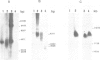Abstract
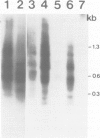
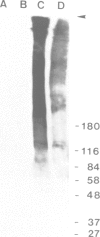
We report the identification of the product of the Plasmodium falciparum Pf11-1 gene and demonstrate that it is a gametocyte-specific protein that has a potential role in the rupture of the host erythrocyte and emergence of the gametes (gametogenesis). The Pf11-1 gene is a large locus (30 kb) whose sequence predicts a glutamic acid-rich polypeptide. Our identification of the Pf11-1 gene product as gametocyte specific was greatly facilitated by the isolation of a mutant parasite clone in which greater than 90% of the Pf11-1 gene was deleted. Molecular analysis of the mutant locus suggests that the underlying genetic mechanism is chromosome breakage and subsequent healing by the addition of telomere repeats. PCR-based analysis showed that similar DNA rearrangements occur commonly in small subpopulations of most laboratory strains, suggesting that the Pf11-1 locus represents a fragile chromosome region. Northern blot analysis demonstrates that a large Pf11-1 gene-specific transcript (much greater than 10 kb) is present in gametocytes but not in asexual blood stage parasites. The Pf11-1 protein was localized by electron microscopy to granules in the cytoplasm of gametocytes adjacent to the membrane of the parasitophorous vacuole. Following in vitro stimulation of gametogenesis, the Pf11-1 protein was found in the membrane of lysed erythrocytes, suggesting a role for Pf11-1 in erythrocyte rupture within the mosquito gut.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappai R., van Schravendijk M. R., Anders R. F., Peterson M. G., Thomas L. M., Cowman A. F., Kemp D. J. Expression of the RESA gene in Plasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol. 1989 Aug;9(8):3584–3587. doi: 10.1128/mcb.9.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Graves P. M., Creasey A., Byrne K., Read D., Alano P., Fenton B. Plasmodium falciparum: an abundant stage-specific protein expressed during early gametocyte development. Exp Parasitol. 1989 Aug;69(2):140–149. doi: 10.1016/0014-4894(89)90182-3. [DOI] [PubMed] [Google Scholar]
- Carter R., Graves P. M., Keister D. B., Quakyi I. A. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 1990 Nov;12(6):587–603. doi: 10.1111/j.1365-3024.1990.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Gritzmacher C. A., Reese R. T. Reversal of knob formation on Plasmodium falciparum-infected erythrocytes. Science. 1984 Oct 5;226(4670):65–67. doi: 10.1126/science.6382613. [DOI] [PubMed] [Google Scholar]
- Kahane B., Sibilli L., Scherf A., Jaureguiberry G., Langsley G., Ozaki L. S., Guillotte M., Müller-Hill B., Pereira da Silva L., Mercereau-Puijalon O. The polymorphic 11.1 locus of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Nov;26(1-2):77–85. doi: 10.1016/0166-6851(87)90132-0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Corcoran L. M., Coppel R. L., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F. Size variation in chromosomes from independent cultured isolates of Plasmodium falciparum. Nature. 1985 May 23;315(6017):347–350. doi: 10.1038/315347a0. [DOI] [PubMed] [Google Scholar]
- Koenen M., Scherf A., Mercereau O., Langsley G., Sibilli L., Dubois P., Pereira da Silva L., Müller-Hill B. Human antisera detect a Plasmodium falciparum genomic clone encoding a nonapeptide repeat. 1984 Sep 27-Oct 3Nature. 311(5984):382–385. doi: 10.1038/311382a0. [DOI] [PubMed] [Google Scholar]
- Magowan C., Wollish W., Anderson L., Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988 Oct 1;168(4):1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D., Scherf A. The Pf332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene. 1992 Jan 2;110(1):71–79. doi: 10.1016/0378-1119(92)90446-v. [DOI] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Petersen C., Nelson R., Leech J., Jensen J., Wollish W., Scherf A. The gene product of the Plasmodium falciparum 11.1 locus is a protein larger than one megadalton. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):189–195. doi: 10.1016/0166-6851(90)90161-e. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Quakyi I. A., Matsumoto Y., Carter R., Udomsangpetch R., Sjolander A., Berzins K., Perlmann P., Aikawa M., Miller L. H. Movement of a falciparum malaria protein through the erythrocyte cytoplasm to the erythrocyte membrane is associated with lysis of the erythrocyte and release of gametes. Infect Immun. 1989 Mar;57(3):833–839. doi: 10.1128/iai.57.3.833-839.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
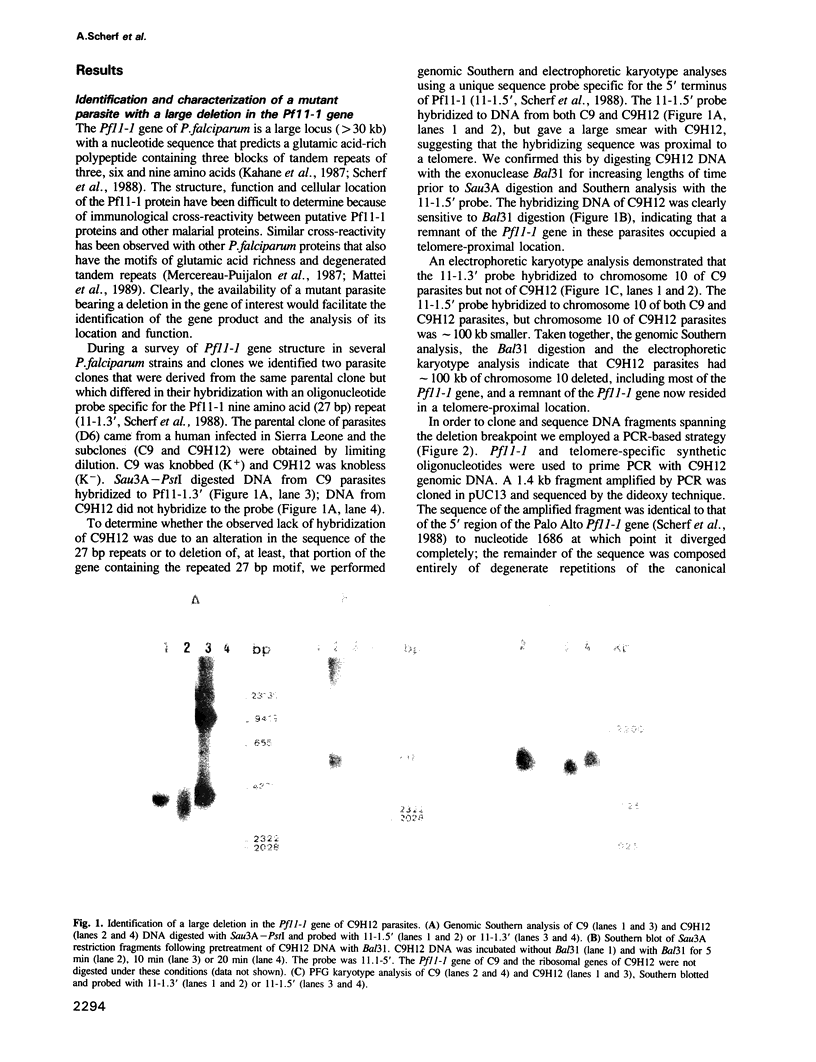
- Scherf A., Hilbich C., Sieg K., Mattei D., Mercereau-Puijalon O., Müller-Hill B. The 11-1 gene of Plasmodium falciparum codes for distinct fast evolving repeats. EMBO J. 1988 Apr;7(4):1129–1137. doi: 10.1002/j.1460-2075.1988.tb02922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Lundgren K., Berzins K., Wåhlin B., Perlmann H., Troye-Blomberg M., Carlsson J., Wahlgren M., Perlmann P., Björkman A. Human monoclonal antibodies to Pf 155, a major antigen of malaria parasite Plasmodium falciparum. Science. 1986 Jan 3;231(4733):57–59. doi: 10.1126/science.3510452. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., McCutchan T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol Biochem Parasitol. 1988 Mar;28(2):85–94. doi: 10.1016/0166-6851(88)90055-2. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H., Monks B. The controlling sequence for site-specific chromosome breakage in Tetrahymena. Cell. 1990 Nov 16;63(4):763–772. doi: 10.1016/0092-8674(90)90142-2. [DOI] [PubMed] [Google Scholar]