Abstract
A 55-year-old man, nonsmoker, with a HIV-positive history came to our attention in February 2017. He was on treatment with StribildTM, 1 capsule daily (150 mg elvitegravir, 150 mg cobicistat, 200 mg emtricitabine, and 245 mg tenofovir disoproxil). The CD4+/CD8+ cellular count was 326/µL (normal values: 404–1,612); the CD3+/CD8+ cellular count was 819/µL (normal values: 220–1,219). The CD4/CD8 ratio was 0.40 (normal value: >1). Several typical genital wart lesions were present at the penis shaft and at the level of the neck and the corona of glans. These lesions were present for 2 years. Several cryotherapy sessions (a total of 10 procedures) had been performed with partial success. At the initial visit a total of 5 lesions were present. Treatment with topical Polyphenon E 10% 3 times a day was prescribed and started. After 1 month of treatment the lesions were reduced to 2. Treatment was very well tolerated. After 8 weeks of treatment no more lesions were observed and therefore a complete clearance was obtained. Local tolerability was evaluated to be very good by the patient.
Keywords: AIDS, Condylomata acuminata, Polyphenon E
Introduction
Genital warts (GW; condylomata acuminata) are a very common and contagious sexually transmitted disease caused by skin infection with human papillomavirus (HPV) [1]. HPV has a specific tropism for squamous stratified epithelium [2]. This infection is in general acquired through direct genital contact. Minor trauma facilitates the penetration of the virus. Penile shaft, glans, vulvar and perianal regions are commonly affected [3]. HPV infection is characterized by a relevant immune evasion mechanism that inhibits and delays the host immune response to the virus [4]. Genotypes 6 and 11 are the most common HPV involved in GW formation [5]. GW lesions manifest as variable in size and shape. Lesions could be soft papules or plaques on anogenital skin. Cryotherapy, podophyllotoxin, imiquimod, and laser therapy are commonly used in GW [6]. However, available treatments are in general still unsatisfactory due to low efficacy and clearance rate, low local tolerability, and high recurrence rate [7]. Topical Polyphenon E 10% has been recently evaluated in immunocompetent subjects with multiple GW [8]. Polyphenon E, applied 3 times a day for up to 16 weeks, induced a complete clearance rate in more than 50% of patients [9]. In addition, the recurrence rate at 3 months after the conclusion of the therapy was particularly low (<6%) [9]. Polyphenon E is a quantified extract of green tea leaves (Camellia sinensis) where the main active substance is epigallocatechin gallate (EGCG), a polyphenol substance which represents the major component of the catechin fraction [10]. EGCG has potent antioxidant, anti-inflammatory and proapoptotic actions, which could explain its antiviral properties [11]. Recent data have shown that EGCG is able to modulate the cellular genes involved in Toll-like receptor production and in the mechanisms of apoptosis [12]. The product has shown a general good safety profile. Local skin reactions like erythema and edema, in general mild to moderate, were reported in most treated subjects [13]. No data are available regarding the efficacy and the tolerability of Polyphenon E in immunocompromised subjects. We report the clinical efficacy and the tolerability of Polyphenon E 10% ointment (VeregenTM; Medigene, Germany) in an HIV-positive subject with multiple “difficult-to-treat” GW.
Case Description
A 55-year-old man, nonsmoker, with a HIV-positive history came to our attention in February 2017. He was on treatment with StribildTM, 1 capsule daily (150 mg elvitegravir, 150 mg cobicistat, 200 mg emtricitabine, and 245 mg tenofovir disoproxil). The CD4+/CD8+ cellular count was 326/µL (normal values: 404–1,612); the CD3+/CD8+ cellular count was 819/µL (normal values: 220–1,219). The CD4/CD8 ratio was 0.40 (normal value: >1). Several typical GW lesions were present at the penis shaft and at the level of the neck and the corona of glans (Fig. 1). These lesions were present for 2 years. Several cryotherapy sessions (a total of 10 procedures) had been performed with partial success. At the initial visit a total number of 5 lesions were present (Fig. 1). Treatment with topical Polyphenon E 10% 3 times a day was prescribed and started. After 1 month of treatment the lesions were reduced to 2. Treatment was very well tolerated. After 8 weeks of treatment no more lesions were observed and therefore a complete clearance was obtained (Fig. 2). Local tolerability was evaluated to be very good by the patient.
Fig. 1.
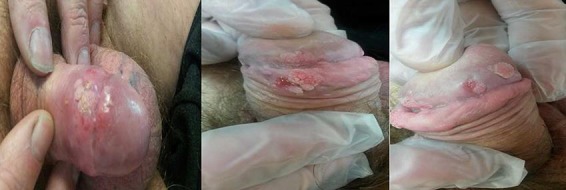
Genital warts at baseline. Multiple lesions.
Fig. 2.
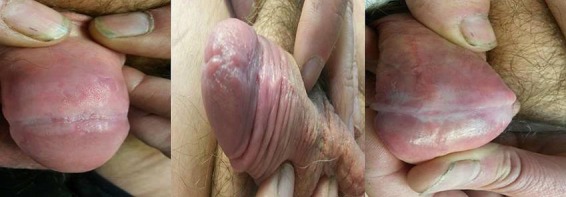
Genital warts after 2 months of treatment with Polyphenon E.
Discussion
Polyphenon E ointment is considered an effective, well-tolerated, self-applicable topical treatment of GW in immunocompetent subjects [14]. Clinical efficacy and tolerability was evaluated in randomized vehicle-controlled phase III trials in more than 1,000 patients [15]. A complete clearance rate was observed in 51% of the treated subjects. The mean treatment duration for complete clearance was between 14 and 16 weeks [16]. GW are very common in immunocompromised subjects [17]. HIV infection appears to alter the natural history of HPV-associated lesions and oncogenesis [18], and HIV-positive patients have a substantial high risk of suffering from GW in comparison with HIV-negative immunocompetent subjects. HIV-positive patients often develop multiple papillomatous lesions in the genital area and also in the extragenital area such as the oral cavity [19]. Goldstone et al. [20] have demonstrated that in HIV subjects the prevalence of any tested HPV type was 18.5% in the penis, 17.1% in the scrotum, 33.0% in the perineal/perianal region, 42.4% in the anal canal, and 48.0% at any site. Antiretroviral therapy is not able to prevent the development of HPV-associated lesions and does not eliminate HPV infection [21]. The treatment of GW in immunocompromised subjects can be particularly disappointing. Data regarding the efficacy of Polyphenon E in immunocompromised subjects are so far lacking. EGCG has an antiviral action through pathways triggering cell growth arrest and increasing proapoptotic molecules like p53 and p16 [22]. EGCG is able to interfere with the cellular activity of E6 and E7, 2 HPV virus proteins [23]. E6 and E7 are involved in the process of apoptosis inhibition and in the mechanisms of immune evasion [24]. Therefore, Polyphenon E could express anti-HPV action through multiple mechanisms. The gene modulation expressed by EGCG could be of help in fighting HPV lesions in HIV patients. In this case report, we have shown a high clinical efficacy of Polyphenon E in the treatment of multiple “difficult-to-treat” GW in an HIV-positive subject. It is remarkably that complete clearance of GW lesions was achieved in only 8 weeks of treatment, which is a shorter treatment period in comparison with the mean duration of treatment for GW clearance in immunocompetent patients. This case report suggests that topical Polyphenon E 10% could be an effective therapeutic strategy in subjects with GW and immunosuppression. Specific controlled trials in this specific population are warranted.
Statement of Ethics
The authors have no ethical conflicts to declare.
Disclosure Statement
M.M. is an employee of Difa Cooper Spa.
References
- 1.Handsfield HH. Clinical presentation and natural course of anogenital warts. Am J Med. 1997;102:16–20. doi: 10.1016/s0002-9343(97)00179-4. [DOI] [PubMed] [Google Scholar]
- 2.Majewski S, Jablonska S. Human papillomavirus-associated tumors of the skin and mucosa. J Am Acad Dermatol. 1997;36:659–685. doi: 10.1016/s0190-9622(97)80315-5. [DOI] [PubMed] [Google Scholar]
- 3.Ferenczy A, Mitao M, Nagai N, Silverstein SJ, Crum CP. Latent papillomavirus and recurring genital warts. New Engl J Med. 1985;313:784–788. doi: 10.1056/NEJM198509263131304. [DOI] [PubMed] [Google Scholar]
- 4.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–64. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 5.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnürch HG, Zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci USA. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterling JC, Handfield-Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144:4–11. doi: 10.1046/j.1365-2133.2001.04066.x. [DOI] [PubMed] [Google Scholar]
- 7.Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician. 2004;70:2335–2342. [PubMed] [Google Scholar]
- 8.Tatti S, Stockfleth E, Beutner KR, Tawfik H, Elsasser U, Weyrauch P, Mescheder A. Polyphenon E®: a new treatment for external anogenital warts. Br J Dermatol. 2010;162:176–184. doi: 10.1111/j.1365-2133.2009.09375.x. [DOI] [PubMed] [Google Scholar]
- 9.Stockfleth E, Beti H, Orasan R, Grigorian F, Mescheder A, Tawfik H, Thielert C. Topical Polyphenon® E in the treatment of external genital and perianal warts: a randomized controlled trial. Br J Dermatol. 2008;158:1329–1338. doi: 10.1111/j.1365-2133.2008.08520.x. [DOI] [PubMed] [Google Scholar]
- 10.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002;277:34933–34940. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 11.Valcic S, Muders A, Jacobsen NE, Liebler DC, Timmermann BN. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechin gallate with peroxyl radicals. Chem Res Toxicol. 1999;12:382–386. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- 12.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 13.Tatti S, Swinehart JM, Thielert C, Tawfik H, Mescheder A, Beutner KR. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet Gynecol. 2008;111:1371–1379. doi: 10.1097/AOG.0b013e3181719b60. [DOI] [PubMed] [Google Scholar]
- 14.Tzellos TG, Sardeli C, Lallas A, Papazisis G, Chourdakis M, Kouvelas D. Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2011;25:345–353. doi: 10.1111/j.1468-3083.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Daigle D. Sinecatechins 10% ointment: a green tea extract for the treatment of external genital warts. Pain. 2015;46:14–15. [PubMed] [Google Scholar]
- 16.Stockfleth E, Meyer T. The use of sinecatechins (Polyphenon E) ointment for treatment of external genital warts. Expert Opin Biol Ther. 2012;12:783–793. doi: 10.1517/14712598.2012.676036. [DOI] [PubMed] [Google Scholar]
- 17.Jin F, Prestage GP, Kippax SC, Pell CM, Donovan B, Templeton DJ, Grulich AE. Risk factors for genital and anal warts in a prospective cohort of HIV-negative homosexual men: the HIM study. Sex Transm Dis. 2007;34:488–493. doi: 10.1097/01.olq.0000245960.52668.e5. [DOI] [PubMed] [Google Scholar]
- 18.Del Mistro A, Bianchi LC. HPV-related neoplasias in HIV-infected individuals. Eur J Cancer. 2001;37:1227–1235. doi: 10.1016/s0959-8049(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 19.Greenspan D, Viliers EM, Greenspan JS, Souza YG, Hausen H. Unusual HPV types in oral warts in association with HIV infection. J Oral Pathol Med. 1988;17:482–487. doi: 10.1111/j.1600-0714.1988.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldstone S, Palefsky JM, Giuliano AR, Moreira ED, Aranda C, Jessen H, Marshall JB. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J Infect Dis. 2011;203:66–74. doi: 10.1093/infdis/jiq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Mistro A, Bertorelle R, Franzetti M, Cattelan A, Torrisi A, Giordani MT, Minucci D. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis. 2004;38:737–742. doi: 10.1086/381681. [DOI] [PubMed] [Google Scholar]
- 22.Thakur VS, Amin AR, Paul RK, Gupta K, Hastak K, Agarwal MK, Agarwal ML. p53-dependent p21-mediated growth arrest pre-empts and protects HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer Lett. 2010;296:225–232. doi: 10.1016/j.canlet.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao Y, Cao J, Xie L, Shi X. Cell growth inhibition and gene expression regulation by (–)-epigallocatechin-3-gallate in human cervical cancer cells. Arch Pharm Res. 2009;32:1309–1315. doi: 10.1007/s12272-009-1917-3. [DOI] [PubMed] [Google Scholar]
- 24.Park JS, Kim EJ, Kwon HJ, Hwang ES, Namkoong SE, Um SJ. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275:6764–6769. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]


