanti S, Natale F, Clericov A, Schiavetti Aet al. Long-term follow-up of the “wait and see” approach to localized perinatal adrenal neuroblastoma. World J Surg. 2013; 37(2): 459–65. [DOI] [PubMed] [Google Scholar]
- Oberthuer A, Juraeva D, Hero B, Volland R, Sterz C, Schmidt Ret al. Revised risk estimation and treatment stratification of low- and intermediate- risk neuroblastoma patients by integrating clinical and molecular prognostic markers. Clin Cancer Res. 2015; 21(8): 1904–15. [DOI] [PubMed] [Google Scholar]
- Bagatell R, Rumcheva P, London WB, Cohn SL, Look AT, Brodeur GMet al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J Clin Oncol. 2005; 23(34): 8819–27. [DOI] [PubMed] [Google Scholar]
- Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton Aet al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010; 363(14): 1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015; 62(1): 225–56. [DOI] [PubMed] [Google Scholar]
- Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KKet al. Children’s oncology group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer. 2013; 60(6): 985–93. [DOI] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HXet al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010; 363(14): 1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner BH, Modak S, Kramer K, LaQuaglia MP, Yataghene K, Basu EMet al. Striking dichotomy in outcome of MYCN-amplified neuroblastoma in the contemporary era. Cancer. 2014; 120(13): 2050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Teshiba R, Ikegaki N, Tang XX, Naranjo A, London WBet al. Augmented expression of MYC and/or MYCN protein defines highly aggressive MYC-driven neuroblastoma: a Children’s Oncology Group study. Br J Cancer. 2015; 113(1): 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ML Lal A Seeger RC Maris JM Shimada H O’Leary M. et al. Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified MYCN neuroblastoma: a Children’s Cancer Group Study. J Clin Oncol. 2005; 23(27): 6474–80. [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Matsuoka K, Okita H, Iwafuchi H, Hori H, Kumagai M. Neuroblastomas with discordant genotype-phenotype relationships: report of four cases with MYCN amplification and favorable histology. Pediatr Dev Pathol. 2011; 14(2): 87–92. [DOI] [PubMed] [Google Scholar]
- Weinstein JL, Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. Oncologist. 2003; 8(3): 278–92. [DOI] [PubMed] [Google Scholar]
- London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada Het al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol. 2005; 23(27): 6459–65. [DOI] [PubMed] [Google Scholar]
- Kretschmar CS, Frantz CN, Rosen EM, Cassady JR, Levey R, Sallan SE. Improved prognosis for infants with stage IV neuroblastoma. J Clin Oncol. 1984; 2(7): 799–803. [DOI] [PubMed] [Google Scholar]
- Hero B, Simon T, Spitz R, Ernestus K, Gnekow AK, Scheel-Walter HGet al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008; 26(9): 1504–10. [DOI] [PubMed] [Google Scholar]
- Vaitkeviciene G, Matuzeviciene R, Stoskus M, Zvirblis T, Rageliene L, Schmiegelow K. Cure rates of childhood acute lymphoblastic leukemia in Lithuania and the benefit of joining international treatment protocol. Medicina (Kaunas). 2014; 50(1): 28–36. [DOI] [PubMed] [Google Scholar]