Abstract
Persister cells are phenotypic variants within a microbial population, which are dormant and transiently tolerant to stress. Persistence has been studied extensively in bacteria, and in eukaryotes to a limited extent, however, it has never been observed in archaea. Using the model haloarchaeon, Haloferax volcanii DS2, we demonstrated persister cell formation in this domain, with time-kill curves exhibiting a characteristic biphasic pattern following starvation or exposure to lethal concentrations of various biocidal compounds. Repeated challenges of surviving cells showed that, as with bacteria, persister formation in H. volcanii was not heritable. Additionally, as previously shown with bacteria, persister formation in H. volcanii was suppressed by exogenous indole. The addition of spent culture media to assays conducted on planktonic cells showed that H. volcanii-conditioned media stimulated persistence, whereas conditioned media of other haloarchaea or halophilic bacteria did not, suggesting the involvement of a species-specific signal. Using a TLC overlay assay, the quorum sensing bioreporter Agrobacterium tumefaciens ATCC BAA-2240 detected the presence of C4 and C6 acyl homoserine lactone-like signal molecules in a H. volcanii culture extract. While synthetic bacterial AHLs did not induce persistence, this is potentially due to structural differences between bacterial and archaeal signals, and does not discount a quorum sensing component in haloarchaeal persister formation. The observation of persister cell formation by this haloarchaeon may provide some insights into the survival of these organisms in stressful or dynamic environments.
Keywords: persister, stress response, haloarchaea, Haloferax volcanii, biofilm, quorum sensing
Introduction
When microbial populations are exposed to stress which exceeds the capacity of genetically encoded protective responses, the majority are killed. Killing is commonly quantified by measuring the proportion of viable cells as a function of exposure time to the condition of interest, and generally exhibits a non-linear, biphasic killing pattern, with an initial exponential decline followed by a plateau or period of slower decline, with the latter part of the killing curve indicating the presence of a small subpopulation of specialized survivor cells, known as persisters (Balaban et al., 2004; Keren et al., 2004a). Kim and Wood (2016) proposed a working definition of persisters as “those cells that remain after the actively respiring cells are vanquished by antibiotics (usually seen as an unchanging or slowly decreasing concentration of viable cells)”.
Unlike resistant mutants, persisters are genetically identical to the cells which are killed, but are phenotypic variants which have entered a transiently quiescent state in which they are metabolically inactive, neither growing nor dying when exposed to lethal stress (Lewis, 2010). This process is reversible, therefore persisters act as a reservoir which, when favorable conditions are restored, gives rise to a new population of cells. The new population is as susceptible as the original, and possesses a similarly small proportion of persister cells; this differentiates persisters from resistant mutants, which are characterized by their stable and heritable insensitivity (Balaban et al., 2004; Keren et al., 2004a).
The phenomenon of persistence has been observed repeatedly since it was first demonstrated during an early study on the mechanism of action of penicillin by Hobby et al. (1942) that it was ineffective against non-growing Staphylococcus cells. This was later confirmed by the work of Bigger (1944), who showed that inhibiting the growth of Staphylococcus cultures by lowering the temperature, removing nutrients, or adding bacteriostatic concentrations of boric acid resulted in the generation of more persisters. More recently, Kwan et al. (2013) induced high levels of persistence (10–100%) from an initial population of 0.01% in Escherichia coli by halting transcription, translation, or ATP synthesis, using rifampin, tetracycline, and carbonyl cyanide m-chlorophenylhydrazone, respectively, confirming that persistence is the result of cells being in an inactive state.
While some previous explanations for the presence of persister cells is their stochastic generation in microbial populations (Balaban et al., 2004), or as a “bet-hedging strategy” ensuring the survival of the population in the event that lethal conditions should arise, their formation is now accepted to be a stress response, as persistence is not a random event and has frequently been shown to be induced by various environmental cues. Initially, it was demonstrated that maintaining a bacterial culture in early exponential phase by repeated dilution led to the elimination of persisters, and that the number of persister cells increased dramatically from mid-exponential to stationary phase, suggesting that persister formation was dependent on growth phase (Keren et al., 2004a). Although mainly studied with regard to antibiotic treatment, it has now been proposed that exposure to any stress condition could induce the formation of surviving persisters (Lewis, 2010). In addition to antibiotic exposure (Dörr et al., 2010; Kwan et al., 2013; Van den Bergh et al., 2016), persister formation has been shown to be stimulated by starvation (Maisonneuve et al., 2013), carbon source transitions (Amato et al., 2013), oxidative stress (Wu et al., 2012), toxic metals (Harrison et al., 2007), quorum sensing (Möker et al., 2010), host macrophages (Helaine et al., 2014), and the SOS response (Bernier et al., 2013). Additionally, persisters are not found in early phase microbial cultures, with any reports of persisters in early phase cultures assumed to be a result of carry-over from a stationary phase inoculum (Keren et al., 2004a). Gene pairs known as toxin–antitoxin (TA) systems, comprising a toxin and cognate antitoxin, have generally been accepted to be effectors and modulators of persistence. During normal growth conditions, the activity of toxins are neutralized by the antitoxins, but under conditions of stress, the antitoxins are degraded, enabling the toxins to exert their effects, which involve corruption of an essential cellular process, such as DNA replication or protein translation, leading to growth arrest and dormancy (Keren et al., 2004b; Shah et al., 2006; Page and Peti, 2016). A study by Chowdhury et al. (2016) also showed that the alarmone guanosine tetraphosphate (ppGpp), a key metabolite commonly linked to persistence, was not in fact required for persister formation in E. coli. These findings suggest that there are further, less explored or as yet unknown, modulators of persistence.
Previous research on persisters has almost exclusively been in a clinical context, focusing on the tenacity of pathogenic bacteria following antibiotic treatment. Persisters have been shown to have a major role in recurrent or chronic bacterial infections (Lewis, 2010; Fauvart et al., 2011), but it is likely that they also play a role in the survival and preservation of natural microbial populations, and it has been suggested that these dormant cells can act as a “seed bank,” enabling populations to recover and repopulate their environment following a catastrophic event (Lennon and Jones, 2011). The environments inhabited by haloarchaea are both extreme and dynamic, and consequently, they are exposed to stressful conditions including fluctuations in salinity (Becker et al., 2014; Thombre et al., 2016), intense solar radiation (Baliga et al., 2004; McCready et al., 2005), low dissolved oxygen (Grant, 2004), pH extremes (Mesbah et al., 2007; Mormile et al., 2009), and even entrapment for millennia (Grant et al., 1998; McGenity et al., 2000; Stan-Lotter et al., 2002; Lowenstein et al., 2011). In any dynamic environment, or when faced with sudden or transient stress, the phenotypic plasticity of microbial populations may be key in ensuring their survival. Published research has shown that archaeal genomes contain toxin–antitoxin loci (Gerdes et al., 2005; Pandey and Gerdes, 2005), suggesting a capacity for persister cell formation, but none as yet have provided experimental evidence to demonstrate the presence of the phenotype in this domain. Using the model haloarchaeon, Haloferax volcanii DS2, this study aimed to investigate whether persister cell formation is a strategy employed by haloarchaeal populations to withstand and recover from lethal stress.
Materials and Methods
Growth Medium and Conditions
Haloferax volcanii DS2 (DSM 3757) was obtained from DSMZ (Braunschweig, Germany) and used for all experiments. H. volcanii was grown and maintained aerobically at 37°C in broth containing, in g L-1, NaCl (150), MgSO4.7H2O (20), KCl (2), FeSO4.4H2O (0.036), MnCl2.4H2O (3.6 × 10-3), yeast extract (10), casein hydrolysate (7.5), trisodium citrate (3), at pH 7.4, with 1.5% agar added for solid media.
Antimicrobial Susceptibility Testing
Sterile aqueous stock solutions of the biocides H2O2, NaClO, 1-dodecyl-3-methylimidazolium chloride ([C12mim]Cl), chlorhexidine, and the antibiotic rifampicin were used to prepare minimum inhibitory concentration (MIC) assays. Serial doubling dilutions of each biocide (six replicates) were prepared in 100 μL sterile broth in 96-well microtitre plates. An inoculum was prepared by adjusting the turbidity of an exponential phase broth culture of H. volcanii to correspond with a cell density of 2 × 105 cfu mL-1, which was verified by total viable count. 100 μL was added to each well of the microtitre plates. Positive and negative growth controls were also included in each plate. Plates were incubated at 37°C until copious growth was evident in the positive control wells, and MIC values were determined for each compound as the lowest concentration at which growth was inhibited. Minimum biocidal concentrations (MBC) were determined by inoculating plates of the above medium with 20 μL spots of broth from clear wells and incubating at 37°C for 5 days, and were defined as the lowest concentration which reduced the initial inoculum by >99.9%, as determined by total viable counts.
Persister Cell Assays
The OD590 of a stationary phase liquid culture of H. volcanii DS2 was adjusted to 0.1 in sterile broth. For the biocides, 10 mL volumes of the cell suspension were transferred to 50 mL microcentrifuge tubes, and MBC concentrations of each of the chosen compounds (0.005% H2O2, 5 μg mL-1 NaClO, 2 μg mL-1 [C12mim]Cl, 10 μg mL-1 chlorhexidine, and 50 μg mL-1 rifampicin) were added (three replicates). For the starvation assay, 10 mL samples of the cell suspensions were centrifuged, the broth discarded, and the pellets resuspended in 10 mL sterile 15% NaCl. All tubes were incubated at 37°C and at specific time intervals, three 200 μL samples were taken and serially diluted to obtain total viable counts for each time point, and cell viability expressed as a percentage of Time 0 samples. To determine persister heritability, colonies were picked from plates showing growth following the persister assays, re-grown in broth to an OD590 of 1.0, and the assays performed again as above.
Persister Cell Formation in Biofilms
Biofilms of H. volcanii were grown using the MBEC device (Innovotech). The OD590 of a H. volcanii liquid culture was adjusted to 0.1, and 150 μL was inoculated into each well of the plate supplied with the device, which was then incubated for 3 weeks at 37°C, with the lid being placed weekly into a new plate containing fresh broth. Following incubation, three pegs were removed using sterilized pliers for each condition to be tested. The pegs were rinsed by placing into the wells of a 96-well plate containing 200 μL sterile 15% NaCl to remove any loosely adhered or planktonic cells. The pegs were then placed in 200 μL of broth containing MBC concentrations of H2O2, NaClO, and chlorhexidine in a 96-well microtitre plate. Following incubation for 6 h, the pegs were placed in the top row of a 96-well plate containing 200 μL 15% NaCl in the top row, and 180 μL in the other rows. The plate was sonicated in an ultrasonic bath for 30 min, the pegs discarded, and serial dilutions were performed to obtain total viable counts, expressed in cfu peg-1. Plates were incubated at 37°C for 5 days and counts were expressed for each treatment as a percentage of those obtained for untreated pegs.
Conditioned Media
A stationary phase liquid culture of H. volcanii was centrifuged and the pellet resuspended in broth containing double concentrations of yeast extract and casein hydrolysate to an OD590 of 0.2. 5 mL aliquots of this suspension were added to 50 mL centrifuge tubes, along with 5 mL sterile filtered spent broth from stationary phase cultures of H. volcanii, other haloarchaea (Halococcus dombrowskii, Halorubrum trapanicum, and Halobacterium noricense), or halophilic bacteria (Halomonas janggokensis, Chromohalobacter canadensis, and Staphylococcus succinus), from our own strain library of isolates from a Triassic salt mine. A control with fresh, sterile broth added was also included. All tubes were incubated at 37°C for 1 h, then H2O2 was added to a final concentration of 0.005%. Viable counts were obtained following incubation for 6 h and were expressed as a percentage of counts obtained at Time 0.
Effect of Indole on Persister Cell Formation
The persister assay was conducted with 0.005% H2O2 as before, except prior to challenge, cell suspensions were incubated at 37°C for 1 h, either in the presence or absence of 500 μM indole. The percentage of survivors at 6 h was compared with and without indole.
Determination of Acyl Homoserine Lactone Production by H. volcanii DS2
A stationary phase culture of H. volcanii was extracted with three volumes of EtOAc by stirring overnight. The EtOAc layer was removed and dried under reduced pressure, weighed, and then reconstituted in EtOAc to create a 50 mg mL-1 stock solution. 0.25 mg of the extract was run on a C18 reversed-phase TLC plate, along with standards of N-butyryl, -hexanoyl -octanoyl (1 μL of 100 μM), and -dodecanoyl (1 μL of 10 mM) -DL-homoserine lactones (Sigma-Aldrich, United Kingdom), using a mobile phase of 55:45:0.001 MeOH:H2O:CH3COOH. Agrobacterium tumefaciens ATCC BAA-2240 was grown in LBB with 30 μg mL-1 gentamicin overnight at 28°C. Glass tubes containing 10 mL LB with 0.5% agar were boiled to liquefy the agar and then cooled to 55°C. 80 μL of a 10 mg mL-1 X-gal solution and 1 mL A. tumefaciens broth culture were added to each tube. The dried TLC plates were overlaid with the molten agar in a sterile Petri dish and incubated at 28°C.
Effect of Acyl Homoserine Lactones on Persister Cell Formation
Synthetic acyl homoserine lactones (N-butyryl, -hexanoyl -octanoyl and -dodecanoyl -DL-homoserine lactone) were added to 10 mL samples of an early stage broth culture of H. volcanii DS2 (OD590 0.1), to a final concentration of 10 μM, and incubated for 1 h. A control with no AHL was also included. 0.005% H2O2 was added and total viable counts were obtained after 6 h, and expressed as a percentage of Time 0 values.
Identification of Toxin–Antitoxin Loci
The complete sequences of the genome of H. volcanii DS2 and its four plasmids were downloaded from NCBI GenBank. Toxin–antitoxin loci were identified using TAfinder1 using the default parameters (Shao et al., 2011).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6. All values in figures are expressed as the mean of three replicates ± one standard deviation. Kruskal–Wallis one-way analysis of variance (ANOVA) with Dunn’s post hoc multiple comparisons test was used to analyze statistical differences between test samples and controls; a paired t-test (two-tailed) was used to analyze differences between pairs of data. For all analyses, a value of p < 0.05 was considered significant.
Results and Discussion
Persister Cell Formation by Haloferax volcanii
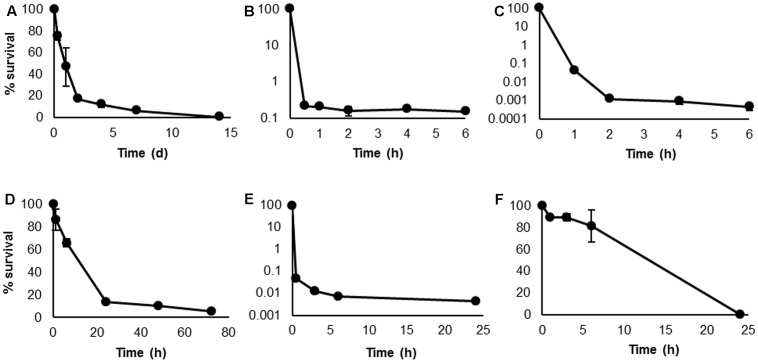
Persister cell formation by H. volcanii was determined based on total viable counts at specific time points, and under the conditions of starvation, and treatment with biocidal concentrations of H2O2, NaClO, [C12mim]Cl, and chlorhexidine, a characteristic, biphasic killing pattern was observed, with a rapid initial decline followed by a distinct plateau indicative of small surviving persister subpopulations (Figures 1A–E). This killing pattern is the definitive indicator of persister formation (Keren et al., 2004a; Maisonneuve and Gerdes, 2014), and provides confirmation of this phenotype in the domain Archaea.
FIGURE 1.
Kill curves of Haloferax volcanii following biocidal treatments. Survival was determined by total viable counts at specific time points, under conditions of starvation (A), 0.005% H2O2 (B), 5 μg mL-1 NaClO (C), 2 μg mL-1 [C12mim]Cl (D), 10 μg mL-1 chlorhexidine (E), and 50 μg mL-1 rifampicin (F). Plotted values are the mean of triplicate measurements and error bars represent ± SD.
The majority of studies on persister cells are conducted using clinically relevant bacteria and focus on their response to antibiotic treatment. However, as the focus of this study was an environmental haloarchaeal species with no clinical relevance and susceptibility to few antibiotics, this approach is less appropriate. We therefore chose stimuli which may be more relevant to haloarchaea: starvation, oxidative stress induced by H2O2 and NaClO, and membrane disruption induced by [C12mim]Cl and chlorhexidine. Rifampicin was also selected as it is one of the few antibiotics to which most haloarchaeal genera show susceptibility (Bonelo et al., 1984). In contrast to the other treatments, rifampicin-treated cells did not exhibit a logarithmic killing pattern, but a steady, linear decline until survival reached 0% (Figure 1F). As the other treatments indicated the presence of persisters in this species, it appears that rifampicin eradicated the persisters in addition to the bulk population of cells. Recently, rifampicin has been used as a pre-treatment in bacterial studies to convert exponentially growing cells to the persister phenotype (Kwan et al., 2013, 2015a; Lee et al., 2016). However, the effects of rifampicin on bacteria and archaea are not comparable. In bacteria, the mechanism of action of rifampicin is inhibition of DNA-dependent RNA polymerase, while in archaea, rifampicin induces cell lysis by exerting a detergent effect on the cell membrane (Pfeifer, 1988). Based on the results obtained here, it is apparent that rifampicin is destructive to all H. volcanii cells, including persisters.
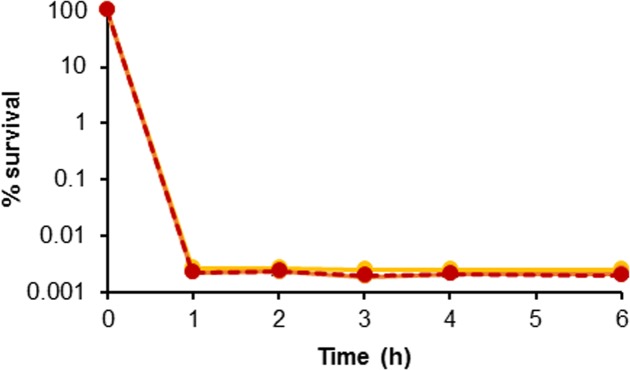
The second major identifier of persistence is non-heritability of the trait (Keren et al., 2004a). In order to demonstrate that the surviving population was a result of the phenotypic response of persister cell formation and not genetically acquired tolerance or resistance, colonies were picked from the plates on which surviving cells were enumerated; these were grown as broth cultures and were tested again as before. Figure 2 shows a challenge with H2O2 repeated three times. No inherited resistance was observed following repeated challenges of surviving cells, as three almost-identical biphasic kill curves were obtained. This confirms that persistence in H. volcanii is a non-heritable phenotypic trait, as enrichment or increase in the surviving population was not possible, and indicates that the survival mechanism is likely similar to the phenotypic response frequently described for bacteria.
FIGURE 2.
Evaluation of persister cell heritability. Kill curves of H. volcanii following repeated challenges of surviving colonies in 0.005% H2O2. Yellow, orange, and red lines are replicate 1, 2, and 3, respectively. Plotted values are the mean of triplicate measurements and error bars represent ± SD.
Persister Cells in H. volcanii Biofilms
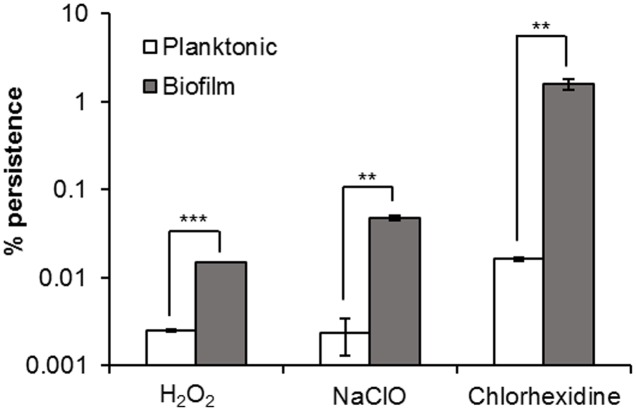
Considerably greater proportions of persister cells were observed in H. volcanii biofilms in comparison to stationary phase planktonic cultures for the three biocides tested. Challenge with H2O2 resulted in 0.0025 and 0.015% survival in planktonic culture and biofilm respectively, with NaClO resulting in 0.002 and 0.047%, respectively, and chlorhexidine 0.016 and 1.6% respectively (Figure 3). Six hours was deemed to be an appropriate time point to determine persister numbers, as based on the kill curves shown in Figure 1, for the three biocides, they had all unquestionably reached a plateau by this time. For cells challenged with H2O2, there was little to no variation in cell number after 30 min–1 h, for NaClO, 2 h, and for chlorhexidine, 6 h. Taking data from studies on bacteria into consideration, the marked difference in persister cells between planktonic and biofilm cultures is not what would be expected, as it has been shown that stationary phase planktonic cultures and biofilms should contain a similar proportion of persisters (Spoering and Lewis, 2001; Lewis, 2008). Haloarchaeal biofilms were only recently described and investigated (Fröls et al., 2012), and while biofilms of H. volcanii DS2 have been shown to have many phenotypic features in common with bacterial biofilms, they also appear to possess some unique features (Chimileski et al., 2014). As haloarchaeal biofilms are currently not well-studied in comparison to bacterial biofilms, it is unclear whether they should be expected to behave in the same manner.
FIGURE 3.
Persister cells in H. volcanii biofilms. Comparison of percentage survival of planktonic and biofilm cultures of H. volcanii, following incubation for 6 h in biocidal concentrations of H2O2, NaClO, and chlorhexidine. Plotted values are the mean of triplicate measurements and error bars represent ± SD. Asterisks denote significance values as determined by paired t-tests: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Spoering and Lewis (2001) suggested that the maintenance of a subpopulation of persister cells is strongly dependent on the population density, and that the common method of conducting assays on diluted stationary phase cultures leads to a collapse in the number of persisters. This indicates that the dense populations of biofilms or stationary phase cultures favors persister formation, and that the common misconception that biofilms are more resistant to killing, or have more persisters than planktonic cultures, derives from experiments using either logarithmic or diluted stationary phase cultures, and are therefore based upon a false comparison, while a more direct comparison should be made between biofilms and undiluted stationary phase cultures. As diluted stationary phase cultures were used for planktonic cell assays, this could provide a reason for the difference in persister cell number, however, as the cell suspensions were treated immediately following dilution, this seems unlikely to be the sole explanation. It is known that extended incubation of diluted stationary phase cultures prior to treatment will cause some persisters to revert and become sensitive. However, the rate of resuscitation of dormant cells appears to be dependent on various factors, such as the composition of the medium into which they are diluted, and the time between dilution and challenge (Jõers et al., 2010). Additionally, Kim et al. (2011) showed that reversion of E. coli and Pseudomonas aeruginosa persisters to normal cells was not instantaneous but took several hours. The slow growth rate of haloarchaea in comparison to fast-growing bacterial species such as E. coli, and their immediate challenge following dilution make dilution even less likely to have been a factor. It could transpire that this difference is the result of an as-yet-unknown feature of haloarchaeal biofilms favoring persister cell formation.
Influence of the Interkingdom Signal Indole on Persistence
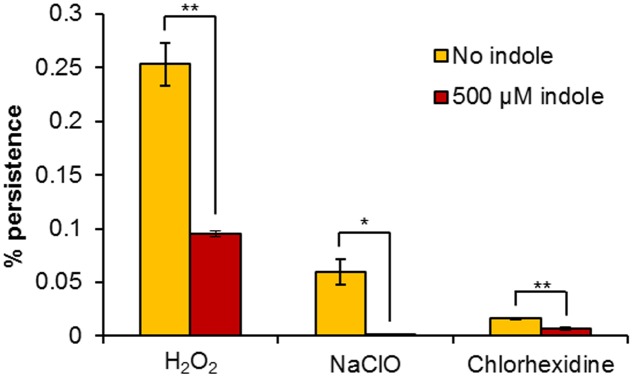
Indole is known as an interspecies and interkingdom signaling molecule with numerous biological roles in microbial communities, including spore formation, plasmid stability, drug resistance, biofilm formation, and virulence among species which produce indole, and even in some cases, those which do not (Lee and Lee, 2010). Vega et al. (2012) showed that incubation of E. coli cells for 1 h in 500 μM indole prior to an antibiotic challenge resulted in a large increase in persister cell formation, which was attributed to the induction of oxidative stress and phage-shock responses, suggesting that indole signaling has a role in the stimulation of persister cell formation. Subsequent studies have shown that indole does have a role in persistence, but it is more likely to be involved in suppression. We conducted our study under the same conditions (incubation with 500 μM indole for 1 h prior to treatment). We also confirmed that the concentration of indole used had no effect on the growth and survival of H. volcanii, with an MIC value in excess of 2 mM. Pre-incubation of H. volcanii cell suspensions with indole prior to challenge with biocidal concentrations of H2O2, NaClO, or chlorhexidine resulted in 2.7-, 188-, and 2.3-fold decreases respectively in persister cell formation in comparison to cells which were not pre-treated. While two of these decreases were very small, they were statistically significant (Figure 4). These results indicate that indole appears to reduce the proportion of persister cells in H. volcanii, which is in agreement with more recent studies showing an inversely proportional relationship between indole concentration and persistence. Hu et al. (2015) showed that the toxin YafQ increases persister cell formation in E. coli by cleaving tryptophanase (TnaA) mRNA and inhibiting the ability of cells to produce indole, TnaA deletion decreased persistence, and that the addition of exogenous indole reduced persister formation, as did the addition of tryptophan. Kwan et al. (2015b) showed that production of DosP (direct oxygen sensing phosphodiesterase) increased persistence in E. coli by degrading another cyclic nucleotide signal, cyclic adenosine monophosphate (cAMP), which results in lower indole levels due to reduced TnaA activity. Lee et al. (2016) showed that indole and modified indoles reduced persisters in E. coli and Staphylococcus aureus, or even eradicated them completely. It has been previously reported that H. volcanii tryptophanase functions as an inducible promoter in this species, with expression increasing rapidly following the addition of tryptophan to the medium (Large et al., 2007). Further research could explore the role of tryptophan and tryptophan-inducible gene expression in persister formation in H. volcanii.
FIGURE 4.
Influence of exogenous indole on H. volcanii persistence. Percentage survival of H. volcanii pre-incubated for 1 h with or without 500 μM indole, prior to 6 h challenge with biocidal concentrations of H2O2, NaClO, and chlorhexidine. Plotted values are the mean of triplicate measurements and error bars represent ± SD. Asterisks denote significance values as determined by paired t-tests: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Toxin–Antitoxin Loci in H. volcanii
Searching the genome and plasmid sequences of H. volcanii in TAfinder highlighted some putative TA loci (Table 1). The TA system with the highest sequence similarity to known TA genes contained a PIN domain (nuclease) toxin and RHH domain antitoxin, with a match of 84.01%. Also highlighted were the common prokaryotic vapBC system (65.84%), the translation repressors RelE with an RHH antitoxin (53.55%), and HicAB (49.01%). Archaea have previously been shown to possess TA systems (Pandey and Gerdes, 2005; Yamaguchi et al., 2011), and the toxin RelE and the antitoxin RelB from the hyperthermophilic archaeon Pyrococcus horikoshii were structurally elucidated and shown to be homologous but with numerous differences to those found in E. coli (Takagi et al., 2005), so it is possible that haloarchaeal toxins and antitoxins are considerably divergent to those which are known. While TA systems are known to be important factors in the archaeal stress response – deletion of a specific vapBC locus in the thermophilic archaeon Sulfolobus solfataricus rendered it heat shock labile (Cooper et al., 2009), there is no current knowledge regarding their role in archaeal persister cell formation. Further work is required to determine the role of these loci and indeed whether the effect of indole addition resulted in their corruption, as has been shown previously.
Table 1.
Toxin–antitoxin loci identified on Haloferax volcanii DS2 chromosome and plasmids.
| TA no. | T/A | Location | Length (aa) | Strand | Family | Domain | T score/A score | TA score |
|---|---|---|---|---|---|---|---|---|
| Chromosome (NC_013967.1) | ||||||||
| 1 | T | 180588–180998 | 136 | - | PRK09831 | 10.98 | 7.1 | |
| A | 180124–180504 | 126 | - | pfam12840 | 1.29 | |||
| 2 | T | 237887–237988 | 33 | + | HicA-like domain | 54.55 | 49.01 | |
| A | 237481–237741 | 86 | + | HicB-like domain | 40.7 | |||
| 3 | T | 437012–437659 | 215 | + | vapC | 11.16 | 13.77 | |
| A | 437774–438166 | 130 | + | ArsR-like domain | 17.69 | |||
| 4 | T | 1813914–1814372 | 152 | + | vapC | 71.05 | 65.84 | |
| A | 1813650–1813895 | 81 | + | vapB | 58.02 | |||
| Plasmid pHV3 (NC_013964.1) | ||||||||
| 1 | T | 81215–81886 | 223 | - | vapC | 12.56 | 12.31 | |
| A | 81961–82491 | 176 | - | Xre-like domain | 11.93 | |||
| Plasmid pHV4 (NC_013966.1) | ||||||||
| 1 | T | 189115–189552 | 145 | - | cd09886 | 16.6 | 13.98 | |
| A | 189549–189824 | 91 | - | COG3905 | 10.05 | |||
| 2 | T | 379569–379850 | 93 | - | relE-like domain | COG2026 | 54.07 | 53.55 |
| A | 379843–380097 | 84 | - | RHH-like domain | COG3609 | 52.76 | ||
| 3 | T | 445961–446530 | 189 | + | PIN-like domain | 83.6 | 84.01 | |
| A | 445533–445964 | 143 | + | RHH like domain | 84.62 | |||
Influence of Conditioned Media on Persister Formation
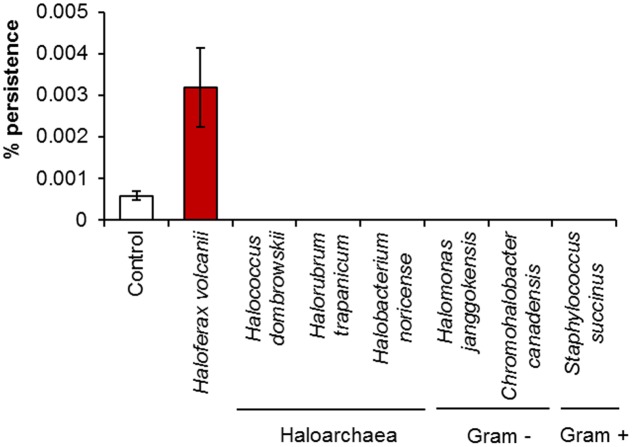
When H. volcanii was challenged with 0.005% H2O2 in the presence of its own spent medium, it showed a fourfold increase in the number of persisters in comparison to fresh broth (Figure 5). It has already been established that persister cell formation is growth phase-dependent, increasing at late exponential to stationary phase (Keren et al., 2004a), and has been attributed to stress resulting from nutrient depletion, or increased cell signaling due to the higher population density. As the assays were conducted in 50% spent broth and 50% fresh broth with double concentrations of nutrients, nutrient levels in the assay conditions should have been comparable to fresh medium, which suggests that in this case, the elevated level of persistence was not induced by nutrient depletion during the pre-incubation period. No such increase in persistence was observed when the assay was conducted in spent media of other halophilic species (other haloarchaea plus Gram-positive and Gram-negative bacteria), all of which resulted in 0% survival after 6 h, which may suggest that a species-specific signal is involved in the induction of persistence. In addition to possibly not containing the specific signal which may be required, the spent media of the other species may also have contained compounds such as halocins or bacteriocins, or some other non-specific antimicrobial metabolite resulting in the complete eradication of the cells. Although differences were not statistically significant (p > 0.05) as determined by Kruskal–Wallis ANOVA, it is still apparent that the addition of conditioned media impacted persister formation in this species. The addition of spent growth medium has previously been shown to stimulate a significant increase in persister cell formation in P. aeruginosa PA14, and was attributed to the presence of various signaling molecules present in the medium. The same effect was not seen when the spent medium of P. aeruginosa PA14 was added to E. coli or S. aureus cultures, however, these species were also unaffected by the addition of their own spent medium (Möker et al., 2010). This suggests that persister cell formation is a response to a specific signal in some species, but the precise mechanisms for others may be different.
FIGURE 5.
The effect of spent culture medium on persister cell formation by H. volcanii. Percentage survival of H. volcanii following incubation for 1 h in the presence of its own spent culture medium or the spent culture media of other haloarchaea or halophilic bacteria, followed by challenge for 6 h with 0.005% H2O2. Cells suspended in fresh broth served as a negative control. Plotted values are the mean of triplicate measurements and error bars represent ± SD.
Acyl Homoserine Lactone Production by H. volcanii
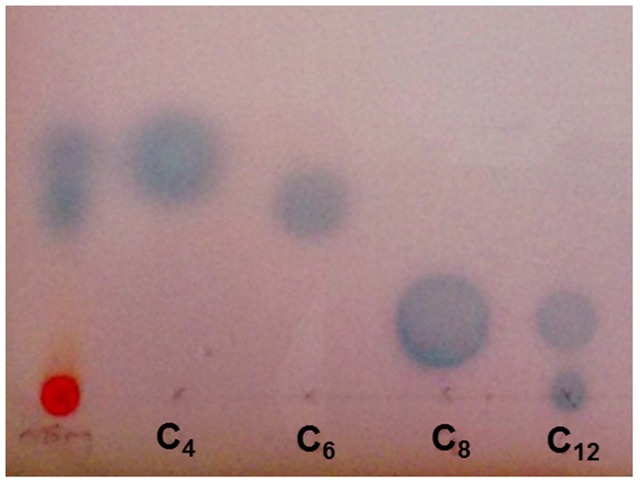
The EtOAc extract of H. volcanii, when separated by TLC and overlaid with the bacterial biosensor A. tumefaciens ATCC BAA-2240, showed activation in two locations, which corresponded with the C4 and C6 AHL standards (Figure 6), suggesting the production of these two molecules by this haloarchaeon. Quorum sensing has been studied previously in archaea, but to a very limited extent. A. tumefaciens has previously been reported to detect quorum sensing signals from the haloalkaliphilic archaeon Natronococcus occultus (Paggi et al., 2003). Additionally, the methanogenic archaeon Methanosaeta harundinacea was shown to produce modified AHL molecules with an additional carboxyl moiety on the N atom of the homoserine lactone ring. These carboxylated AHLs induced a change in cellular morphology from short to filamentous, with Gram negative bacterial AHL molecules failing to induce the same effect (Zhang et al., 2012). While the mechanism of AHL production in archaea does not appear to be the same as in bacteria – they are not known to possess LuxI or LuxR genes – it was suggested that FilI, a histidine kinase, is an AHL synthase in this species. It has also been shown that diketopiperazines produced by the halophilic archaeon Haloterrigena hispanica could activate AHL bioreporters (Tommonaro et al., 2012). However, the spots produced on the TLC by the H. volcanii extract aligned with AHL standards, suggesting that they are in fact AHLs or AHL-like molecules. The extract did not activate the more specific AHL bioreporter Chromobacterium violaceum CV026 (not shown), suggesting that, while the signaling molecules produced are certainly AHL-like, they may not be identical to those used by bacteria, and it is quite possible that these too are carboxylated or carry some other slight modification, and further study would be required to elucidate their structures.
FIGURE 6.
Acyl homoserine lactone production by H. volcanii. TLC overlay with Agrobacterium tumefaciens ATCC BAA-2240 showing two zones of activation in response to 0.25 mg EtOAc extract of H. volcanii, alongside standards of N-butyryl, -hexanoyl -octanoyl and -dodecanoyl -DL -homoserine lactone molecules.
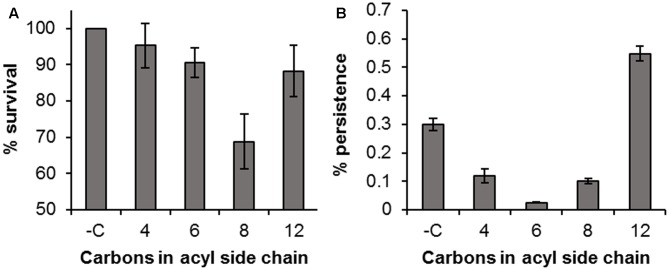
Pre-incubation of H. volcanii with exogenous AHL molecules prior to challenge showed that the AHLs themselves had a considerable antimicrobial activity against H. volcanii, with a reduction of 4.7, 9.4, 31.2, and 11.8% by C4, C6, C8, and C12 AHL respectively, in comparison to the untreated control (Figure 7A). This pattern was still evident, and more pronounced, following the 6 h treatment with H2O2 (Figure 7B). The observed killing pattern was characteristic of the biocidal activity of many classes of compounds bearing hydrophobic side chains (Birnie et al., 2000; Łuczak et al., 2010; Joondan et al., 2014), with activity increasing with chain length, up to a critical length after which it decreases, known as a “cut-off effect.” The results suggest that these AHLs have a biocidal activity against H. volcanii, with a chain length-dependent effect. The number of persisters in the samples incubated with C12 AHL prior to challenge was almost double that of the negative control, suggesting that this particular molecule may have stimulated persister formation, although this is unlikely to have been a quorum sensing effect as H. volcanii is not known to produce a C12 AHL, and is more likely a result of its antimicrobial activity.
FIGURE 7.
Antimicrobial activity of AHLs. Percentage survival of H. volcanii following incubation with 10 μM AHLs for 1 h compared to an untreated control (Time 0) (A); percentage survival following treatment with 0.005% H2O2 in the presence of 10 μM AHLs, compared to the time 0 value (B). Plotted values are the mean of triplicate measurements and error bars represent ± SD.
It was shown by Zhang et al. (2012) that the quorum sensing controlled phenotype observed in Methanosaeta harundinacea could not be induced by bacterial AHLs. Although it appears that H. volcanii does produce AHL-like molecules, the exogenous molecules supplied may not have been sufficiently similar to its own, and not only did these molecules not induce the desired phenotype, they had a detrimental effect on cell viability. It has been shown previously that persister cell formation can be a quorum sensing controlled phenotype in bacteria. RelE-mediated dormancy in E. coli has been shown to be enhanced at high cell densities in comparison to low densities (Tashiro et al., 2012), quorum sensing based on acyl homoserine lactones or pyocyanin can induce persister formation in P. aeruginosa (Möker et al., 2010), and the stress-inducible quorum sensing CSP peptide can induce persister formation in Streptococcus mutans (Leung and Lévesque, 2012). Although not confirmed by this study, based on the results obtained using conditioned media, the possibility remains that persister formation in H. volcanii could still be a quorum sensing controlled phenotype. However, further study would be required to confirm whether or not the mechanism leading to persistence is AHL-based or controlled by another type of signal, and to determine the precise nature of the molecule or molecules responsible.
Conclusion
Our results demonstrate the persister cell phenotype in Archaea. The formation of these specialized survivor cells has therefore now been observed in all three domains of life. Persister formation by haloarchaea could be a strategy employed by these organisms to ensure the survival of populations as a whole following exposure to lethal conditions, environmental fluctuations, or for long-term survival by inducing dormancy when adverse conditions are encountered. While further studies are required to determine the precise mechanisms of haloarchaeal persister formation, the role of toxin–antitoxin systems, indole signaling, and quorum sensing, this initial study may provide some insights into the survival of haloarchaea in stressful or dynamic environments.
Author Contributions
BG and JM conceived and designed the study and experiments. JM conducted the experiments, analyzed the data, and wrote the manuscript. BG critically revised and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by the Irish Marine Institute under the Beaufort Marine Biodiscovery research award funded by the Irish government, under the National Development Plan and the Engineering and Physical Sciences Research Council (EPSRC) grant EP/M027473/1 ‘Building the Queen’s University Belfast AMR Network.’
References
- Amato S. M., Orman M. A., Brynildsen M. P. (2013). Metabolic control of persister formation in Escherichia coli. Mol. Cell 50 475–487. 10.1016/j.molcel.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Balaban N. Q., Merrin J., Chait R., Kowalik L., Leibler S. (2004). Bacterial persistence as a phenotypic switch. Science 305 1622–1625. 10.1126/science.1099390 [DOI] [PubMed] [Google Scholar]
- Baliga N. S., Bjork S. J., Bonneau R., Pan M., Iloanusi C., Kottemann M. C. H., et al. (2004). Systems level insights into the stress response to UV radiation in the halophilic archaeon Halobacterium NRC-1. Genome Res. 14 1025–1035. 10.1101/gr.1993504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. A., Seitzer P. M., Tritt A., Larsen D., Krusor M., Yao A. I., et al. (2014). Phylogenetically driven sequencing of extremely halophilic archaea reveals strategies for static and dynamic osmo-response. PLoS Genet. 10:e1004784 10.1371/journal.pgen.1004784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier S. P., Lebeaux D., DeFrancesco A. S., Valomon A., Soubigou G., Coppée J. Y., et al. (2013). Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet. 9:e1003144 10.1371/journal.pgen.1003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger J. (1944). Treatment of staphylococcal infections with penicillin. Lancet 244 497–500. 10.1016/S0140-6736(00)74210-3 [DOI] [Google Scholar]
- Birnie C. R., Malamud D., Schnaare R. L. (2000). Antimicrobial evaluation of N-Alkyl betaines and N-alkyl-N, N-dimethylamine oxides with variations in chain length. Antimicrob. Agents Chemother. 44 2514–2517. 10.1128/AAC.44.9.2514-2517.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelo G., Ventosa A., Megás M., Ruiz-Berraquero F. (1984). The sensitivity of halobacteria to antibiotics. FEMS Microbiol. Lett. 21 341–345. 10.1016/0378-1097(84)90095-8 [DOI] [Google Scholar]
- Chimileski S., Franklin M. J., Papke R. T. (2014). Biofilms formed by the archaeon Haloferax volcanii exhibit cellular differentiation and social motility, and facilitate horizontal gene transfer. BMC Biol. 12:65 10.1186/s12915-014-0065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N., Kwan B. W., Wood T. K. (2016). Persistence increases in the absence of the alarmone guanosine tetraphosphate by reducing cell growth. Sci. Rep. 6:20519 10.1038/srep20519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. R., Daugherty A. J., Tachdjian S., Blum P. H., Kelly R. M. (2009). Role of vapBC toxin-antitoxin loci in the thermal stress response of Sulfolobus solfataricus. Biochem. Soc. Trans. 37 123–126. 10.1042/BST0370123.Role [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörr T. Vulić M. Lewis K. (2010). Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 8:e1000317 10.1371/journal.pbio.1000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvart M., de Groote V. N., Michiels J. (2011). Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 60 699–709. 10.1099/jmm.0.030932-0 [DOI] [PubMed] [Google Scholar]
- Fröls S., Dyall-Smith M., Pfeifer F. (2012). Biofilm formation by haloarchaea. Environ. Microbiol. 14 3159–3174. 10.1111/j.1462-2920.2012.02895.x [DOI] [PubMed] [Google Scholar]
- Gerdes K., Christensen S. K., Løbner-Olesen A. (2005). Prokaryotic toxin–antitoxin stress response loci. Nat. Rev. Microbiol. 3 371–382. 10.1038/nrmicro1147 [DOI] [PubMed] [Google Scholar]
- Grant W. D. (2004). Life at low water activity. Philos. Trans. R. Soc. B Biol. Sci. 359 1249–1267. 10.1098/rstb.2004.1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D., Gemmell R. T., McGenity T. J. (1998). Halobacteria: the evidence for longevity. Extremophiles 2 279–287. 10.1007/s007920050070 [DOI] [PubMed] [Google Scholar]
- Harrison J. J., Ceri H., Turner R. J. (2007). Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 5 928–938. 10.1038/nrmicro1774 [DOI] [PubMed] [Google Scholar]
- Helaine S., Cheverton A. M., Watson K. G., Faure L. M., Matthews S. A., Holden D. W. (2014). Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343 204–208. 10.1126/science.1244705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobby G., Meyer K., Chaffee E. (1942). Observations on the mechanism of action of penicillin. Proc. Soc. Exp. Biol. Med. 50 281–285. 10.3181/00379727-50-13773 [DOI] [Google Scholar]
- Hu Y., Kwan B. W., Osbourne D. O., Benedik M. J., Wood T. K. (2015). Toxin YafQ increases persister cell formation by reducing indole signalling. Environ. Microbiol. 17 1275–1285. 10.1111/1462-2920.12567 [DOI] [PubMed] [Google Scholar]
- Jõers A., Kaldalu N., Tenson T. (2010). The frequency of persisters in Escherichia coli reflects the kinetics of awakening from dormancy. J. Bacteriol. 192 3379–3384. 10.1128/JB.00056-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joondan N., Jhaumeer-Laulloo S., Caumul P. (2014). A study of the antibacterial activity of l-Phenylalanine and l-Tyrosine esters in relation to their CMCs and their interactions with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DPPC as model membrane. Microbiol. Res. 169 675–685. 10.1016/j.micres.2014.02.010 [DOI] [PubMed] [Google Scholar]
- Keren I., Kaldalu N., Spoering A., Wang Y., Lewis K. (2004a). Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230 13–18. 10.1016/S0378-1097(03)00856-5 [DOI] [PubMed] [Google Scholar]
- Keren I., Shah D., Spoering A., Kaldalu N., Lewis K. (2004b). Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186 8172–8180. 10.1128/JB.186.24.8172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Heo P., Yang T. J., Lee K. S., Cho D. H., Kim B. T., et al. (2011). Selective killing of bacterial persisters by a single chemical compound without affecting normal antibiotic-sensitive cells. Antimicrob. Agents Chemother. 55 5380–5383. 10.1128/AAC.00708-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-S., Wood T. K. (2016). Persistent persister misperceptions. Front. Microbiol. 7:2134 10.3389/fmicb.2016.02134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan B. W., Chowdhury N., Wood T. K. (2015a). Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 17 4406–4414. 10.1111/1462-2920.12873 [DOI] [PubMed] [Google Scholar]
- Kwan B. W., Osbourne D. O., Hu Y., Benedik M. J., Wood T. K. (2015b). Phosphodiesterase DosP increases persistence by reducing cAMP which reduces the signal indole. Biotechnol. Bioeng. 112 588–600. 10.1002/bit.25456 [DOI] [PubMed] [Google Scholar]
- Kwan B. W., Valenta J. A., Benedik M. J., Wood T. K. (2013). Arrested protein synthesis increases persister-like cell formation. Antimicrob. Agents Chemother. 57 1468–1473. 10.1128/AAC.02135-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large A., Stamme C., Lange C., Duan Z., Allers T., Soppa J., et al. (2007). Characterization of a tightly controlled promoter of the halophilic archaeon Haloferax volcanii and its use in the analysis of the essential cct1 gene. Mol. Microbiol. 66 1092–1106. 10.1111/j.1365-2958.2007.05980.x [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Kim Y.-G., Gwon G., Wood T. K., Lee J. (2016). Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Express 6 123 10.1186/s13568-016-0297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Lee J. (2010). Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 34 426–444. 10.1111/j.1574-6976.2009.00204.x [DOI] [PubMed] [Google Scholar]
- Lennon J. T., Jones S. E. (2011). Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 9 119–130. 10.1038/nrmicro2504 [DOI] [PubMed] [Google Scholar]
- Leung V., Lévesque C. M. (2012). A stress-inducible quorum-sensing peptide mediates the formation of persister cells with noninherited multidrug tolerance. J. Bacteriol. 194 2265–2274. 10.1128/JB.06707-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. (2008). “Multidrug tolerance of biofilms and Persister cells,” in Bacterial Biofilms, ed. Romeo T. (Berlin: Springer; ) 107–131. 10.1007/978-3-540-75418-3_6 [DOI] [PubMed] [Google Scholar]
- Lewis K. (2010). Persister cells. Annu. Rev. Microbiol. 64 357–372. 10.1146/annurev.micro.112408.134306 [DOI] [PubMed] [Google Scholar]
- Lowenstein T. K., Schubert B. A., Timofeeff M. N. (2011). Microbial communities in fluid inclusions and long-term survival in halite. GSA Today 21 4–9. 10.1130/GSATG81A.1 [DOI] [Google Scholar]
- Łuczak J., Jungnickel C., Łącka I., Stolte S., Hupka J. (2010). Antimicrobial and surface activity of 1-alkyl-3-methylimidazolium derivatives. Green Chem. 12 593–601. 10.1039/b921805j [DOI] [Google Scholar]
- Maisonneuve E., Castro-Camargo M., Gerdes K. (2013). (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154 1140–1150. 10.1016/j.cell.2013.07.048 [DOI] [PubMed] [Google Scholar]
- Maisonneuve E., Gerdes K. (2014). Molecular mechanisms underlying bacterial persisters. Cell 157 539–548. 10.1016/j.cell.2014.02.050 [DOI] [PubMed] [Google Scholar]
- McCready S., Müller J. A., Boubriak I., Berquist B. R., Ng W. L., DasSarma S. (2005). UV irradiation induces homologous recombination genes in the model archaeon, Halobacterium sp. NRC-1. Saline Systems 1:3 10.1186/1746-1448-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGenity T. J., Gemmell R. T., Grant W. D., Stan-Lotter H. (2000). Origins of halophilic microorganisms in ancient salt deposits. Environ. Microbiol. 2 243–250. 10.1046/j.1462-2920.2000.00105.x [DOI] [PubMed] [Google Scholar]
- Mesbah N. M., Abou-El-Ela S. H., Wiegel J. (2007). Novel and unexpected prokaryotic diversity in water and sediments of the alkaline, hypersaline lakes of the Wadi An Natrun. Egypt. Microb. Ecol. 54 598–617. 10.1007/s00248-006-9193-y [DOI] [PubMed] [Google Scholar]
- Möker N., Dean C. R., Tao J. (2010). Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules. J. Bacteriol. 192 1946–1955. 10.1128/JB.01231-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormile M. R., Hong B.-Y., Benison K. C. (2009). Molecular analysis of the microbial communities of Mars analog lakes in Western Australia. Astrobiology 9 919–930. 10.1089/ast.2008.0293 [DOI] [PubMed] [Google Scholar]
- Page R., Peti W. (2016). Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 12 208–214. 10.1038/nchembio.2044 [DOI] [PubMed] [Google Scholar]
- Paggi R. A., Martone C. B., Fuqua C., De Castro R. E. (2003). Detection of quorum sensing signals in the haloalkaliphilic archaeon Natronococcus occultus. FEMS Microbiol. Lett. 221 49–52. 10.1016/S0378-1097(03)00174-5 [DOI] [PubMed] [Google Scholar]
- Pandey D. P., Gerdes K. (2005). Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33 966–976. 10.1093/nar/gki201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F. (1988). “Genetics of halobacteria,” in Halophilic Bacteria ed. Rodriguez-Valera F. (Boca Raton, FL: CRC Press; ) 105–133. [Google Scholar]
- Shah D., Zhang Z., Khodursky A., Kaldalu N., Kurg K., Lewis K. (2006). Persisters: a distinct physiological state of E. coli. BMC Microbiol. 6:53 10.1186/1471-2180-6-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Harrison E. M., Bi D., Tai C., He X., Ou H. Y., et al. (2011). TADB: a web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res. 39 606–611. 10.1093/nar/gkq908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoering A. L., Lewis K. (2001). Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183 6746–6751. 10.1128/JB.183.23.6746-6751.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan-Lotter H., Radax C., Gruber C., Legat A., Pfaffenhuemer M., Wieland H., et al. (2002). Astrobiology with haloarchaea from Permo-Triassic rock salt. Int. J. Astrobiol. 1 271–284. 10.1017/S1473550403001307 [DOI] [Google Scholar]
- Takagi H., Kakuta Y., Okada T., Yao M., Tanaka I., Kimura M. (2005). Crystal structure of archaeal toxin-antitoxin RelE–RelB complex with implications for toxin activity and antitoxin effects. Nat. Struct. Mol. Biol. 12 327–331. 10.1038/nsmb911 [DOI] [PubMed] [Google Scholar]
- Tashiro Y., Kawata K., Taniuchi A., Kakinuma K., May T., Okabe S. (2012). RelE-mediated dormancy is enhanced at high cell density in Escherichia coli. J. Bacteriol. 194 1169–1176. 10.1128/JB.06628-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombre R. S., Shinde V. D., Oke R. S., Dhar S. K., Shouche Y. S. (2016). Biology and survival of extremely halophilic archaeon Haloarcula marismortui RR12 isolated from Mumbai salterns, India in response to salinity stress. Sci. Rep. 6:25642 10.1038/srep25642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommonaro G., Abbamondi G. R., Iodice C., Tait K., De Rosa S. (2012). Diketopiperazines produced by the halophilic archaeon, Haloterrigena hispanica, activate AHL bioreporters. Microb. Ecol. 63 490–495. 10.1007/s00248-011-9980-y [DOI] [PubMed] [Google Scholar]
- Van den Bergh B., Michiels J. E., Wenseleers T., Windels E. M., Vanden Boer P., Kestemont D., et al. (2016). Frequency of antibiotic application drives rapid evolutionary adaptation of Escherichia coli persistence. Nat. Microbiol. 1:16020 10.1038/nmicrobiol.2016.20 [DOI] [PubMed] [Google Scholar]
- Vega N. M., Allison K. R., Khalil A. S., Collins J. J. (2012). Signaling-mediated bacterial persister formation. Nat. Chem. Biol. 8 431–433. 10.1038/nchembio.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Vulić M., Keren I., Lewis K. (2012). Role of oxidative stress in persister tolerance. Antimicrob. Agents Chemother. 56 4922–4926. 10.1128/AAC.00921-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Park J.-H., Inouye M. (2011). Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 45 61–79. 10.1146/annurev-genet-110410-132412 [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang F., Ding G., Li J., Guo X., Zhu J., et al. (2012). Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. ISME J. 6 1336–1344. 10.1038/ismej.2011.203 [DOI] [PMC free article] [PubMed] [Google Scholar]