Abstract
The association between Hashimoto thyroiditis (HT) and papillary thyroid carcinoma (PTC) has been originally suggested by retrospective pathological studies and has recently been re-evaluated and proposed on the basis of several fine-needle aspiration cytology (FNAC) studies. In FNAC studies, the association between HT and PTC is based on the comparison of anti-thyroid autoantibodies (ATA) (anti-thyroperoxidase [TPOAb] and anti-thyroglobulin [TgAb]), thyroid function (TSH), and cytology with histology of thyroid nodules and lymphocytic thyroid infiltration (LTI) of operated thyroid glands. Most of the pathological studies found a high prevalence rate of PTC in HT. In most FNAC studies, the risk ratio of PTC in HT patients was evaluated using multivariate statistical analysis: increased TSH levels represented the main and common independent risk factor of malignancy, although it resulted not consistently related to HT. On the other hand, several studies provided a positive relationship between ATA and PTC, particularly with TgAb. Two recent FNAC studies from the same referral center clearly demonstrated an independent risk for thyroid malignancy conferred by both TPOAb and TgAb, confirming the role of increased TSH levels, and found a significant association between PTC and ATA and diffuse LTI at histology. These studies are consistent with the hypothesis that autoimmune thyroid inflammation and increased serum TSH concentration may be involved in thyroid tumor growth. The complex relationship between HT and PTC, which involves immunological/hormonal pathogenic links, needs to be further investigated with prospective studies.
Keywords: Autoimmune thyroiditis, TSH, Thyroid cytology, Papillary thyroid carcinoma
Introduction
Hashimoto thyroiditis (HT) is an autoimmune organ-specific disease characterized by a wide spectrum of morphological and functional alterations. The pathogenesis of HT is based on an abnormal humoral (autoantibodies) and cellular immune response against thyroid autoantigens, with the contribution of genetic and environmental factors [1, 2, 3].
Thyroid nodules (TN) are very frequently observed, but only a small part of them are malignant tumors, mostly (85%) papillary thyroid carcinomas (PTC).
An association between HT and PTC has strongly been suggested by retrospective analyses of surgical series [4, 5, 6, 7, 8, 9] and more recently re-evaluated in several studies carried out in consecutive patients submitted to fine-needle aspiration cytology (FNAC) which are believed to be less subject to the potential selection bias of surgical series [10, 11]. However, according to FNAC studies, the link between PTC and HT appears less evident. The aim of the present review will be to briefly summarize the clinical and biological evidence supporting this link.
Background: Chronic Inflammation and Thyroid Cancer
Inflammatory or infectious diseases may be strongly associated with human tumors. Several inflammatory/immune cells, such as lymphocytes, macrophages, mast cells, and myeloid-derived suppressor cells, have been identified in cancer tissues [12, 13]. Tumor cells secrete several molecules, such as cytokines and chemokines promoting cancer cell growth, apoptosis, autophagy, angiogenesis, and metastasis, and attract inflammatory-immune cells into tumor sites. In response to these stimuli, leukocytes produce and secrete highly reactive metabolites inducing the production of peroxynitrite and other mutagenic agents which could cause genetic alterations (point mutations, rearrangements in the DNA) in proliferating cells [14, 15, 16] that may be implicated in cancerogenesis. On the other hand, an immune response might be important in preventing metastasis and recurrence of the tumor [17, 18].
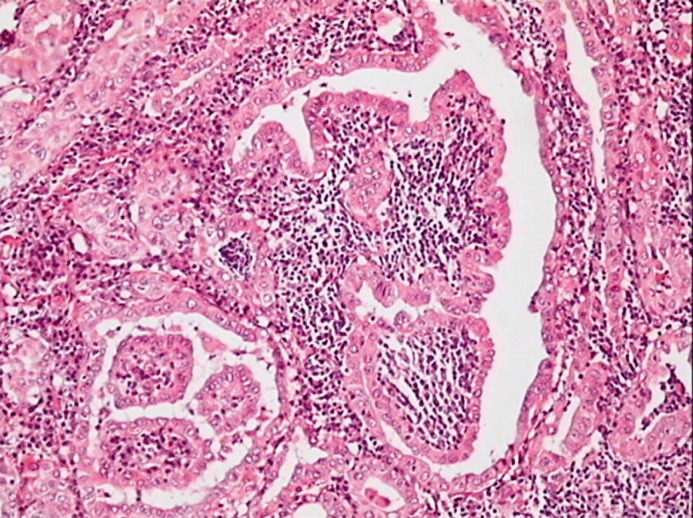
Regarding thyroid carcinoma, it has been noticed for a long time [5, 19] that diffuse lymphocytic thyroid infiltration (LTI) is frequently associated with PTC (Fig. 1), but the role of HT in cancer growth and progression is still debated. Pathological series have looked at the presence of LTI in thyroid glands harboring thyroid cancer and reported a rate of associated HT, histologically defined, ranging between 5 and 48% [5, 20, 21]. This wide distribution may be due, at least in part, to differences in the level of histological examination and criteria of autoimmunity definition, or indication for surgery. An additional confounding factor is that the sole presence of tumoral or peritumoral LTI is not distinguished from the true presence of diffuse HT. Differentiation between these 2 pathological features is a critical point, since a limited LTI may represent just the response to tumor antigens rather than true HT [22]. A commonly used approach to study the in vivo interaction between tumors and the immune system is to quantify the numbers and define the subsets of the lymphocytes in the infiltration, and to study their relation with tumor characteristics and outcome. Analysis of specific lymphocyte subsets revealed that T regulatory cells (Tregs) suppress anti-tumor immune responses [23], are consistently found within and surrounding thyroid tumors, and their number correlates with disease severity. These findings are in contrast with previous studies reporting correlations between LTI and reduced invasion and recurrence of thyroid carcinoma [24, 25]. However, these studies have the limitation of not distinguishing between tumor-associated lymphocytes and background lymphocytic thyroiditis.
Fig. 1.
Histology of a case of papillary thyroid carcinoma associated with diffuse lymphocytic thyroid infiltration.
Also, in several clinical studies (detailed in the next paragraphs), a significant association between HT and differentiated thyroid carcinoma, particularly with the most invasive forms of PTC [26], has been observed, while other studies suggest that HT may exert a protective effect preventing tumor progression [27]. These studies support the concept that in some patients, the immune response may play a protective effect preventing thyroid cancer progression, while in other patients, it may enhance tumor development and unfavorable prognosis [12].
Association of PTC and HT: Clinical Studies
The data supporting the association of PTC and HT are based on experimental and molecular studies, which are briefly summarized in the following paragraphs.
Molecular Studies
The potential pathogenic link between PTC and HT is supported by molecular genetic studies on the effects of RET/PTC (one of the main oncogenes involved in PTC) activation on the immune system [10, 28, 29, 30].
The oncogenic potential of RET in PTC derives from a genetic rearrangement between the RET tyrosine kinase domain and heterologous genes. The consequence of these rearrangements is the constitutive activation of RET due to dimerization/oligomerization of the oncoprotein induced by the different RET-fused genes. Both point mutations and genetic rearrangements cause constitutive activation of the tyrosine kinase activity of RET in the absence of ligands [31]. RET-induced transcriptional activity depends almost entirely on the integrity of the Y1062 residue and on activation of the RAS/BRAF/MAPK pathway [32]. RET/PTC activation could also activate the immune system, in keeping with a series of experimental studies.
Russell et al. [33] reported that the expression of the RET/PTC3 isoform in a rat thyroid cell line (PC Cl3) induced an increase in NF-κB DNA-binding activity and a consequent increase in proinflammatory cytokine secretion. In particular, CXCL1/ Groα, CCL2/mcp-1, and GM-CSF were upregulated upon RET/PTC3 expression, and this increase depended on the integrity of residue 1062 of RET [33]. GDNF stimulation of the neuroectodermal tumor cell line SK-N-MC, ectopically expressing the human wild-type RET, induced the production of high levels of IL8, a proinflammatory, mitogenic, and proangiogenic chemokine [34]. Other cytokines related to RET/PTC3 activation in thyroid cancer cells are IL1α, IL1β, IL6, IL24 [35, 36], prostaglandin E2 (PGE2), microsomal prostaglandin E synthase1 (mPGES1), cyclooxygenase2 (COX2), and several other genes involved in immune response and inflammation [37, 38]. The relevance of RET/PTC in the association between PTC and HT is also underscored by a retrospective pathological study by Muzza et al. [30]. In this study, RET/PTC1 rearrangement was more frequently observed in PTC associated with HT when compared to PTC without HT (31 vs. 13%, p = 0.02), while BRAF (V600E) mutation, another important mutated oncogene associated with PTC [39], was found more often in PTC not associated with HT (38 vs. 22%; p = 0.07). No evidence of a significant association in PTC between BRAF mutation and HT has also been confirmed in some recent papers [40, 41]. On the other hand, it has been shown that thyroid cell lines expressing both RET/PTC and BRAF may induce genes coding for molecules involved in the immune response [36, 38, 42] including chemokines binding to the chemokine receptor CXCR3, such as CXCL10 that plays an important role in the first steps of HT lymphocytic infiltration [43, 44]. Other proinflammatory proteins (several cytokines and chemokines) induced by these mutated genes are relevant for the mobility, proliferation survival, invasiveness of tumor cells, stimulation of angiogenesis, and reduction of anti-tumoral immune response [12].
Surgical and Pathological Studies
The association between HT and PTC in surgical and pathological studies was proposed on the basis of the evidence of LTI combined with thyroid cancer at histological examination. The relationship between HT and PTC has originally been suggested based on retrospective pathological studies on surgical series, as listed in Table 1. Dailey et al. [4] in 1955 firstly reported an unexpectedly high prevalence (12.6%) of PTC in patients with HT. This link was confirmed 40 years later by an extensive study carried out by Okayasu et al. [8] in a very large number of thyroid surgical specimens from Japanese and US American patients. In addition, the degree of association between HT and thyroid carcinoma was dependent on ethnic origin and gender, being more evident in females compared to males and in Japanese patients compared to both White American and African-American patients [8]. This study also provided evidence that the association of HT was significant only for PTC and not observed for follicular thyroid carcinoma.
Table 1.
Summary of surgical and pathological thyroid studies
| First author, year [Ref.] | Study method | Country | Sample size, n | Prevalence of gender | Prevalence of PTC in HT, % | LTI in PTC vs. non-PTC1 |
|---|---|---|---|---|---|---|
| Dailey, 1955 [4] | Retrospective | USA | 278 | NA | 12.6 | NA |
| Okayasu, 1995 [8] | Retrospective | USA, Japan | 1,046 | NA | 46.2–76.0 | p < 0.01, p < 0.001 |
| Büyükaûık, 2011 [45] | Retrospective | Turkey | 917 | NA | 19.5 | NA |
| Yoon, 2012 [46] | Retrospective | South Korea | 195 | F | 28.7 | NA |
| Consorti, 2010 [64] | Retrospective | Italy | 404 | NA | 36.2 | 1.62 (p = 0.02) |
| Cipolla, 2005 [47] | Retrospective | Italy | 225 | NA | 27.6 | p < 0.02 |
| Larson, 2007 [21] | Retrospective | USA | 812 | F | 37.7 | NA |
| Bradly, 2009 [48] | Retrospective | USA | 678 | F | 12.0 | p < 0.01 |
| Siriweera, 2010 [49] | Retrospective | Sri Lanka | 5,357 | F | 9.46 | 4.16 (95% CI 2.87–6.04) p < 0.0001 |
| Kim, 2011 [20] | Retrospective | South Korea | 1,329 | F | 29.9 | 2.96 (95% CI 1.81–4.85) p < 0.001 |
| Jankovic, 2013 [50] | Review | USA | 9,431 (8 studies) | F | 27.56 (mean) | Average RR = 1.59 |
| Chen, 2013 [65] | Retrospective | Taiwan | 7,605 | F | NA | OR = 11.8 (p < 0.001) |
PTC, papillary thyroid carcinoma; LTI, lymphocytic thyroid infiltration; NA, not available data.
Expanded as odds ratio or risk ratio, when available.
After this pivotal study, several other pathological reports [8, 20, 21, 45, 46, 47, 48, 49, 50] provided similar results summarized in 2 meta-analyses published several years apart [9, 51], reporting very similar odds ratios (OR) for PTC in the presence of HT versus non-HT: 2.77 in the study by Singh et al. [9] of 1999 and 2.8 in the more recent (2013) work by Lee et al. [51].
These results clearly show that PTC and HT are significantly associated in patients’ surgical specimens. However, this association may be emphasized by the bias due to patient selection for thyroidectomy. To circumvent this problem, further studies were more recently performed on unselected patients submitted to FNAC for the presence of TN. These studies are summarized in the following paragraph.
Cytological Studies
The association between HT and PTC is suggested by several large FNAC series in retrospective and prospective studies. As shown in Table 2, several parameters, such as serum anti-thyroid autoantibodies (ATA), TSH levels, cytology, and histology in operated patients, have been compared in these studies in an attempt to clarify the mechanism(s) involved in the association of PTC with HT.
Table 2.
Thyroid autoimmunity and thyroid cancer: risk factors for malignancy identified in recent large retrospective FNAC studies
| First author, year [Ref.] | TSH | ATA | TPOAb | TgAb | LTI |
|---|---|---|---|---|---|
| Boi, 2005 [10] | NA | + (2.29) | NA | NA | NA |
| Boelaert, 2006 [52] | + (2.72–11.18) | – | – | – | NA |
| Fiore, 2009 [53] | + (2.66–4.29) | – | – | – | NA |
| Kim, 2010 [59] | + (1.72–1.98) | NA | – | + (1.61) | – |
| Fiore, 2011 [22] | + (1.1) | NA | – | + (1.001) | NA |
| Boi, 2013 [11] | + (3.33–4.39) | + (2.21) | + (2.15) | + (1.67) | + |
| Azizi, 2014 [54] | + (1.49) | NA | – | + (2.24) | NA |
Values in parentheses are risk ratios or odds ratios for papillary thyroid carcinoma risk. +, positive; –, negative; ATA, anti-thyroid autoantibodies; TPOAb, anti-thyroperoxidase; TgAb, anti-thyroglobulin; LTI, lymphocytic thyroid infiltration; NA, not assessed.
In the first of these studies, Boi et al. [10] in 2005 retrospectively described a higher prevalence (18.8%) of suspicious/malignant cytology in patients with positive serum anti-thyroperoxidase (TPOAb) and/or anti-thyroglobulin (TgAb) than in patients with negative ATA (9.28%). By univariate analysis, ATA positivity conferred a significant risk (OR 2.29) for suspicious/malignant cytology independently from age and sex. In patients submitted to total thyroidectomy, PTC was more prevalent in ATA-positive (13.7%) compared to ATA-negative (8.4%) patients.
Using a similar retrospective approach in patients submitted to FNAC, Boelaert et al. [52] found no significant correlation between PTC and ATA, while increased serum TSH concentrations were significantly and independently associated with malignancy (PTC). Moreover, the OR for malignancy was directly related to serum TSH concentrations reaching 11.2 for TSH levels >5.5 mUI/L.
Fiore and colleagues [22, 53], in 2 large retrospective FNAC studies, further confirmed that serum TSH concentration was significantly higher in PTC than in benign TN both in the cytological and histological series, and significantly adjusted OR for the diagnosis of malignancy was revealed by logistic regression analysis in subjects with the highest values of TSH.
In contrast to the study by Boi et al. [10], the presence of circulating ATA – although it was associated with a significant increase of TSH – did not contribute significantly to thyroid cancer risk. In keeping with Fiore et al.'s results, Kim et al. [20] observed that the prevalence of detectable TPOAb was not higher in malignant nodules, while both TPOAb and TgAb were well correlated with TSH levels and LTI at histology. In this study, however, no correlation was found at histology between PTC and LTI.
More recently, in an extended retrospective study, Boi et al. [11] reported a full concordance of all HT parameters with malignancy. In this study, cytological classes were compared to ATA (both TgAb and TPOAb), TSH levels, and histological data in patients submitted to total thyroidectomy. The results obtained confirmed that the prevalence of malignant cytology was significantly related to the presence of high serum ATA titers and to higher serum TSH concentrations. In particular, the mean TSH concentration was higher in ATA-positive patients than in ATA-negative patients. In the group of thyroidectomized patients, a higher prevalence of PTC was confirmed in ATA-positive TN (66%) compared to ATA-negative TN (52.7%, p < 0.05) and a higher prevalence of PTC associated with diffuse LTI (60.3%) compared to negative LTI (44.5%, p < 0.05) was found. In partial agreement with this study, in a prospective cytological study, Azizi et al. [54] reported a significant association between PTC, TSH levels, and serum TgAb, but not TPOAb.
Taken together, all these FNAC studies suggest a potential positive association between HT and PTC, although the relative role of ATA, thyroid function (TSH), and LTI remains to be further elucidated.
Some retrospective cytological studies analyzed by Castagna et al. [55] and Matesa-Anić et al. [56] did not find an increased risk of thyroid cancer in patients with HT. In the latter study, the prevalence of PTC was lower in HT patients (1.9%) than in patients without HT (2.7%); the same result was also confirmed by a previous FNAC prospective study [57].
Discussion
This review shows different rates of the relative risk of PTC in the presence of HT between surgical and FNAC studies. The highest prevalence rate of PTC in HT in surgical series may be explained because of the bias due to patient selection for thyroidectomy and the absence of homogeneous control groups. Furthermore, the wide distribution of the prevalence of PTC in HT may be related to the ethnic, geographic, and gender differences of the clinical records and may be also due to different histological criteria used to define HT. In fact, as a critical point, in some studies [12], diffuse LTI was not clearly reported and not differentiated from LTI surrounding thyroid cancer, which may represent just the response to tumor rather than true HT.
Some retrospective FNAC studies [22, 52, 58] suggest increased TSH levels as the main potential link between PTC and HT, although TSH concentrations were not always related to ATA positivity or to clinically relevant HT. Interestingly, 2 studies from the same Institution [10, 11] clearly demonstrated an increased independent risk ratio for malignancy conferred by any ATA, TPOAb and TgAb. In the last largest study [11], strong evidence was provided for the association between HT and PTC comparing serological parameters with pathological data by multivariate logistic regression analysis. Not only the positivity, but also the titers of serum ATA (both TPOAb and TgAb) conferred a significant and independent predictive risk for thyroid cancer; the association of ATA positivity and risk of malignancy was not affected by gender. This study also demonstrated the importance of increased TSH levels (>1.0 μU/mL) as an independent predictive risk factor for PTC, which was consistently higher in ATA-positive compared to ATA-negative TN, confirming the known relationship between ATA titers and increased serum TSH levels. The association between HT and PTC was clearly confirmed at histology in a representative subgroup of operated patients, in whom PTC was significantly associated with both ATA positivity and diffuse LTI. As expected, a high significant correlation was also demonstrated between serum ATA and diffuse LTI, suggesting that in most cases ATA were markers of underlying relevant autoimmune thyroiditis. In conclusion, this study found a full concordance of all autoimmune parameters (TPOAb, TgAb, TSH, and LTI) with PTC. Finally, this study is in agreement with a recent paper reporting increased risk of thyroid malignancy for TgAb combined to increased TSH levels [53, 59].
The discrepancies of the relationship between TSH levels with ATA positivity and HT diagnosis reported in the above studies are mostly due to differences in ATA assays performances, in the predictivity for HT of ATA tests employed in different studies, as well as in the “background” thyroid autoimmunity linked to genetic/environmental factors of the population studied. Interestingly, the studies that reported a strong association between HT and PTC [10, 11] and the most aggressive behavior of PTC in HT [26] were performed in countries (Sardinia, Turkey, and Greek) from the same geographical area of the Mediterranean Basin, which may share similar genetic/environmental HT backgrounds potentially linked to the development and progression of PTC.
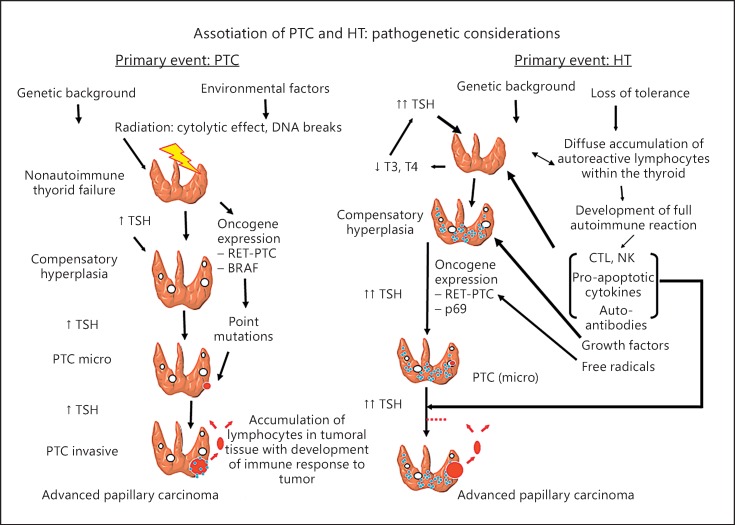
The pathogenic links between environmental factors (radiation, etc.), genetic background, autoimmune inflammation, and thyroid cancer development are displayed in Figure 2. It is well known that thyroid carcinogenesis induced by radiation is a phenomenon mainly related to rearrangements of RET-PTC oncogenes and more rarely with point BRAF mutations. Increased TSH due to nonautoimmune thyroid failure, induced by radiation, may play an important role as cofactor in promoting PTC development and progression. The complex relations between HT and PTC are still debated. The above studies provide evidence that multiple factors related to thyroid autoimmunity (serum ATA, inflammatory molecules, and free radicals with secondary RET/PTC rearrangements), genetic/environmental conditions, and increased TSH represent independent risk factors for PTC, supporting the notion that overt HT rather than nonspecific serologic reactivity, may be linked to thyroid malignancy. Thus, thyroid autoimmunity could exert a promoting effect on thyroid tumor growth either directly or through secondary increase of TSH values as a consequence of initial autoimmune thyroid failure. The pathogenic link between inflammation and cancer has been well described in different models of human carcinogenesis [60, 61], and the relevant role of TSH of as a trophic factor on thyroid cancer growth and progression has been well documented [62, 63]. Furthermore, several biomolecular markers, such as RET/PTC rearrangements, may be potentially involved in neoplastic transformation from HT to PTC [12], although, so far, no causal genetic linkage has been confirmed.
Fig. 2.
The relationships between Hashimoto thyroiditis (HT) and papillary thyroid carcinoma (PTC) with their immunological/hormonal pathogenic links. The left part shows the relevance of environmental factors and genetic background in thyroid carcinogenesis. Radiation may cause PTC development by RET-PTC oncogene rearrangements and more rarely with point BRAF mutations. Increased TSH levels due to nonautoimmune thyroid failure induced by radiation play an important role both in promotion and progression of PTC. The right part displays the complex relation between HT and PTC. Multiple factors related to thyroid autoimmunity (serum anti-thyroid autoantibodies, inflammatory molecules, free radicals with secondary RET/PTC rearrangements), genetic/environmental conditions, and high TSH values (consequence of autoimmune thyroid failure) are involved.
In summary, this review shows that several clinical and pathological parameters related to HT could be associated with PTC and some of these (particularly TSH and ATA) represent independent predictor markers of thyroid malignancy in HT patients with TN. This evidences is consistent with the hypothesis that autoimmune thyroid inflammation is per se tumorigenic and HT-related hypothyroidism may also cause tumor growth through TSH receptor stimulation.
Disclosure Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
This work was partially supported by PRIN 2012 (Research Grant: #2012Z3F7HE from “Ministero Università e Ricerca,” Roma, Italy) and by funds of “Regione Autonoma della Sardegna” to the Centro Studio per la Prevenzione e Terapia delle Malattie della Tiroide.
References
- 1.Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003;148:1–9. doi: 10.1530/eje.0.1480001. [DOI] [PubMed] [Google Scholar]
- 2.Tomer Y. Genetic dissection of familial autoimmune thyroid diseases using whole genome screening. Autoimmun Rev. 2002;1:198–204. doi: 10.1016/s1568-9972(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 3.Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150:605–618. doi: 10.1530/eje.0.1500605. [DOI] [PubMed] [Google Scholar]
- 4.Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- 5.Hirabayashi RN, Lindsay S. The relation of thyroid carcinoma and chronic thyroiditis. Surg Gynecol Obstet. 1965;121:243–252. [PubMed] [Google Scholar]
- 6.Ott RA, Calandra DB, McCall A, Shah KH, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in patients with Hashimoto's thyroiditis and solitary cold nodules. Surgery. 1985;98:1202–1206. [PubMed] [Google Scholar]
- 7.Ott RA, McCall AR, McHenry C, Jarosz H, Armin A, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in Hashimoto's thyroiditis. Am Surg. 1987;53:442–445. [PubMed] [Google Scholar]
- 8.Okayasu I, Fujiwara M, Hara Y, Tanaka Y, Rose NR. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312–2318. doi: 10.1002/1097-0142(19951201)76:11<2312::aid-cncr2820761120>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto's thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999;126:1070–1076. doi: 10.1067/msy.2099.101431. [DOI] [PubMed] [Google Scholar]
- 10.Boi F, Lai ML, Marziani B, Minerba L, Faa G, Mariotti S. High prevalence of suspicious cytology in thyroid nodules associated with positive thyroid autoantibodies. Eur J Endocrinol. 2005;153:637–642. doi: 10.1530/eje.1.02020. [DOI] [PubMed] [Google Scholar]
- 11.Boi F, Minerba L, Lai ML, Marziani B, Figus B, Spanu F, Borghero A, Mariotti S. Both thyroid autoimmunity and increased serum TSH are independent risk factors for malignancy in patients with thyroid nodules. J Endocrinol Invest. 2013;36:313–320. doi: 10.3275/8579. [DOI] [PubMed] [Google Scholar]
- 12.Guarino V, Castellone MD, Avilla E, Melillo RM. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321:94–102. doi: 10.1016/j.mce.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Vella V, Mineo R, Frasca F, Mazzon E, Pandini G, Vigneri R, Belfiore A. Interleukin-4 stimulates papillary thyroid cancer cell survival: implications in patients with thyroid cancer and concomitant Graves’ disease. J Clin Endocrinol Metab. 2004;89:2880–2889. doi: 10.1210/jc.2003-031639. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;6917:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 16.Okayasu I. The relationship of lymphocytic thyroiditis to the development of thyroid carcinoma. Endocr Pathol. 1997;8:225–230. doi: 10.1007/BF02738789. [DOI] [PubMed] [Google Scholar]
- 17.Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, Matsuzuka F, Kakudoh K, Kuma K, Tamai H. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995;80:3421–3424. doi: 10.1210/jcem.80.12.8530576. [DOI] [PubMed] [Google Scholar]
- 18.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 19.Okayasu I. The relationship of lymphocytic thyroiditis to the development of thyroid carcinoma. Endocr Pathol. 1997;8:225–230. doi: 10.1007/BF02738789. [DOI] [PubMed] [Google Scholar]
- 20.Kim KW, Park YJ, Kim EH, Park SY, Park do J, Ahn SH, Park do J, Jang HC, Cho BY. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck. 2011;33:691–695. doi: 10.1002/hed.21518. [DOI] [PubMed] [Google Scholar]
- 21.Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Ak pathway. J Am Coll Surg. 2007;204:764–773. doi: 10.1016/j.jamcollsurg.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiore E, Rago T, Latrofa F, Provenzale MA, Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L, Pinchera A, Vitti P. Hashimoto's thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocr Relat Cancer. 2011;18:429–437. doi: 10.1530/ERC-11-0028. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 24.Kashima K, Yokoyama S, Noguchi S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998;8:197–202. doi: 10.1089/thy.1998.8.197. [DOI] [PubMed] [Google Scholar]
- 25.Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:458–463. doi: 10.1210/jcem.84.2.5443. [DOI] [PubMed] [Google Scholar]
- 26.Iliadou PK, Effraimidis G, Konstantinos M, Grigorios P, Mitsakis P, Patakiouta F, Pazaitou-Panayiotou K. Chronic lymphocytic thyroiditis is associated with invasive characteristics of differentiated thyroid carcinoma in children and adolescents. Eur J Endocrinol. 2015;173:827–833. doi: 10.1530/EJE-14-1046. [DOI] [PubMed] [Google Scholar]
- 27.Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab. 2013;98:2409–2414. doi: 10.1210/jc.2013-1309. [DOI] [PubMed] [Google Scholar]
- 28.Modi J, Patel A, Terrell R, Tuttle RM, Francis GL. Papillary thyroid carcinomas from young adults and children contain a mixture of lymphocytes. J Clin Endocrinol Metab. 2003;88:4418–4425. doi: 10.1210/jc.2003-030342. [DOI] [PubMed] [Google Scholar]
- 29.Nikiforova MN, Caudill CM, Biddinger P, Nikiforov YE. Prevalence of RET/PTC rearrangements in Hashimoto's thyroiditis and papillary thyroid carcinomas. Int J Surg Pathol. 2002;10:15–22. doi: 10.1177/106689690201000104. [DOI] [PubMed] [Google Scholar]
- 30.Muzza M, Degl'Innocenti D, Colombo C, Perrino M, Ravasi E, Rossi S, Cirello V, Beck-Peccoz P, Borrello MG, Fugazzola L. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf) 2010;72:702–708. doi: 10.1111/j.1365-2265.2009.03699.x. [DOI] [PubMed] [Google Scholar]
- 31.Santoro M, Carlomagno F, Melillo RM, Fusco A. Dysfunction of the RET receptor in human cancer. Cell Mol Life Sci. 2004;61:2954–2964. doi: 10.1007/s00018-004-4276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R, Kruhoffer M, Orntoft T, Fusco A, Santoro M. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115:1068–1081. doi: 10.1172/JCI22758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Russell JP, Shinohara S, Melillo RM, Castellone MD, Santoro M, Rothstein JL. Tyrosine kinase oncoprotein, RET/PTC3, induces the secretion of myeloid growth and chemotactic factors. Oncogene. 2003;22:4569–4577. doi: 10.1038/sj.onc.1206759. [DOI] [PubMed] [Google Scholar]
- 34.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 35.Russell JP, Engiles JB, Rothstein JL. Proinflammatory mediators and genetic background in oncogene mediated tumor progression. J Immunol. 2004;172:4059–4067. doi: 10.4049/jimmunol.172.7.4059. [DOI] [PubMed] [Google Scholar]
- 36.Shinohara S, Rothstein JL. Interleukin 24 is induced by the RET/PTC3 oncoprotein and is an autocrine growth factor for epithelial cells. Oncogene. 2004;23:7571–7579. doi: 10.1038/sj.onc.1207964. [DOI] [PubMed] [Google Scholar]
- 37.Puxeddu E, Mitsutake N, Knauf JA, Moretti S, Kim HW, Seta KA, Brockman D, Myatt L, Millhorn DE, Fagin JA. Microsomal prostaglandin E2 synthase-1 is induced by conditional expression of RET/PTC in thyroid PCCL3 cells through the activation of the MEK ERK pathway. J Biol Chem. 2003;278:52131–52138. doi: 10.1074/jbc.M306003200. [DOI] [PubMed] [Google Scholar]
- 38.Puxeddu E, Knauf JA, Sartor MA, Mitsutake N, Smith EP, Medvedovic M, Tomlinson CR, Moretti S, Fagin JA. RET/PTC-induced gene expression in thyroid PCCL3 cells reveals early activation of genes involved in regulation of the immune response. Endocr Relat Cancer. 2005;12:319–334. doi: 10.1677/erc.1.00947. [DOI] [PubMed] [Google Scholar]
- 39.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 40.Kim KH, Suh KS, Kang DW, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto's thyroiditis. Pathol Int. 2005;55:540–545. doi: 10.1111/j.1440-1827.2005.01866.x. [DOI] [PubMed] [Google Scholar]
- 41.Sargent R, LiVolsi V, Murphy J, Mantha G, Hunt JL. BRAF mutation is unusual in chronic lymphocytic thyroiditis-associated papillary thyroid carcinomas and absent in non-neoplastic nuclear atypia of thyroiditis. Endocr Pathol. 2006;17:235–241. doi: 10.1385/ep:17:3:235. [DOI] [PubMed] [Google Scholar]
- 42.Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P, Greco A, Collini P, Pilotti S, Cassinelli G, Bressan P, Fugazzola L, Mantovani A, Pierotti MA. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci. 2005;102:14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 44.Antonelli A, Rotondi M, Fallahi P, Grosso M, Boni G, Ferrari SM, Romagnani P, Serio M, Mariani G, Ferrannini E. Iodine-131 given for therapeutic purposes modulates differently interferon-gamma-inducible alpha-chemokine CXCL10 serum levels in patients with active Graves’ disease or toxic nodular goiter. J Clin Endocrinol Metab. 2007;92:1485–1490. doi: 10.1210/jc.2006-1571. [DOI] [PubMed] [Google Scholar]
- 45.Büyükaşık O, Hasdemir AO, Yalçın E, Celep B, Sengül S, Yandakçı K, Tunç G, Küçükpınar T, Alkoy S, Cöl C. The association between thyroid malignancy and chronic lymphocytic thyroiditis: should it alter the surgical approach? Endokrynol Pol. 2011;62:303–308. [PubMed] [Google Scholar]
- 46.Yoon YH, Kim HJ, Lee JW, Kim JM, Koo BS. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngol. 2012;269:1013–1017. doi: 10.1007/s00405-011-1732-6. [DOI] [PubMed] [Google Scholar]
- 47.Cipolla C, Sandonato L, Graceffa G, Fricano S, Torcivia A, Vieni S, Latteri S, Latteri MA. Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg. 2005;71:874–878. [PubMed] [Google Scholar]
- 48.Bradly DP, Reddy V, Prinz RA, Gattuso P. Incidental papillary carcinoma in patients treated surgically for benign thyroid diseases. Surgery. 2009;146:1099–1104. doi: 10.1016/j.surg.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 49.Siriweera EH, Ratnatunga NV. Profile of Hashimoto's thyroiditis in Sri Lankans: is there an increased risk of ancillary pathologies in Hashimoto's thyroiditis? J Thyroid Res. 2010;2010:124264. doi: 10.4061/2010/124264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98:474–482. doi: 10.1210/jc.2012-2978. [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168:343–349. doi: 10.1530/EJE-12-0903. [DOI] [PubMed] [Google Scholar]
- 52.Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91:4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 53.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, Pinchera A, Vitti P. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251–1260. doi: 10.1677/ERC-09-0036. [DOI] [PubMed] [Google Scholar]
- 54.Azizi G, Keller JM, Lewis M, Piper K, Puett D, Rivenbark KM, Malchoff CD. Association of Hashimoto's thyroiditis with thyroid cancer. Endocr Relat Cancer. 2014;21:845–852. doi: 10.1530/ERC-14-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castagna MG, Belardini V, Memmo S, Maino F, Di Santo A, Toti P, Carli AF, Pacini F. Nodules in autoimmune thyroiditis are associated with increased risk of thyroid cancer in surgical series, but not in cytological series: evidence for selection bias. J Clin Endocrinol Metab. 2014;99:3193–3198. doi: 10.1210/jc.2014-1302. [DOI] [PubMed] [Google Scholar]
- 56.Matesa-Anić D, Matesa N, Dabelić N, Kusić Z. Coexistence of papillary carcinoma and Hashimoto's thyroiditis. Acta Clin Croat. 2009;48:9–12. [PubMed] [Google Scholar]
- 57.Anil C, Goksel S, Gursoy A. Hashimoto's thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid. 2010;20:601–606. doi: 10.1089/thy.2009.0450. [DOI] [PubMed] [Google Scholar]
- 58.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Miccoli P, Grasso L, Pinchera A, Vitti P. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27 914 patients. Endocr Relat Cancer. 2010;17:231–239. doi: 10.1677/ERC-09-0251. [DOI] [PubMed] [Google Scholar]
- 59.Kim ES, Lim DJ, Baek KH, Lee JM, Kim MK, Kwon HS, Song KH, Kang MI, Cha BY, Lee KW, Son HY. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid. 2010;20:885–891. doi: 10.1089/thy.2009.0384. [DOI] [PubMed] [Google Scholar]
- 60.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Kang DY, Kim KH, Kim JM, Kim SH, Kim JY, Baik HW, Kim YS. High prevalence of RET, RAS, and ERK expression in Hashimoto's thyroiditis and in papillary thyroid carcinoma in the Korean population. Thyroid. 2007;17:1031–1038. doi: 10.1089/thy.2007.0035. [DOI] [PubMed] [Google Scholar]
- 62.Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer. 2009;16:1065–1072. doi: 10.1677/ERC-09-0150. [DOI] [PubMed] [Google Scholar]
- 63.Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J, Jaffiol C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab. 1996;81:4318–4823. doi: 10.1210/jcem.81.12.8954034. [DOI] [PubMed] [Google Scholar]
- 64.Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto's thyroiditis. Eur Surg Res. 2010;45:333–337. doi: 10.1159/000320954. [DOI] [PubMed] [Google Scholar]
- 65.Chen YK, Lin CL, Cheng FT, Sung FC, Kao CH. Cancer risk in patients with Hashimoto's thyroiditis: a nationwide cohort study. Br J Cancer. 2013;29:2496–2501. doi: 10.1038/bjc.2013.597. [DOI] [PMC free article] [PubMed] [Google Scholar]