Abstract
Objective
To report MRI outcomes and explore the relationship between clinical remission and MRI inflammation in patients with axial spondyloarthritis (axSpA) from the RAPID-axSpA trial, including radiographic (r-)axSpA and non-radiographic (nr-)axSpA.
Methods
RAPID-axSpA (NCT01087762) was double-blind and placebo-controlled to week 24, dose-blind to week 48 and open-label to week 204. Patients were randomised to certolizumab pegol (CZP) or placebo. Placebo patients entering dose-blind were rerandomised to CZP. MRIs performed at baseline, weeks 12, 48 and 96 were scored by 2 reviewers independently: Spondyloarthritis Research Consortium of Canada (SPARCC) for sacroiliac (SI) joints; Berlin modification of the Ankylosing Spondylitis spine MRI scoring system for disease activity (Berlin) for spine. Inflammation thresholds: SPARCC≥2; Berlin>2. Remission thresholds: SPARCC<2 (SI joints); Berlin≤2 (spine); Ankylosing Spondylitis Disease Activity Score (ASDAS) inactive disease (<1.3, clinical).
Results
Across 163 patients in the MRI set (109 CZP; 54 placebo), week 12 mean changes from baseline in MRI scores were greater for CZP versus placebo: SPARCC: −4.8 (SD 8.6) vs −1.6 (7.8; p<0.001); Berlin: −2.9 (4.2) vs 0.2 (4.8; p<0.001). Improvements were maintained to week 96. Week 12 MRI remission was achieved by 52.6% of patients with baseline MRI inflammation in SI joints, 62.0% in the spine and 37.9% of patients with both. MRI remission rates were sustained to week 96, with similar trends in r-axSpA and nr-axSpA. At week 96, 57.5% vs 65.9% of patients achieving versus not achieving clinical remission had MRI remission.
Conclusions
CZP reduced inflammation in the spine and SI joints in patients with r-axSpA and nr-axSpA, with improvements maintained over 96 weeks. Substantial proportions of patients achieved MRI remission. Concordance between clinical remission and current definitions of absence of MRI inflammation was limited.
Trial registration number
NCT01087762; Post-results.
Keywords: Ankylosing Spondylitis, Magnetic Resonance Imaging, Anti-TNF, Treatment
Key messages.
What is already known about this subject?
A relationship between MRI inflammation in the sacroiliac (SI) joints and structural progression from non-radiographic to radiographic axial spondyloarthritis (axSpA) has been shown. However, the relationship between improvement in clinical disease activity and MRI inflammation is not well established.
While certolizumab pegol (CZP) has demonstrated efficacy in clinical and patient-reported outcomes in radiographic and non-radiographic axSpA, with improvements maintained to 4 years, its effect on MRI inflammation has not previously been reported.
What does this study add?
In patients with both radiographic and non-radiographic axSpA, CZP rapidly reduced inflammation in the spine and SI joints, with improvements sustained over 96 weeks. A substantial proportion of patients achieved MRI remission, using stipulated definitions.
No strong associations were seen between clinical remission and the absence of MRI inflammation, either in patients with MRI inflammation at baseline, or in all patients irrespective of baseline MRI status.
How might this impact on clinical practice?
The lack of a strong association between clinical remission and the absence of MRI inflammation suggests that one cannot be used as a surrogate for the other by the treating physician.
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease characterised by inflammation of the sacroiliac (SI) joints and the spine. AxSpA encompasses patients with structural changes due to sacroiliitis detectable by conventional radiographs and fulfilling the modified New York (mNY) criteria,1 classified as having radiographic axSpA (r-axSpA), or ankylosing spondylitis (AS), and those not meeting the mNY criteria, classified as having non-radiographic axSpA (nr-axSpA).
MRI has emerged as an integral diagnostic tool for axSpA, as it can identify both active inflammation and structural changes, whereas other imaging modalities (conventional radiography and CT) can only demonstrate structural changes.2 This has allowed physicians to diagnose axSpA earlier, and thus offers the possibility to initiate early treatment for better symptom relief, aiming for the prevention of structural damage or halting of structural progression.3
In addition to the use of MRI as a diagnostic and classification tool for patients with axSpA, it can also be used as an outcome measure to assess the level of inflammation in the spine and SI joints, which potentially aids the decision-making process when considering treatment with an antitumour necrosis factor (TNF).4 5 A relationship between inflammation in the SI joints as assessed by MRI and structural progression from nr-axSpA to r-axSpA has been shown,6 7 and there is accumulating evidence showing an association between MRI inflammation in the spine and syndesmophyte formation.8–12 Currently, clinical disease activity is used to monitor treatment efficacy in clinical practice. It is still unclear what role improvements in inflammation, as assessed by MRI, should play in axSpA treatment,13 14 as the relationship between improvement in clinical disease activity and improvement in MRI inflammation is not well established.
Certolizumab pegol (CZP) is a pegylated Fc-free anti-TNF with proven efficacy in clinical and patient-reported outcomes in r-axSpA and nr-axSpA.15–17 Here, we use MRI data from 96 weeks of the RAPID-axSpA trial to address three questions:
What is the effect of CZP treatment on inflammation in the spine and SI joints in patients with axSpA, compared with placebo treatment to week 12, and during open-label treatment to week 96?
What proportion of CZP-treated patients with axSpA achieve MRI remission in the spine and SI joints?
Does clinical remission correlate with the absence of MRI inflammation, that is, can clinical remission be used as a surrogate for ongoing imaging?
Methods
Patients
Detailed inclusion and exclusion criteria of the RAPID-axSpA trial have been previously reported.15 In brief, eligible patients were aged ≥18 years with a diagnosis of adult-onset axSpA, fulfilling the Assessment of Spondyloarthritis (ASAS) criteria,18 of ≥3 months' duration and previous treatment failure with ≥1 non-steroidal anti-inflammatory drug. All patients (both r-axSpA and nr-axSpA) must have had active disease (Bath AS Disease Activity Index (BASDAI)19 ≥4 and spinal pain ≥4) and objective signs of inflammation (C reactive protein (CRP) levels >Upper Limit of Normal (ULN) or evidence of sacroiliitis on MRI). Patients who had prior exposure to >2 previous biological agents (>1 anti-TNF) for the treatment of axSpA were excluded. Patients with r-axSpA and nr-axSpA were included in the study; patients were stratified based on the presence of radiographic sacroiliitis at baseline.
Study design
The RAPID-axSpA trial was a 204-week phase 3 multicentre trial in patients with axSpA that was double-blind and placebo-controlled to week 24, dose-blind to week 48 and open-label to week 204. Certain investigator sites participated in the imaging substudy of the main RAPID-axSpA trial, which involved MRI of the SI joints and spine, and spinal X-ray assessments. Here, we report MRI outcomes from the imaging substudy through to week 96 of the trial.
Patients were randomised 1:1:1 to placebo, or CZP 400 mg at weeks 0, 2 and 4 (loading dose) followed by either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks. Patients originally randomised to CZP in the double-blind phase continued on their assigned dose in the dose-blind and open-label phases. Patients originally randomised to placebo, who were non-responders according to the ASAS20 response criteria at weeks 14 and 16, or who completed the 24-week double-blind phase, entered the dose-blind phase and were rerandomised 1:1 to CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks following the CZP loading dose (see online supplementary figure 1A).
rmdopen-2017-000430supp001.pdf (992.1KB, pdf)
Study assessment
The primary end point of RAPID-axSpA was the week 12 ASAS20 response, which was met and is reported elsewhere.15 Here, we report imaging data over 96 weeks of the trial. MRIs of the SI joints and spine were performed at sites participating in the imaging substudy using short-τ-inversion-recovery (STIR) sequences at baseline (±2 weeks), weeks 12, 48 and 96 (see online supplementary figure 1A). The Spondyloarthritis Research Consortium of Canada (SPARCC) scoring method20 was used for lesions found in the SI joints on MRI. Total SI joint SPARCC is scored on a 0–72 scale. The Berlin modification of the Ankylosing Spondylitis spine MRI scoring system for disease activity (Berlin)21–24 was used for lesions found in the spine on MRI. Total spine score in the Berlin modification is scored from 0–69.
Data are reported as average scores of the readers (XB, K-GH, PMM) from the week 96 reading campaign, where all MRIs from the four MRI assessment time points (baseline, weeks 12, 48 and 96) were scored by two expert readers independently, without adjudication (two of the three readers scored each MRI, based on reader availability). Average scores of the two readers were also used to determine whether a patient achieved the specified thresholds for remission. Readers were blinded both to the time point and the treatment group.
A priori MRI parameters included change from baseline in SPARCC SI joint scores and Berlin scores for the spine. Post hoc MRI parameters included the achievement of MRI remission, defined using the validated cut-off of SPARCC<2 for the SI joints,25 and the unvalidated cut-off of Berlin score ≤2 for the spine. The Ankylosing Spondylitis Disease Activity Score (ASDAS)-CRP was calculated as per van der Heijde26 at week 96, and clinical remission was defined as ASDAS inactive disease (ASDAS ID: ASDAS<1.3).27
Statistical analysis
Demographic and baseline characteristics are presented for the imaging substudy: defined as patients with a valid baseline and ≥1 postbaseline assessment for either of the MRI parameters or spinal X-ray. Only observed data are reported for patients in the imaging set, with no imputation of missing data.
In this publication, we undertake three different analyses, to examine three different questions. The specific methodology of each of these is described below.
MRI outcomes
In a prespecified analysis, MRI outcomes at week 12 were compared between CZP and placebo using an analysis of covariance model with treatment, region, mNY criteria (yes or no) and prior anti-TNF exposure (yes or no) as factors and baseline MRI score as the covariate. Week 12 MRI outcomes are reported separately for patients originally randomised to CZP (doses combined) or placebo. Week 48 and 96 outcomes are reported for all patients, regardless of baseline randomisation (ie, including patients switching from placebo (PBO) to CZP).
Achievement of MRI remission
Achievement of MRI remission was analysed in those patients with MRI evidence of inflammation at baseline only. Evidence of inflammation, as assessed by MRI, was based on the validated threshold of SPARCC≥2 for the SI joints,25 and the unvalidated thresholds of Berlin score >2 for the spine. Combined data are reported for all patients, regardless of baseline randomisation.
Association between clinical remission and the absence of MRI inflammation
In order to explore whether a correlation exists between clinical remission and the absence of MRI inflammation, patients from the imaging set in clinical remission at week 96 were identified, and subsequently the proportions with/without MRI inflammation were calculated (irrespective of whether MRI inflammation was present at baseline). Combined data are reported, regardless of baseline randomisation. An additional analysis included only those patients with evidence of MRI inflammation at baseline, to ask whether patients achieving clinical remission also achieved a reduction in their MRI inflammation.
Results
Patient disposition and baseline characteristics
Of the 325 patients randomised, 163 were included in the imaging set. Of these patients, 158 (97%) remained in the study to week 24, 146 (90%) to week 48 and 132 (81%) to week 96 (see online supplementary figure 1B).16
Patient demographics and characteristics in the imaging set (table 1) were comparable to those of the overall study population at baseline.15 In the imaging set, there were more men in the r-axSpA group, and patients with r-axSpA had higher CRP and Bath Ankylosing Spondylitis Metrology Index (BASMI), but other baseline demographics and characteristics were similar between the r-axSpA and nr-axSpA subpopulations. With regard to MRI outcomes, there was a higher proportion of patients with r-axSpA with spinal inflammation on MRI when compared with the nr-axSpA subpopulation, though SI joint inflammation was slightly more common in patients with nr-axSpA (table 1).
Table 1.
Baseline demographics and patient characteristics for all patients included in the imaging set
| r-axSpA |
nr-axSpA |
|||||
|---|---|---|---|---|---|---|
| Overall r-axSpA (n=95) |
Week 0 CZP (dose combined) (n=63) | Placebo (n=32) | Overall nr-axSpA (n=68) |
Week 0 CZP (dose combined) (n=46) | Placebo (n=22) | |
| Except where indicated otherwise, values are n (%) at baseline | ||||||
| Age, years, mean (SD) | 40.6 (11.6) | 40.5 (10.3) | 40.8 (13.8) | 36.6 (12.9) | 36.7 (12.8) | 36.2 (13.5) |
| Gender, male | 69 (72.6) | 44 (69.8) | 25 (78.1) | 36 (52.9) | 24 (52.2) | 12 (54.5) |
| BMI, mean (SD) | 27.2 (5.3) | 27.3 (5.5) | 26.9 (5.0) | 26.4 (5.8) | 26.9 (5.8) | 25.5 (5.9) |
| Symptom duration, years, median (minimum, maximum) | 9.5 (0.3, 50.9) | 8.9 (0.3, 44.8) | 9.8 (0.5, 50.9) | 5.2 (0.3, 39.6) | 5.4 (0.3, 31.4) | 5.0 (0.5, 39.6) |
| Symptom duration, <5 years | 33 (34.7) | 23 (36.5) | 10 (31.3) | 34 (50.0) | 23 (50.0) | 11 (50.0) |
| Smoking status | ||||||
| Current | 21 (22.1) | 15 (23.8) | 6 (18.8) | 20 (29.4) | 13 (28.3) | 7 (31.8) |
| Former | 18 (18.9) | 8 (12.7) | 10 (31.3) | 13 (19.1) | 9 (19.6) | 4 (18.2) |
| Never | 56 (58.9) | 40 (63.5) | 16 (50.0) | 35 (51.5) | 24 (52.2) | 11 (50.0) |
| Positive for HLA-B27 | 78 (82.1) | 51 (81.0) | 27 (84.4) | 53 (77.9) | 34 (73.9) | 19 (86.4) |
| CRP mg/L, median | 14.2 | 14.0 | 16.0 | 10.5 | 10.5 | 10.5 |
| SPARCC SI joint score, mean (SD) | 7.2 (11.9) | 7.2 (11.9) | 12.6 (17.1) | 7.2 (9.8) | 7.2 (9.8) | 11.8 (13.5) |
| SI joint inflammation on MRI (SPARCC≥2) | 49 (52.7) | 33 (54.1) | 16 (50.0) | 41 (68.3) | 26 (65.0) | 15 (75.0) |
| Berlin, mean (SD) | 5.3 (5.9) | 5.3 (5.9) | 5.5 (7.0) | 2.8 (4.2) | 2.8 (4.2) | 3.2 (7.7) |
| Spinal inflammation on MRI (Berlin >2) | 54 (58.1) | 36 (59.0) | 18 (56.3) | 19 (31.7) | 14 (35.0) | 5 (25.0) |
| SI joint and spinal inflammation on MRI (SPARCC≥2 and Berlin >2) | 32 (34.4) | 23 (37.7) | 9 (28.1) | 11 (19.0) | 8 (21.1) | 3 (15.0) |
| BASDAI, mean (SD) | 6.6 (1.5) | 6.5 (1.5) | 6.8 (1.7) | 6.5 (1.5) | 6.5 (1.5) | 6.4 (1.4) |
| BASFI, mean (SD) | 6.0 (2.1) | 5.9 (2.1) | 6.3 (1.9) | 4.8 (2.2) | 4.8 (2.3) | 4.7 (2.0) |
| BASMI, mean (SD) | 4.4 (1.5) | 4.2 (1.5) | 4.8 (1.5) | 2.9 (1.4) | 3.0 (1.4) | 2.8 (1.3) |
| ASDAS, mean (SD) | 4.0 (0.9) | 3.9 (0.8) | 4.2 (1.0) | 3.7 (0.9) | 3.7 (0.8) | 3.8 (1.0) |
| Peripheral arthritis* | 36 (37.9) | 20 (31.7) | 16 (50.0) | 29 (42.6) | 16 (34.8) | 13 (59.1) |
| Enthesitis† | 72 (75.8) | 46 (73.0) | 26 (81.3) | 50 (73.5) | 33 (71.7) | 17 (77.3) |
| Extraspinal features of axSpA (either patient history or current diagnosis) Defined by the ASAS classification criteria screening assessment | ||||||
| Heel enthesitis | 38 (40.0) | 24 (38.1) | 14 (43.8) | 25 (36.8) | 14 (30.4) | 11 (50.0) |
| Uveitis | 22 (23.2) | 12 (19.0) | 10 (31.3) | 14 (20.6) | 9 (19.6) | 5 (22.7) |
| Psoriasis | 3 (3.2) | 2 (3.2) | 1 (3.1) | 5 (7.4) | 4 (8.7) | 1 (4.5) |
| Inflammatory bowel disease | 6 (6.3) | 3 (4.8) | 3 (9.4) | 1 (1.5) | 0 | 1 (4.5) |
*Defined as ≥1 swollen joint in a 44-joint assessment.
†Defined as a Maastricht Ankylosing Spondylitis Entheses Score (MASES) score >0.
ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMI, body mass index; CRP, C reactive protein; CZP, certolizumab pegol; HLA, human leucocyte antigen; nr-axSpA, non-radiographic axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis; SI, sacroiliac; SPARCC, Spondyloarthritis Research Consortium of Canada.
At baseline, 43 (28.5%) patients had inflammation in the SI joints and spine, 46 (30.5%) patients had inflammation in the SI joints only, 28 (18.5%) patients had inflammation in the spine only, and 34 (22.5%) patients did not have inflammation in either the SI joints or the spine, as demonstrated by MRI (see online supplementary table S1). Of patients without sacroiliac joint (SIJ) inflammation at baseline, 6/18 patients with nr-axSpA (33.3%) and 22/44 patients with r-axSpA (50.0%) had evidence of spinal inflammation.
At baseline, more spinal inflammation on MRI (ie, a higher Berlin score) was observed among patients with evidence of inflammation in the SI joints (SPARCC≥2) compared with those without (see online supplementary figure 2A). Similarly, a greater degree of SI joint inflammation on MRI (ie, higher SPARCC SI joint scores) was observed among patients with evidence of inflammation in the spine compared with those without (see online supplementary figure 2B).
MRI outcomes
At week 12, the observed mean change from baseline (SD) in the SPARCC SI joint score was significantly greater for CZP-treated patients than patients given placebo: −4.8 (8.6) for patients treated with CZP (dose combined) compared with −1.6 (7.8) for placebo (p<0.001; table 2). Corresponding improvements were seen in the spine for CZP-treated patients, with observed mean change from baseline scores (SD) in the Berlin score at week 12 of −2.9 (4.2) in the CZP-treated group (dose combined) compared with 0.2 (4.8) in the placebo group (p<0.001; table 2). Similar improvements in MRI scores were observed with both CZP dosing regimens at week 12 and were also similar in the r-axSpA and nr-axSpA subpopulations (table 2).
Table 2.
Improvement from baseline in SPARCC (SI joints) and Berlin (spine) at week 12 of the RAPID-axSpA trial for all patients in the imaging set (observed data)
| CZP (dose combined) |
Placebo |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean score at baseline (SD)* | Mean score at week 12 (SD) | Mean change from baseline (SD) | N | Mean score at baseline (SD)* | Mean score at week 12 (SD) | Mean change from baseline (SD) | p Value† | |
| SPARCC (SI joints) | |||||||||
| axSpA | 97 | 6.9 (10.4) | 2.1 (4.1) | −4.8 (8.6) | 45 | 12.9 (16.6) | 11.2 (14.5) | −1.6 (7.8) | <0.001 |
| r-axSpA | 58 | 6.5 (10.8) | 1.5 (3.6) | −5.0 (9.1) | 28 | 13.3 (18.0) | 9.9 (14.4) | −3.4 (8.9) | <0.001 |
| nr-axSpA | 39 | 7.4 (9.9) | 2.9 (4.7) | −4.4 (7.9) | 17 | 12.2 (14.5) | 13.4 (14.9) | 1.2 (4.6) | <0.001 |
| Berlin (spine) | |||||||||
| axSpA | 99 | 4.4 (5.4) | 1.4 (2.4) | −2.9 (4.2) | 49 | 4.9 (7.4) | 5.2 (7.3) | 0.2 (4.8) | <0.001 |
| r-axSpA | 60 | 5.3 (5.9) | 1.8 (2.8) | −3.6 (4.7) | 32 | 5.5 (7.0) | 5.8 (6.5) | 0.2 (5.8) | <0.001 |
| nr-axSpA | 39 | 2.9 (4.2) | 0.9 (1.4) | −2.0 (3.2) | 17 | 3.7 (8.3) | 4.0 (8.7) | 0.3 (1.6) | 0.006 |
*Matched baseline readings; in the week 96 reading campaign, MRIs from each previous visit were reread.
†Difference to placebo in mean change from baseline scores using an ANCOVA model with treatment, region, mNY criteria (yes or no; for the axSpA analysis only) and prior anti-TNF exposure (yes or no) as factors and baseline score as covariate.
ANCOVA, analysis of covariance; axSpA, axial spondyloarthritis; CZP, certolizumab pegol; mNY, modified New York; nr-axSpA, non-radiographic axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis; SI, sacroiliac; SPARCC, Spondyloarthritis Research Consortium of Canada; TNF, tumour necrosis factor.
Improvements to week 12 with both MRI outcome measures were maintained through the dose-blind (to week 48) and the open-label (to week 96) trial periods (table 3), with greater improvements seen in patients with higher levels of inflammation at baseline (figure 1).
Table 3.
Improvement from baseline scores in SPARCC (SI joints) and Berlin (spine) at weeks 48 and 96 of the RAPID-axSpA trial for all patients in the imaging set (observed data)
| All CZP (dose combined) |
||||
|---|---|---|---|---|
| N | Mean score at baseline (SD)* | Mean score (SD) | Mean change from baseline (SD) | |
| SPARCC (SI joints) | ||||
| Week 48 | ||||
| axSpA | 113 | 9.0 (13.3) | 2.7 (5.7) | −6.3 (12.7) |
| r-axSpA | 61 | 9.6 (14.9) | 2.0 (4.5) | −7.6 (13.0) |
| nr-axSpA | 52 | 8.3 (11.3) | 3.5 (6.9) | −4.8 (12.2) |
| Week 96 | ||||
| axSpA | 125 | 9.2 (13.1) | 2.1 (6.2) | −7.2 (12.6) |
| r-axSpA | 77 | 9.5 (14.1) | 1.4 (5.6) | −8.1 (12.7) |
| nr-axSpA | 48 | 8.8 (11.4) | 3.2 (7.0) | −5.6 (12.4) |
| Berlin (spine) | ||||
| Week 48 | ||||
| axSpA | 114 | 3.8 (5.7) | 1.4 (2.6) | −2.4 (4.4) |
| r-axSpA | 62 | 4.6 (5.6) | 1.7 (3.2) | −2.9 (4.0) |
| nr-axSpA | 52 | 2.9 (5.7) | 1.0 (1.5) | −1.9 (4.7) |
| Week 96 | ||||
| axSpA | 126 | 4.7 (6.2) | 1.4 (2.5) | −3.3 (5.1) |
| r-axSpA | 78 | 5.6 (6.3) | 1.6 (2.9) | −4.0 (5.1) |
| nr-axSpA | 48 | 3.3 (5.9) | 1.0 (1.7) | −2.3 (5.0) |
Patients randomised to placebo at baseline received CZP from week 16 (if escaping early), or week 24 (if completing double-blind phase) and are included in weeks 48 and 96 results.
*Matched baseline readings; in the week 96 reading campaign, MRIs from each previous visit were re-read.
axSpA, axial spondyloarthritis; CZP, certolizumab pegol; nr-axSpA, non-radiographic axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis; SI, sacroiliac; SPARCC, Spondyloarthritis Research Consortium of Canada.
Figure 1.
Baseline scores and corresponding changes to week 96 of the RAPID-axSpA trial for all CZP-treated patients included in the imaging substudy for: (A) SPARCC SI joint scores, (B) Berlin score for the spine. Patients randomised to placebo at baseline received CZP from week 16 (if escaping early), or week 24 (if completing a double-blind phase) and are included in these results. axSpA, axial spondyloarthritis; CZP, certolizumab pegol; SI, sacroiliac; SPARCC, Spondyloarthritis Research Consortium of Canada.
Achievement of MRI remission
Placebo-controlled period (week 12 data)
At week 12, among patients with MRI inflammation at baseline, higher proportions of CZP-randomised versus placebo-randomised patients achieved MRI remission: SI joint remission was achieved by 30 of 57 (52.6%) CZP-randomised patients versus 0 of 26 placebo-randomised patients (figure 2A), and spinal remission was achieved by 31 of 50 (62.0%) CZP-randomised and 3 of 23 (13.0%) placebo-randomised patients (figure 2B). Of patients with both SI joint and spinal inflammation at baseline, 11/29 (37.9%) CZP patients and 0/11 (0.0%) placebo patients achieved MRI remission of both sites (figure 2C). SI joint remission rates were numerically higher in patients with r-axSpA (61.3%), compared with patients with nr-axSpA (42.3%); spinal remission rates were similar between groups (r-axSpA: 61.1%; nr-axSpA: 64.3%).
Figure 2.
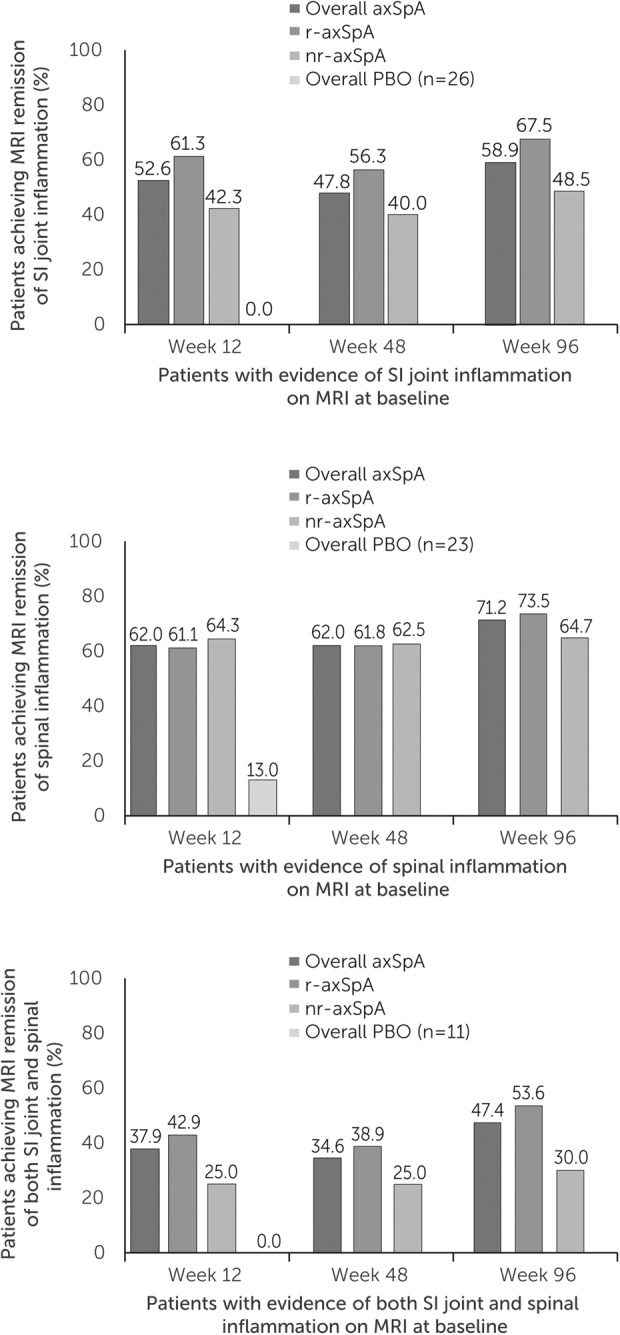
MRI remission in the imaging set of patients from the RAPID-axSpA trial. Remission rates to week 96 in patients with (A) SI joint inflammation, (B) spinal inflammation and (C) both SI joint and spinal. Observed case data shown at week 12 for patients randomised to CZP at baseline and for all patients regardless of baseline randomisation thereafter. axSpA, axial spondyloarthritis; CZP, certolizumab pegol; nr-axSpA, non-radiographic axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis; SI, sacroiliac.
Notably, among patients without evidence of SI joint inflammation on MRI at baseline, 1 of 40 CZP-treated patients (2.5%) developed inflammation at week 12, compared with 2 of 19 (10.5%) placebo-treated patients (all had r-axSpA). Similarly, among patients without MRI evidence of spinal inflammation at baseline, 1 of 49 CZP-treated patients with r-axSpA (2.0%) developed inflammation at week 12 compared with 3 of 26 (11.5%) placebo-treated patients (1 r-axSpA and 2 nr-axSpA).
Dose-blind and early open-label periods (data to week 96)
MRI remission rates at week 12 were sustained to week 96 for all patients treated with CZP (ie, including patients rerandomised from placebo to CZP). Similar trends were observed for patients with r-axSpA and nr-axSpA, although, as at week 12, patients with r-axSpA tended to have higher rates of MRI remission of the SI joints than patients with nr-axSpA (figure 2A–C). The majority of patients who achieved the specified thresholds for remission at week 12 sustained these results to week 96 (60.0% of those with SI joint remission, 88.2% of those with Berlin spine remission). Among those who did not achieve remission at week 12, only 26.4% (SPARCC SI joint) and 33.3% (Berlin spine) subsequently achieved remission at weeks 48 and 96 (data not shown). A summary of patients achieving SI joint, Berlin spine and both SI joint and Berlin spine remission is shown in online supplementary figure S3.
In patients with MRI evidence of inflammation in the SI joints and spine at baseline, MRI remission of SI joints and spinal inflammation occurred with similar frequency to week 96 (24 (63.2%) and 26 (68.4%) of 38 patients, respectively).
Associations between clinical and MRI remission
Further investigations assessed whether achievement of clinical remission was associated with the absence of MRI inflammation. All patients were included, irrespective of whether MRI inflammation was present at baseline.
A third of all patients achieved clinical remission at week 96 (33.3%; 41/123). Of these patients, 63.4% (26/41) also did not have MRI inflammation (SPARCC<2) of the SI joints (figure 3A). However, of the 82 patients who did not achieve clinical remission, 65 (79.3%) also did not have MRI inflammation of the SI joints, suggesting that clinical remission and MRI SI joint inflammation are not closely associated.
Figure 3.
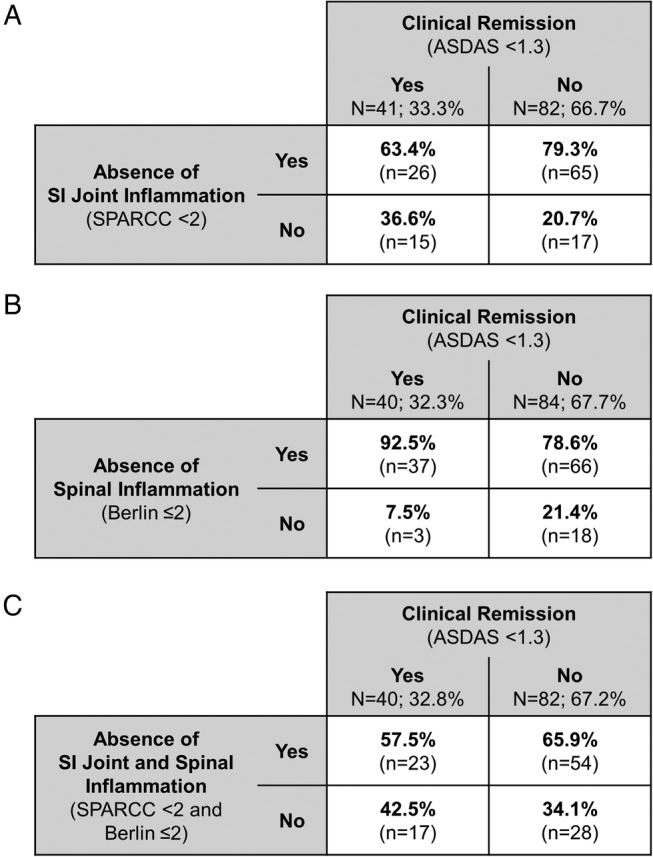
Correlations between clinical remission and MRI inflammation of the (A) SI joints, (B) spine and (C) both SI joints and spine, at week 96 of the RAPID-axSpA trial for all patients with axSpA in the imaging set. ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; SI, sacroiliac; SPARCC, Spondyloarthritis Research Consortium of Canada.
High proportions of patients both achieving and not achieving clinical remission at week 96 had no spinal inflammation on MRI (92.5% of patients achieving, and 78.6% of patients not achieving clinical remission; figure 3B), again indicating a lack of correlation between clinical remission and MRI inflammation.
When considering MRI inflammation of the SI joints and the spine, this was achieved with similar frequency regardless of the week 96 clinical remission state (figure 3C). Achievement of MRI and clinical remission did not differ greatly between the r-axSpA and nr-axSpA subpopulations (see online supplementary figure S4).
In addition to determining whether patients achieving/not achieving clinical remission met the specified thresholds for MRI inflammation, we examined summary statistics of MRI parameters in patients with clinical remission or other categories of disease activity (ASDAS moderate disease activity, high disease activity and very high disease activity). Again, no strong correlations were seen between MRI parameters and disease activity (see online supplementary table S2).
In order to examine a possible association between clinical remission and MRI remission (ie, a reduction in MRI inflammation), we assessed only those patients with evidence of MRI inflammation at baseline. Similar to the analyses above, no strong associations were seen between clinical remission and MRI remission: spinal remission was seen in only slightly higher proportions of patients achieving versus not achieving clinical remission (82.4% of patients achieving vs 68.8% of patients not achieving clinical remission). Trends were similar in patients with r-axSpA and nr-axSpA (see online supplementary figure S5). When looking at SI joint remission, the opposite (weak) trend was observed; SIJ remission was achieved by 44.4% of patients who did achieve clinical remission, versus 68.9% of patients who did not.
Discussion
The results reported here from the RAPID-axSpA trial demonstrate that CZP treatment of patients with axSpA significantly reduced MRI inflammation in the SI joints and spine over 12 weeks; these improvements were seen across the broad axSpA population, including patients with r-axSpA and nr-axSpA. Improvements in MRI scores were maintained through to week 96 for patients treated with CZP, including patients originally randomised to placebo. Furthermore, when stipulated thresholds for remission of inflammation, as demonstrated by MRI, were applied to these data, over half of all patients with baseline inflammation in either the SI joints or spine, and at least a third of patients with inflammation in both, achieved week 12 MRI remission, which was sustained to week 96.
Results from previous studies have demonstrated the effectiveness of anti-TNFs in improving long-term MRI outcomes in patients with r-axSpA,28–31 and short-term MRI outcomes in patients with nr-axSpA.32 33 Additionally, results from the ESTHER trial have demonstrated similar responses in MRI outcomes in patients with r-axSpA and nr-axSpA over 3 years of treatment with etanercept, though the patient numbers in this study were small (31 patients with r-axSpA and 30 with nr-axSpA).34 This is the first report demonstrating long-term (2 years) maintenance of improvements in MRI outcomes in a large patient population (N=163) containing both patients with r-axSpA and nr-axSpA treated with an anti-TNF.
At baseline, there was no difference observed in the level of inflammation in the SI joints between the r-axSpA and nr-axSpA subpopulations (ie, mean SPARCC score), but a slightly higher proportion of patients with nr-axSpA had definite evidence of inflammation (ie, SPARCC score ≥2) than patients with r-axSpA. In contrast, patients with r-axSpA had a greater level of inflammation in the spine at baseline than patients with nr-axSpA, with approximately one-half of patients with r-axSpA compared with one-third of patients with nr-axSpA having definite evidence of inflammation in the spine at baseline. We also noted that a substantial proportion (33.3%) of patients with nr-axSpA without SI joint inflammation had evidence of spinal inflammation on MRI. This is in agreement with data from the ABILITY-1 trial,35 which concluded that spinal inflammation may be present in up to half of the patients with nr-axSpA without SIJ inflammation.
In the overall axSpA population, baseline data suggested a relationship between inflammation in the SI joints and the spine. Presence of SI joint inflammation at baseline was associated with higher spinal MRI scores than in those with absence of inflammation at baseline. The same was true for spinal inflammation and SI joint MRI scores, with greater SI joint MRI scores observed in patients with spinal inflammation at baseline than those without.
Since it is impractical to conduct frequent MRIs in clinical practice, the question of whether patients in clinical remission have concurrent absence of axial inflammation is an important one to help determine whether ongoing imaging is necessary. The findings from the present study suggest a lack of correlation between the achievement of clinical remission and lack of MRI evidence of inflammation at week 96 in patients with axSpA treated with CZP. Similar conclusions have been drawn in a previous study in patients with r-axSpA, which found that both ASAS responders and non-responders showed significant reductions in MRI inflammation over 52 weeks of treatment.31 These findings suggest that clinical remission cannot be taken as a surrogate of MRI remission by the treating physician.
Ongoing inflammation in the spine has been linked to structural progression,13 36 and while incorporating imaging into future treatment targets has been proposed, paucity of evidence precludes such a recommendation, where monitoring of MRI inflammation also has limited application in clinical practice.37 Further research into the use of MRI outcomes as targets for treatment is required to fully understand the extent to which this inflammation relates to structural progression and long-term outcomes.
The analyses reported here are not without limitation. The exploratory, post hoc analyses evaluating the association between clinical and MRI remission at week 96 should be interpreted with care, as patient withdrawal over the course of the study could bias the remaining population and impact on these results. Additionally, it should be noted that the definition of spinal remission used here (Berlin ≤2) has not been validated, and recent data suggest that at least five inflammatory (or fatty) lesions detectable by MRI may be required to differentiate patients with axSpA from those with no SpA, at a level of 95% specificity.38 However, here we use this threshold not to differentiate between patients with and without axSpA, but within a population of patients already diagnosed with axSpA, to assess their MRI inflammation.
In summary, CZP treatment was shown to rapidly reduce inflammation in the spine and SI joints in patients with axSpA, including both patients with r-axSpA and nr-axSpA, with these improvements maintained over 96 weeks. A substantial proportion of patients also achieved MRI remission of inflammation. Furthermore, it was demonstrated that clinical remission was not associated with the absence of MRI inflammation, suggesting that one cannot be used as a surrogate for the other.
Acknowledgments
This study was funded by UCB Pharma. The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to this study. The authors acknowledge Alvaro Arjona, PhD, UCB Pharma, Brussels, Belgium, for publication coordination, and Sana Eljamel, MBChB, and Helen Chambers, DPhil, from Costello Medical Consulting, Cambridge, UK, for medical writing and editorial assistance in preparing this manuscript for publication based on the authors' input and direction. All costs associated with the development of this manuscript were funded by UCB Pharma.
Footnotes
Funding: UCB Pharma funded this study and manuscript. It reviewed only for scientific and legal accuracy.
Competing interests: JB received consultant fees/research support from Abbott, Bristol Myers Squibb, Celgene, Celltrion, Chugai, Johnson & Johnson, MSD, Novartis, Pfizer, Roche and UCB Pharma. XB received consultancy/speaker fees/research grants from AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Chugai, Janssen, MSD, Novartis, Pfizer and UCB Pharma. K-GH received lecture fees from AbbVie, MSD, Pfizer and UCB Pharma. RL received consultancy fees from Abbott, Ablynx, Amgen, Astra-Zeneca, Bristol Myers Squibb, Centocor, Glaxo-Smith-Kline, Novartis, Merck, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth, research grants from Abbott, Amgen, Centocor, Novartis, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth, and speaker's bureau from Abbott, Amgen, Bristol Myers Squibb, Centocor, Merck, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth. PMM received consultancy/speaker's fees/research grants from AbbVie, Centocor, Janssen, Merck, Novartis, Pfizer and UCB Pharma. WPM received consultant and/or speaker fees and/or grants from AbbVie, Amgen, Eli-Lilly, Janssen, Merck, Pfizer, Synarc, Sanofi and UCB Pharma. OD is the employee and stockholder of UCB Pharma. BH is the employee of UCB Pharma. TN is the consultant for UCB Pharma. CS is the employee of UCB Pharma. DvdH received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB Pharma, and is the Director of Imaging Rheumatology BV.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 2.Hermann KG, Bollow M. Magnetic resonance imaging of sacroiliitis in patients with spondyloarthritis: correlation with anatomy and histology. Rofo 2014;186:230–7. 10.1055/s-0033-1350411 [DOI] [PubMed] [Google Scholar]
- 3.Baraliakos X, Braun J. [MRI examinations for axial and peripheral spondyloarthritis]. Z Rheumatol 2012;71:27–37. 10.1007/s00393-011-0894-3 [DOI] [PubMed] [Google Scholar]
- 4.Hamilton L, Barkham N, Bhalla A, et al. . BSR and BPHR Guideline for the Treatment of Axial Spondyloarthritis (Including Ankylosing Spondylitis) with Biologics. Rheumatology (Oxford) 2017;56:313–6. https://academic.oup.com/rheumatology/article/56/2/313/2631549/BSR-and-BHPR-guideline-for-the-treatment-of-axial. [DOI] [PubMed] [Google Scholar]
- 5.Rios Rodriguez V, Poddubnyy D. Old and new treatment targets in axial spondyloarthritis. RMD Open 2015;1:e000054 10.1136/rmdopen-2015-000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett AN, McGonagle D, O'Connor P, et al. . Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413–18. 10.1002/art.24024 [DOI] [PubMed] [Google Scholar]
- 7.Dougados M, Demattei C, van den Berg R, et al. . Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheum 2016;68:1904–13. 10.1002/art.39666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado PM, Baraliakos X, van der Heijde D, et al. . MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2016;75:1486–93. 10.1136/annrheumdis-2015-208011 [DOI] [PubMed] [Google Scholar]
- 9.Baraliakos X, Listing J, Rudwaleit M, et al. . The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther 2008;10:R104 10.1186/ar2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. . Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009;60:93–102. 10.1002/art.24132 [DOI] [PubMed] [Google Scholar]
- 11.Maksymowych WP, Morency N, Conner-Spady B, et al. . Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 2013;72:23–8. 10.1136/annrheumdis-2011-200859 [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde D, Machado P, Braun J, et al. . MRI inflammation at the vertebral unit only marginally predicts new syndesmophyte formation: a multilevel analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:369–73. 10.1136/annrheumdis-2011-200208 [DOI] [PubMed] [Google Scholar]
- 13.Ramiro S, van der Heijde D, van Tubergen A, et al. . Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. 10.1136/annrheumdis-2014-205178 [DOI] [PubMed] [Google Scholar]
- 14.Smolen JS, Braun J, Dougados M, et al. . Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis 2014;73:6–16. 10.1136/annrheumdis-2013-203419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landewé R, Braun J, Deodhar A, et al. . Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieper J, Landewé R, Rudwaleit M, et al. . Effect of certolizumab pegol over ninety-six weeks in patients with axial spondyloarthritis: results from a phase III randomized trial. Ann Rheum Dis 2015;67:668–77. 10.1002/art.38973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Heijde D, Dougados M, Landewé R, et al. . Long Term Efficacy, Safety and Patient-Reported Outcomes of Certolizumab Pegol in Axial Spondyloarthritis: 4-Year Outcomes from RAPID-axSpA. Rheumatology (Oxford) 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudwaleit M, van der Heijde D, Landewé R, et al. . The development of assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 19.Garrett S, Jenkinson T, Kennedy LG, et al. . A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 20.Maksymowych WP, Inman RD, Salonen D, et al. . Spondyloarthritis research consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. 10.1002/art.21337 [DOI] [PubMed] [Google Scholar]
- 21.Braun J, Baraliakos X. Imaging of axial spondyloarthritis including ankylosing spondylitis. Ann Rheum Dis 2011;70(Suppl 1):i97–103. 10.1136/ard.2010.140541 [DOI] [PubMed] [Google Scholar]
- 22.Braun J, Baraliakos X, Golder W, et al. . Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003;48:1126–36. 10.1002/art.10883 [DOI] [PubMed] [Google Scholar]
- 23.Braun J, van der Heijde D. Imaging and scoring in ankylosing spondylitis. Best Pract Res Clin Rheumatol 2002;16:573–604. 10.1016/S1521-6942(02)90250-0 [DOI] [PubMed] [Google Scholar]
- 24.Lukas C, Braun J, van der Heijde D, et al. . Scoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: a multireader experiment. J Rheumatol 2007;34:862–70. [PubMed] [Google Scholar]
- 25.van den Berg R, de Hooge M, Bakker PA, et al. . Metric properties of the SPARCC score of the sacroiliac joints—data from baseline, 3-month, and 12-month followup in the SPACE Cohort. J Rheumatol 2015;42:1186–93. 10.3899/jrheum.140806 [DOI] [PubMed] [Google Scholar]
- 26.van der Heijde D, Lie E, Kvien TK, et al. . ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:1811–18. 10.1136/ard.2008.100826 [DOI] [PubMed] [Google Scholar]
- 27.Machado P, Landewé R, Lie E, et al. . Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47–53. 10.1136/ard.2010.138594 [DOI] [PubMed] [Google Scholar]
- 28.Baraliakos X, Davis J, Tsuji W, et al. . Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor alpha receptor fusion protein etanercept. Arthritis Rheum 2005;52:1216–23. 10.1002/art.20977 [DOI] [PubMed] [Google Scholar]
- 29.Braun J, Baraliakos X, Hermann KG, et al. . Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo- controlled GO-RAISE study. Ann Rheum Dis 2012;71:878–84. 10.1136/annrheumdis-2011-200308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun J, Landewé R, Hermann KG, et al. . Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum 2006;54:1646–52. 10.1002/art.21790 [DOI] [PubMed] [Google Scholar]
- 31.Lambert RG, Salonen D, Rahman P, et al. . Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2007;56:4005–14. 10.1002/art.23044 [DOI] [PubMed] [Google Scholar]
- 32.Dougados M, van der Heijde D, Sieper J, et al. . Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2014;66:2091–102. 10.1002/art.38721 [DOI] [PubMed] [Google Scholar]
- 33.Sieper J, van der Heijde D, Dougados M, et al. . Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013;72:815–22. 10.1136/annrheumdis-2012-201766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song IH, Hermann KG, Haibel H, et al. . Consistently good clinical response in patients with early axial spondyloarthritis after 3 years of continuous treatment with etanercept: longterm data of the ESTHER trial. J Rheumatol 2014;41:2034–40. 10.3899/jrheum.140056 [DOI] [PubMed] [Google Scholar]
- 35.van der Heijde D, Sieper J, Maksymowych WP, et al. . Spinal inflammation in the absence of sacroiliac joint inflammation on magnetic resonance imaging in patients with active nonradiographic axial spondyloarthritis. J Rheumatol 2014;66:667–73. 10.1002/art.38283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poddubnyy D, Haibel H, Listing J, et al. . Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondyloarthritis. Arthritis Rheum 2012;64:1388–98. 10.1002/art.33465 [DOI] [PubMed] [Google Scholar]
- 37.Mandl P, Navarro-Compán V, Terslev L, et al. . EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. 10.1136/annrheumdis-2014-206971 [DOI] [PubMed] [Google Scholar]
- 38.de Hooge M, van den Berg R, Navarro-Compán V, et al. . Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis 2016;75:1308–14. 10.1136/annrheumdis-2015-207823 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2017-000430supp001.pdf (992.1KB, pdf)



