Abstract
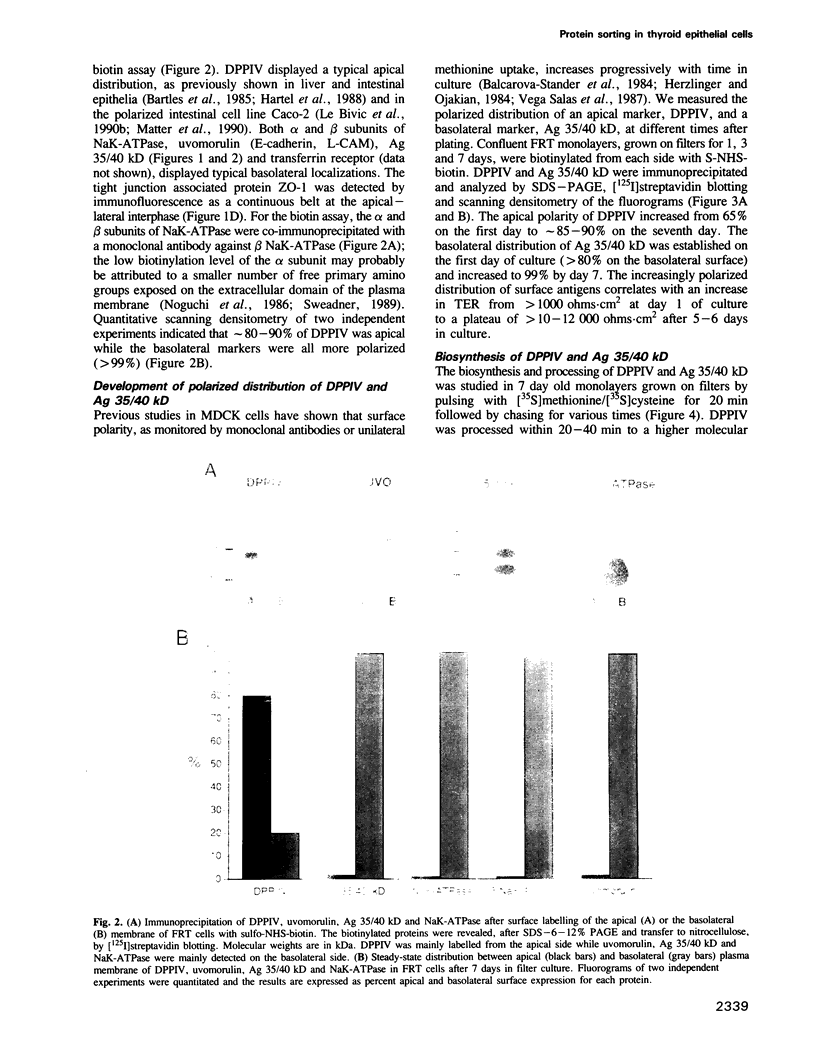
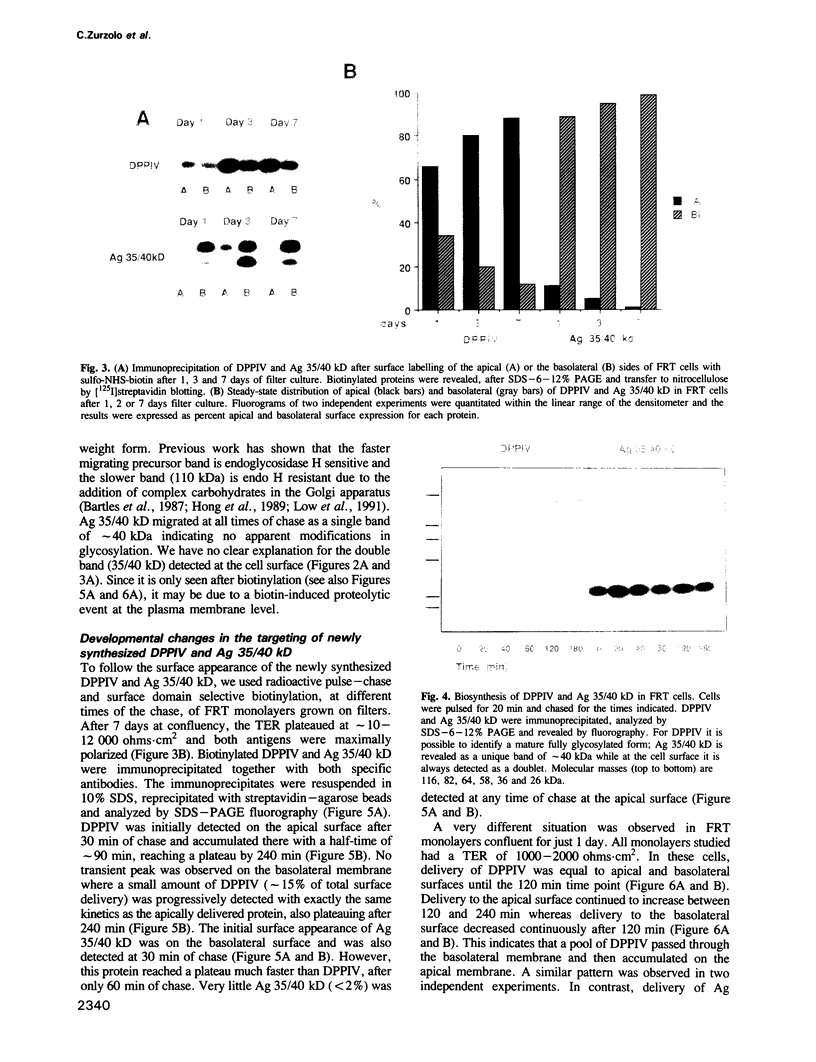
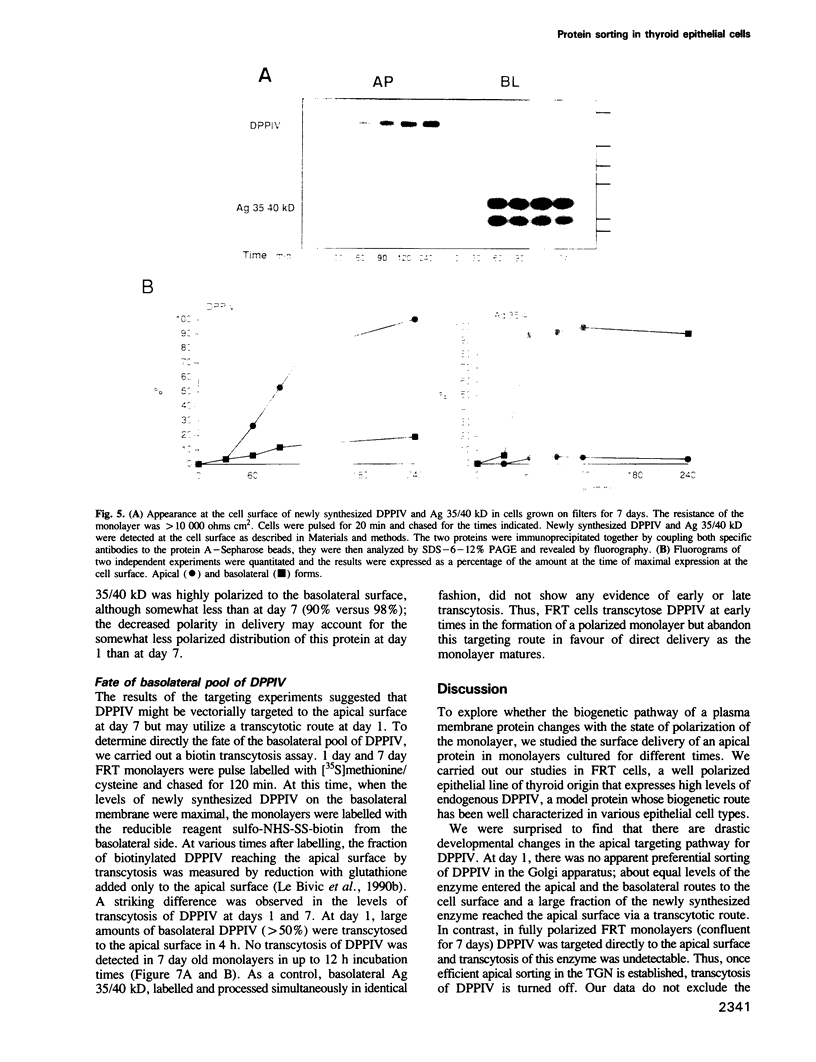
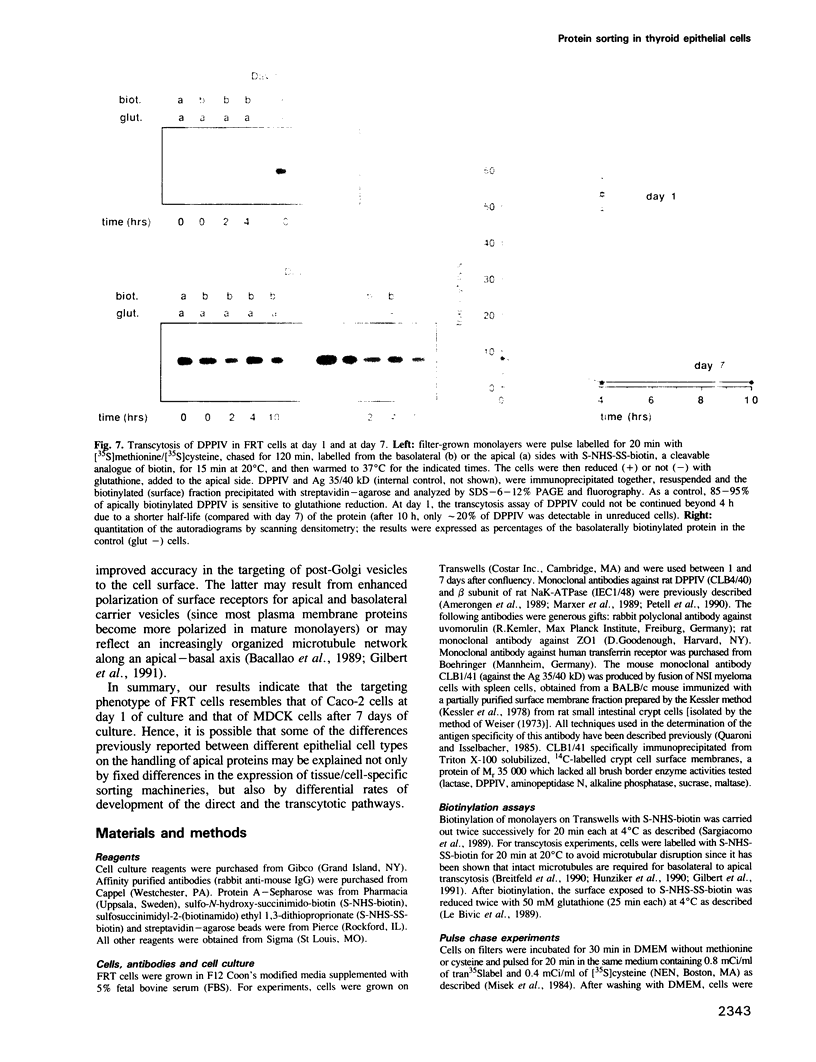
Two biosynthetic pathways exist for delivery of membrane proteins to the apical surface of epithelial cells, direct transport from the trans-Golgi network (TGN) and transcytosis from the basolateral membrane. Different epithelial cells vary in the expression of these mechanisms. Two extremes are MDCK cells, that use predominantly the direct route and hepatocytes, which deliver all apical proteins via the basolateral membrane. To determine how epithelial cells establish a particular targeting phenotype, we studied the apical delivery of endogenous dipeptidyl peptidase IV (DPPIV) at early and late stages in the development of monolayers of a highly polarized epithelial cell line derived from Fischer rat thyroid (FRT). In 1 day old monolayers, surface delivery of DPPIV from the TGN was unpolarized (50%/50%) but a large basal to apical transcytotic component resulted in a polarized apical distribution. In contrast, after 7 days of culture, delivery of DPPIV was mainly direct (85%) with no transcytosis of the missorted component. A basolateral marker, Ag 35/40 kD, on the other hand, was directly targeted (90-98%) at all times. These results indicate that the sorting machinery for apical proteins develops independently from the sorting machinery for basolateral proteins and that the sorting site relocates progressively from the basal membrane to the TGN during development of the epithelium. The transient expression of the transcytotic pathway may serve as a salvage pathway for missorted apical proteins when the polarized phenotype is being established.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amerongen H. M., Mack J. A., Wilson J. M., Neutra M. R. Membrane domains of intestinal epithelial cells: distribution of Na+,K+-ATPase and the membrane skeleton in adult rat intestine during fetal development and after epithelial isolation. J Cell Biol. 1989 Nov;109(5):2129–2138. doi: 10.1083/jcb.109.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E. H., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989 Dec;109(6 Pt 1):2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcarova-Ständer J., Pfeiffer S. E., Fuller S. D., Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J. 1984 Nov;3(11):2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Braiterman L. T., Hubbard A. L. Endogenous and exogenous domain markers of the rat hepatocyte plasma membrane. J Cell Biol. 1985 Apr;100(4):1126–1138. doi: 10.1083/jcb.100.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987 Sep;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Hubbard A. L. Plasma membrane protein sorting in epithelial cells: do secretory pathways hold the key? Trends Biochem Sci. 1988 May;13(5):181–184. doi: 10.1016/0968-0004(88)90147-8. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., McKinnon W. C., Mostov K. E. Effect of nocodazole on vesicular traffic to the apical and basolateral surfaces of polarized MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 1):2365–2373. doi: 10.1083/jcb.111.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Apodaca G., Mostov K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991 Jul 12;66(1):65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Mishumi Y., Ikehara Y., Hubbard A. L., Mostov K. E. Direct apical sorting of rat liver dipeptidylpeptidase IV expressed in Madin-Darby canine kidney cells. J Biol Chem. 1991 Dec 25;266(36):24428–24432. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Gilbert T., Le Bivic A., Quaroni A., Rodriguez-Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol. 1991 Apr;113(2):275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel S., Gossrau R., Hanski C., Reutter W. Dipeptidyl peptidase (DPP) IV in rat organs. Comparison of immunohistochemistry and activity histochemistry. Histochemistry. 1988;89(2):151–161. doi: 10.1007/BF00489918. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlinger D. A., Ojakian G. K. Studies on the development and maintenance of epithelial cell surface polarity with monoclonal antibodies. J Cell Biol. 1984 May;98(5):1777–1787. doi: 10.1083/jcb.98.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. J., Petell J. K., Swank D., Sanford J., Hixson D. C., Doyle D. Expression of dipeptidyl peptidase IV in rat tissues is mainly regulated at the mRNA levels. Exp Cell Res. 1989 May;182(1):256–266. doi: 10.1016/0014-4827(89)90296-6. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Mâle P., Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 1990 Nov;9(11):3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990 Oct;111(4):1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990 May;110(5):1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Patzak A., Patil N., Chao M., Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991 Nov;115(3):607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Gilbert T., Hanzel D., Rodriguez-Boulan E. Vectorial apical delivery and slow endocytosis of a glycolipid-anchored fusion protein in transfected MDCK cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7419–7423. doi: 10.1073/pnas.87.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Le Bivic A., Sargiacomo M., Rodriguez-Boulan E. Steady-state distribution and biogenesis of endogenous Madin-Darby canine kidney glycoproteins: evidence for intracellular sorting and polarized cell surface delivery. J Cell Biol. 1989 Nov;109(5):2117–2127. doi: 10.1083/jcb.109.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Wong S. H., Tang B. L., Subramaniam V. N., Hong W. J. Apical cell surface expression of rat dipeptidyl peptidase IV in transfected Madin-Darby canine kidney cells. J Biol Chem. 1991 Jul 15;266(20):13391–13396. [PubMed] [Google Scholar]
- Marxer A., Stieger B., Quaroni A., Kashgarian M., Hauri H. P. (Na+ + K+)-ATPase and plasma membrane polarity of intestinal epithelial cells: presence of a brush border antigen in the distal large intestine that is immunologically related to beta subunit. J Cell Biol. 1989 Sep;109(3):1057–1069. doi: 10.1083/jcb.109.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey D., Feracci H., Gorvel J. P., Rigal A., Soulié J. M., Maroux S. Evidence for the transit of aminopeptidase N through the basolateral membrane before it reaches the brush border of enterocytes. J Membr Biol. 1987;96(1):19–25. doi: 10.1007/BF01869331. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol. 1984 Dec;99(6):2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Brauchbar M., Bucher K., Hauri H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell. 1990 Feb 9;60(3):429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- Misek D. E., Bard E., Rodriguez-Boulan E. Biogenesis of epithelial cell polarity: intracellular sorting and vectorial exocytosis of an apical plasma membrane glycoprotein. Cell. 1984 Dec;39(3 Pt 2):537–546. doi: 10.1016/0092-8674(84)90460-4. [DOI] [PubMed] [Google Scholar]
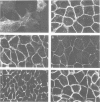
- Nitsch L., Tramontano D., Ambesi-Impiombato F. S., Quarto N., Bonatti S. Morphological and functional polarity of an epithelial thyroid cell line. Eur J Cell Biol. 1985 Jul;38(1):57–66. [PubMed] [Google Scholar]
- Noguchi S., Noda M., Takahashi H., Kawakami K., Ohta T., Nagano K., Hirose T., Inayama S., Kawamura M., Numa S. Primary structure of the beta-subunit of Torpedo californica (Na+ + K+)-ATPase deduced from the cDNA sequence. FEBS Lett. 1986 Feb 17;196(2):315–320. doi: 10.1016/0014-5793(86)80270-8. [DOI] [PubMed] [Google Scholar]
- Pathak R. K., Yokode M., Hammer R. E., Hofmann S. L., Brown M. S., Goldstein J. L., Anderson R. G. Tissue-specific sorting of the human LDL receptor in polarized epithelia of transgenic mice. J Cell Biol. 1990 Aug;111(2):347–359. doi: 10.1083/jcb.111.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petell J. K., Quaroni A., Hong W. J., Hixson D. C., Amarri S., Reif S., Bujanover Y. Alteration in the regulation of plasma membrane glycoproteins of the hepatocyte during ontogeny. Exp Cell Res. 1990 Apr;187(2):299–308. doi: 10.1016/0014-4827(90)90095-r. [DOI] [PubMed] [Google Scholar]
- Powell S. K., Cunningham B. A., Edelman G. M., Rodriguez-Boulan E. Targeting of transmembrane and GPI-anchored forms of N-CAM to opposite domains of a polarized epithelial cell. Nature. 1991 Sep 5;353(6339):76–77. doi: 10.1038/353076a0. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J. Study of intestinal cell differentiation with monoclonal antibodies to intestinal cell surface components. Dev Biol. 1985 Oct;111(2):267–279. doi: 10.1016/0012-1606(85)90482-8. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Plesken H., Rodriguez-Boulan E., Sabatini D. D. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J Cell Biol. 1984 Apr;98(4):1304–1319. doi: 10.1083/jcb.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Salas P. J., Bard E. Intracellular transport of influenza virus hemagglutinin to the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1984 Jan;98(1):308–319. doi: 10.1083/jcb.98.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E. Polarized assembly of enveloped viruses from cultured epithelial cells. Methods Enzymol. 1983;98:486–501. doi: 10.1016/0076-6879(83)98176-4. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989 May 9;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Gundersen D., Rodriguez-Boulan E. Formation of the apical pole of epithelial (Madin-Darby canine kidney) cells: polarity of an apical protein is independent of tight junctions while segregation of a basolateral marker requires cell-cell interactions. J Cell Biol. 1987 Apr;104(4):905–916. doi: 10.1083/jcb.104.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wessels H. P., Hansen G. H., Fuhrer C., Look A. T., Sjöström H., Norén O., Spiess M. Aminopeptidase N is directly sorted to the apical domain in MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 2):2923–2930. doi: 10.1083/jcb.111.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C., Gentile R., Mascia A., Garbi C., Polistina C., Aloj L., Avvedimento V. E., Nitsch L. The polarized epithelial phenotype is dominant in hybrids between polarized and unpolarized rat thyroid cell lines. J Cell Sci. 1991 Jan;98(Pt 1):65–73. doi: 10.1242/jcs.98.1.65. [DOI] [PubMed] [Google Scholar]