Abstract
We studied protein sorting signals which are responsible for the retention of reticuloplasmins in the lumen of the plant endoplasmic reticulum (ER). A non-specific passenger protein, previously shown to be secreted by default, was used as a carrier for such signals. Tagging with C-terminal tetrapeptide sequences of mammalian (KDEL) and yeast (HDEL) reticuloplasmins led to effective accumulation of the protein chimeras in the lumen of the plant ER. Some single amino acid substitutions within the tetrapeptide tag (-SDEL, -KDDL, -KDEI and -KDEV) can cause a complete loss of its function as a retention signal, demonstrating the high specificity of the retention machinery. However, other modifications confer efficient (-RDEL) or partial (-KEEL) retention. It is also shown that the efficiency of protein retention is not significantly impaired by an increased ligand concentration in plants. The efficiently retained chimeras (-KDEL, -HDEL and -RDEL) were shown to be recognized by a monoclonal antibody directed against the C-terminus of the mammalian reticuloplasmin protein disulfide isomerase (PDI). The recognized epitope is also present in several putative reticuloplasmins in microsomal fractions of plant and mammalian cells, suggesting that the antibodies recognize an important structural determinant of the retention signal. In addition, data are discussed which support the view that upstream sequences beyond the C-terminal tetrapeptide can influence or may be part of the structure of reticuloplasmin retention signals.
Full text
PDF


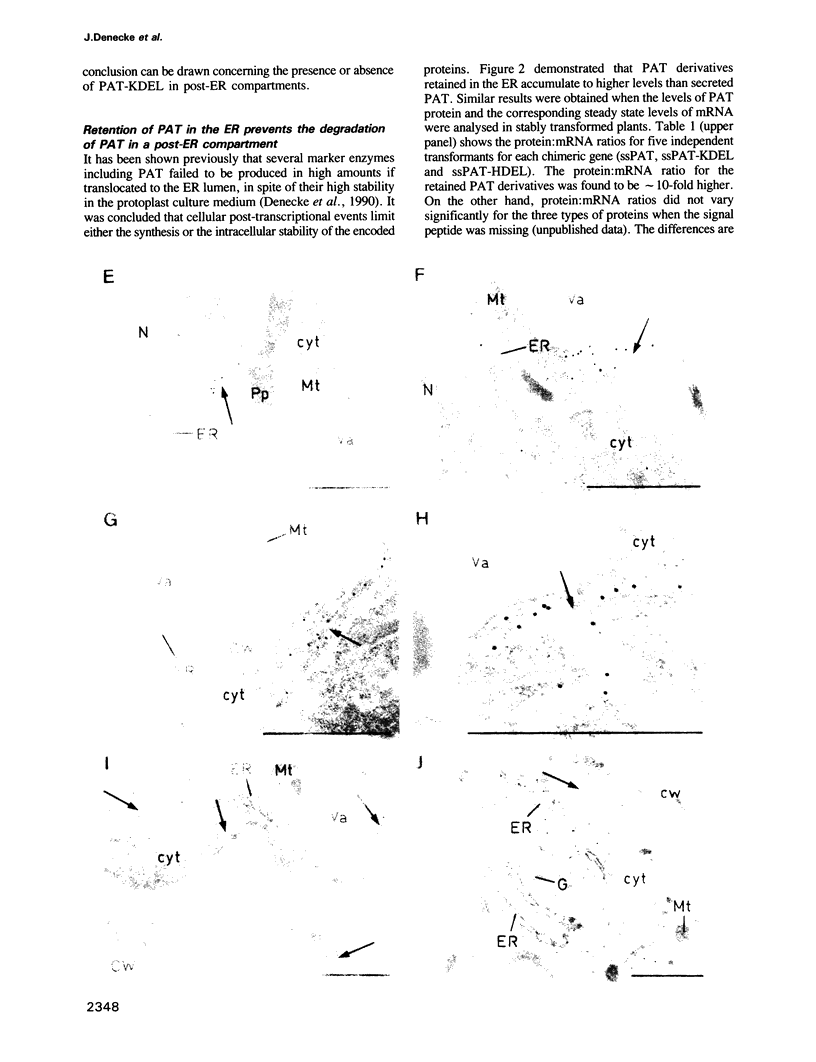
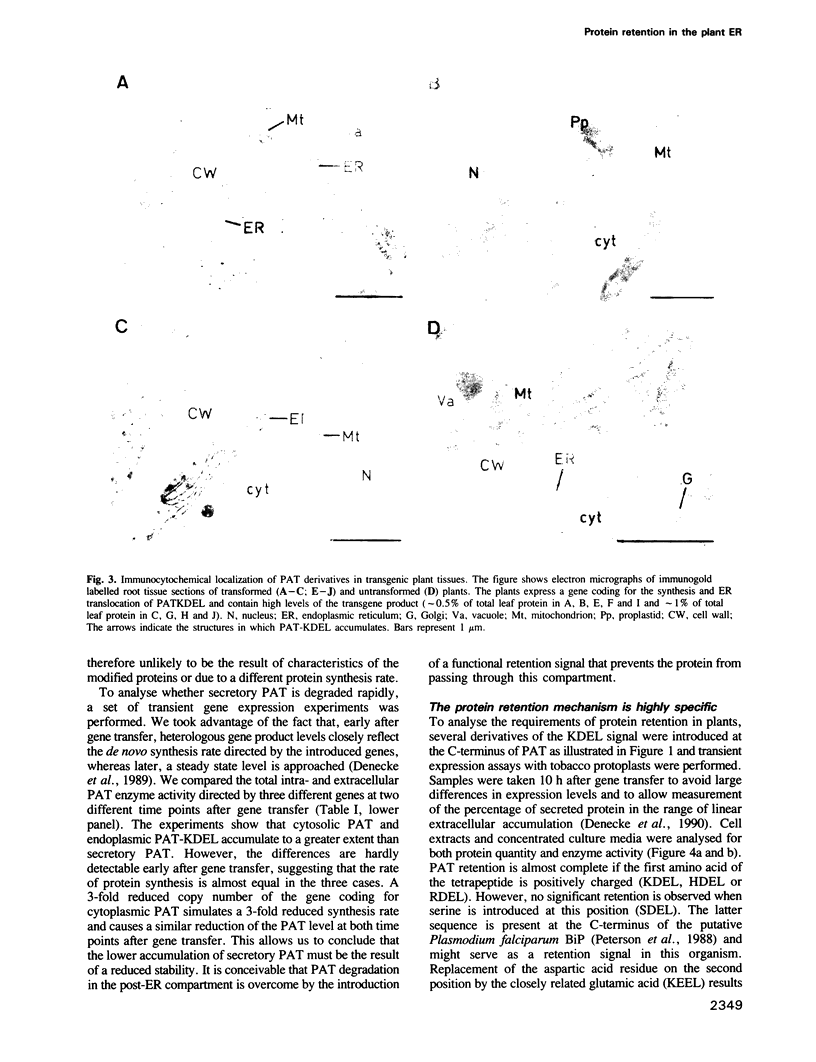
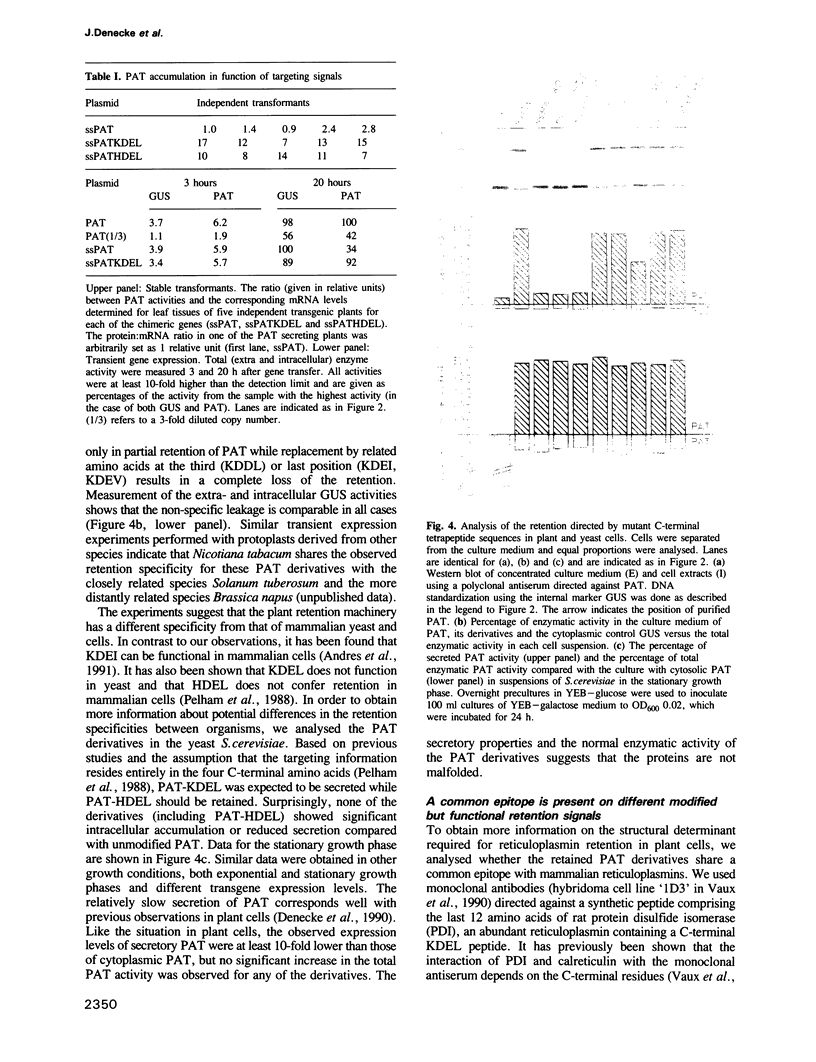





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasofu H., Yamauchi D., Mitsuhashi W., Minamikawa T. Nucleotide sequence of cDNA for sulfhydryl-endopeptidase (SH-EP) from cotyledons of germinating Vigna mungo seeds. Nucleic Acids Res. 1989 Aug 25;17(16):6733–6733. doi: 10.1093/nar/17.16.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres D. A., Dickerson I. M., Dixon J. E. Variants of the carboxyl-terminal KDEL sequence direct intracellular retention. J Biol Chem. 1990 Apr 15;265(11):5952–5955. [PubMed] [Google Scholar]
- Andres D. A., Rhodes J. D., Meisel R. L., Dixon J. E. Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J Biol Chem. 1991 Aug 5;266(22):14277–14282. [PubMed] [Google Scholar]
- Block M. D., Botterman J., Vandewiele M., Dockx J., Thoen C., Gosselé V., Movva N. R., Thompson C., Montagu M. V., Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987 Sep;6(9):2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Botterman J., Gosselé V., Thoen C., Lauwereys M. Characterization of phosphinothricin acetyltransferase and C-terminal enzymatically active fusion proteins. Gene. 1991 Jun 15;102(1):33–37. doi: 10.1016/0378-1119(91)90534-i. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Ceriotti A., Colman A. Binding to membrane proteins within the endoplasmic reticulum cannot explain the retention of the glucose-regulated protein GRP78 in Xenopus oocytes. EMBO J. 1988 Mar;7(3):633–638. doi: 10.1002/j.1460-2075.1988.tb02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen M., Vandewiele M. Nuclear transcriptional activity of the tobacco plastid psbA promoter. Nucleic Acids Res. 1989 Jan 11;17(1):19–29. doi: 10.1093/nar/17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq A., Vandewiele M., De Rycke R., Van Damme J., Van Montagu M., Krebbers E., Vandekerckhove J. Expression and Processing of an Arabidopsis 2S Albumin in Transgenic Tobacco. Plant Physiol. 1990 Apr;92(4):899–907. doi: 10.1104/pp.92.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J., Botterman J., Deblaere R. Protein secretion in plant cells can occur via a default pathway. Plant Cell. 1990 Jan;2(1):51–59. doi: 10.1105/tpc.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J., Goldman M. H., Demolder J., Seurinck J., Botterman J. The tobacco luminal binding protein is encoded by a multigene family. Plant Cell. 1991 Sep;3(9):1025–1035. doi: 10.1105/tpc.3.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Wasley L. C., Raney P., Haugejorden S., Green M., Kaufman R. J. The stress response in Chinese hamster ovary cells. Regulation of ERp72 and protein disulfide isomerase expression and secretion. J Biol Chem. 1990 Dec 15;265(35):22029–22034. [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Apparent Inhibition of beta-Fructosidase Secretion by Tunicamycin May Be Explained by Breakdown of the Unglycosylated Protein during Secretion. Plant Physiol. 1989 Mar;89(3):845–851. doi: 10.1104/pp.89.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K. G., Lewis M. J., Semenza J., Dean N., Pelham H. R. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 1990 Mar;9(3):623–630. doi: 10.1002/j.1460-2075.1990.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugejorden S. M., Srinivasan M., Green M. Analysis of the retention signals of two resident luminal endoplasmic reticulum proteins by in vitro mutagenesis. J Biol Chem. 1991 Apr 5;266(10):6015–6018. [PubMed] [Google Scholar]
- Hesse T., Feldwisch J., Balshüsemann D., Bauw G., Puype M., Vandekerckhove J., Löbler M., Klämbt D., Schell J., Palme K. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J. 1989 Sep;8(9):2453–2461. doi: 10.1002/j.1460-2075.1989.tb08380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N., Shimomura S., Fukui T., Futai M. Auxin-binding protein located in the endoplasmic reticulum of maize shoots: molecular cloning and complete primary structure. Proc Natl Acad Sci U S A. 1989 May;86(10):3564–3568. doi: 10.1073/pnas.86.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Cell biology. Tracking an elusive receptor. Nature. 1990 Jun 7;345(6275):480–481. doi: 10.1038/345480a0. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Harriss J. V., Sharp Z. D., Douglas M. G. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene. 1983 Nov;25(2-3):333–341. doi: 10.1016/0378-1119(83)90238-x. [DOI] [PubMed] [Google Scholar]
- Koch G. L. Reticuloplasmins: a novel group of proteins in the endoplasmic reticulum. J Cell Sci. 1987 May;87(Pt 4):491–492. doi: 10.1242/jcs.87.4.491. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- LaMantia M., Miura T., Tachikawa H., Kaplan H. A., Lennarz W. J., Mizunaga T. Glycosylation site binding protein and protein disulfide isomerase are identical and essential for cell viability in yeast. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4453–4457. doi: 10.1073/pnas.88.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. J., Pelham H. R. A human homologue of the yeast HDEL receptor. Nature. 1990 Nov 8;348(6297):162–163. doi: 10.1038/348162a0. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Sweet D. J., Pelham H. R. The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell. 1990 Jun 29;61(7):1359–1363. doi: 10.1016/0092-8674(90)90699-f. [DOI] [PubMed] [Google Scholar]
- Macer D. R., Koch G. L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J Cell Sci. 1988 Sep;91(Pt 1):61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Mazzarella R. A., Srinivasan M., Haugejorden S. M., Green M. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J Biol Chem. 1990 Jan 15;265(2):1094–1101. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Hardwick K. G., Lewis M. J. Sorting of soluble ER proteins in yeast. EMBO J. 1988 Jun;7(6):1757–1762. doi: 10.1002/j.1460-2075.1988.tb03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Crewther P. E., Thompson J. K., Corcoran L. M., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. A second antigenic heat shock protein of Plasmodium falciparum. DNA. 1988 Mar;7(2):71–78. doi: 10.1089/dna.1988.7.71. [DOI] [PubMed] [Google Scholar]
- Semenza J. C., Hardwick K. G., Dean N., Pelham H. R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990 Jun 29;61(7):1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Stinissen H. M., Peumans W. J., Chrispeels M. J. Posttranslational processing of proteins in vacuoles and protein bodies is inhibited by monensin. Plant Physiol. 1985 Feb;77(2):495–498. doi: 10.1104/pp.77.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Yamauchi D., Minamikawa T. Nucleotide sequence of cDNA for an endopeptidase (EP-C1) from pods of maturing Phaseolus vulgaris fruits. Plant Mol Biol. 1991 Jun;16(6):1083–1084. doi: 10.1007/BF00016081. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Movva N. R., Tizard R., Crameri R., Davies J. E., Lauwereys M., Botterman J. Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J. 1987 Sep;6(9):2519–2523. doi: 10.1002/j.1460-2075.1987.tb02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann U., Viola G., Kayser B., Siemeister G., Hesse T., Palme K., Löbler M., Klämbt D. cDNA clones of the auxin-binding protein from corn coleoptiles (Zea mays L.): isolation and characterization by immunological methods. EMBO J. 1989 Sep;8(9):2463–2467. doi: 10.1002/j.1460-2075.1989.tb08381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Velten J., Velten L., Hain R., Schell J. Isolation of a dual plant promoter fragment from the Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1984 Dec 1;3(12):2723–2730. doi: 10.1002/j.1460-2075.1984.tb02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Salvage receptors: two of a kind? Cell. 1990 Jul 13;62(1):1–2. doi: 10.1016/0092-8674(90)90229-8. [DOI] [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Zagouras P., Rose J. K. Carboxy-terminal SEKDEL sequences retard but do not retain two secretory proteins in the endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2633–2640. doi: 10.1083/jcb.109.6.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]