Abstract
Neosartorya fumigata (Aspergillus fumigatus) is the most common cause of invasive aspergillosis, a frequently fatal lung disease primarily affecting immunocompromised individuals. This opportunistic fungal pathogen produces several classes of specialised metabolites including products of a branch of the ergot alkaloid pathway called fumigaclavines. The biosynthesis of the N. fumigata ergot alkaloids and their relation to those produced by alternate pathway branches in fungi from the plant-inhabiting Clavicipitaceae have been well-characterised, but the potential role of these alkaloids in animal pathogenesis has not been studied extensively. We investigated the contribution of ergot alkaloids to virulence of N. fumigata by measuring mortality in the model insect Galleria mellonella. Larvae were injected with conidia (asexual spores) of two different wild-type strains of N. fumigata and three different ergot alkaloid mutants derived by previous gene knockouts and differing in ergot alkaloid profiles. Elimination of all ergot alkaloids significantly reduced virulence of N. fumigata in G. mellonella (P < 0.0001). Mutants accumulating intermediates but not the pathway end product fumigaclavine C also were less virulent than the wild type (P < 0.0003). The data indicate that ergot alkaloids contribute to virulence of N. fumigata in this insect model and that fumigaclavine C is important for full virulence.
Introduction
Ergot alkaloids are specialised metabolites derived from prenylated tryptophan and produced by several fungi representing different phylogenetic lineages and occupying different ecological niches1–6. Lysergic acid-based ergot alkaloids produced by fungi in the Clavicipitaceae, including the plant pathogen Claviceps purpurea and several Epichloë species that grow as mutualistic symbionts of grasses, have profoundly affected humanity as toxins in agricultural crops5, 6 and as the bases of pharmaceuticals used to treat dementia, migraines, Parkinson’s disease, and hyperprolactinemia7–12. The activities of these alkaloids stem from their abilities to act as agonists or antagonists at receptors for monoamine neurotransmitters including serotonin, dopamine, adrenaline, and noradrenaline. Ecologically the ergot alkaloids of the Clavicipitaceae have been shown to serve as feeding deterrents in mammals and insects and also have insecticidal activities13, 14.
A different branch of the ergot alkaloid pathway leads to the production of fumigaclavines (Fig. 1) in certain members of the Trichocomaceae, including the opportunistic human and animal pathogen Neosartorya fumigata (Aspergillus fumigatus)1–4. Whereas several members of the Trichocomaceae produce representatives of the fumigaclavines, N. fumigata is the only fungus known to produce fumigaclavine C. The activities of the ergot alkaloids of the Trichocomaceae have not been studied as intensively as those of the Clavicipitaceae, but recent data demonstrate that fumigaclavine C inhibits tumor necrosis factor α production in human macrophages15 and leads to reduced expression of several other inflammatory cytokines in mice16. Moreover, fumigaclavine C is cytotoxic to cancer cells17.
Figure 1.
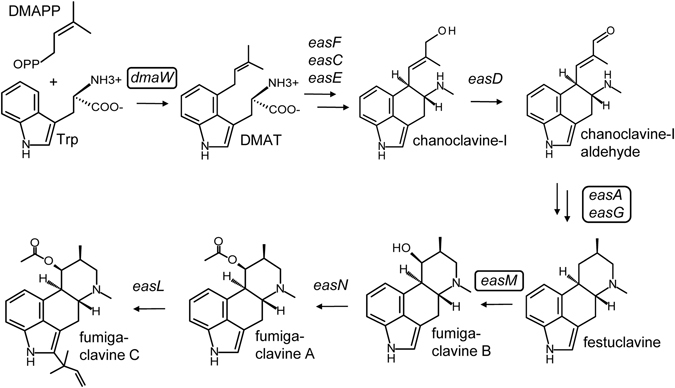
Important intermediates and end product of the ergot alkaloid pathway of N. fumigata. Genes controlling pathway steps are indicated, and genes knocked out in strains included in the present study are outlined. DMAPP, dimethylallylpyrophosphate; Trp, tryptophan, DMAT, dimethylallyltryptophan. Double arrows indicate additional, non-illustrated intermediates.
Neosartorya fumigata is the most frequent cause of invasive aspergillosis, a disease that most frequently affects immunocompromised individuals and that has a high mortality rate18–20. Several lines of evidence indicate roles for specialised metabolites in virulence of N. fumigata, but direct evidence of the contribution of specific metabolites to virulence is scarce. In addition to its family of ergot alkaloids, N. fumigata produces gliotoxin, fumagillin, and fumitremorgins, among other specialised metabolites19, 20. Gene clusters for at least 13 metabolites, including the ergot alkaloids, are controlled by LaeA, a master transcriptional regulator of specialised metabolism21. LaeA-deficient mutants demonstrate reduced accumulation of several metabolites and have greatly reduced virulence in animal models22, 23. These data demonstrate that products of at least some LaeA-regulated genes are required for virulence and indicate roles for specialised metabolites in determining virulence. Among the metabolites produced by N. fumigata, gliotoxin has been most intensively studied because of its immunosuppressive activities19, 20. Tests of the contribution of gliotoxin to virulence in a murine model of invasive aspergillosis have yielded conflicting results, with the contribution of gliotoxin to virulence differing perhaps due to genetic background of the fungus or methods of suppressing host immunity24–27. Neosartorya fumigata also produces the polyketide-derived metabolite fumagillin, which suppresses the innate immune system of the model insect host Galleria mellonella rendering it significantly more susceptible to subsequent infection by the pathogen28.
A link between fumigaclavine C and virulence of N. fumigata can be hypothesised based on previous studies of peptide synthetases Pes1 and PesL. Whereas, in vitro studies indicate that reverse prenyl transferase FgaPT1 (also called EasL) is sufficient to convert fumigaclavine A to fumigaclavine C29, knockout studies indicate that peptide synthetases Pes1 and PesL also are required in some way for the conversion of fumigaclavine A to fumigaclavine C30. Pes1 and PesL mutants had additional altered phenotypes including increased accumulation of fumitremorgins and increased sensitivities to oxidative stress and antifungal azoles30, 31. Whether these additional phenotypes are associated with the loss of fumigaclavine C or with other activities of these peptide synthetases is unclear. Knockout of pesL reduced virulence in the insect model host G. mellonella 30. The role of Pes1 in virulence varied in two fungal strains in which mutants were made. Reeves et al.31 observed a significant reduction in virulence when pes1 was knocked out in an Af293.1 background, but O’Hanlon et al.30 observed no difference in virulence when pes1 was knocked out in an ATCC 46645 background.
Whereas mice have most frequently served as the model host in studies of infectious human pathogens, the larva of the greater wax moth Galleria mellonella has recently been used as a model host for pathogenicity assays of numerous mammalian pathogens32–35. In addition to being inexpensive and easy to maintain, experiments with this invertebrate do not require institutional oversight. In several studies, G. mellonella has been demonstrated to be a reliable and useful model of invasive aspergillosis20, 28, 30, 31. Results obtained with this invertebrate model correlate well with those obtained from tests of the same pathogens in mammalian models33, 35, 36. In one exception to this correlation, melanin-deficient mutants of N. fumigata had greatly reduced virulence in mice but increased virulence in G. mellonella 37.
Our previous research on the biosynthesis of ergot alkaloids in N. fumigata has resulted in the generation of gene knockout mutants that are deficient in the accumulation of some or all ergot alkaloids. Knockout of dmaW, the first gene in the ergot alkaloid pathway, eliminated all ergot alkaloids38 (Fig. 1). Blocking the pathway at the step controlled by easA prevented formation of festuclavine from chanoclavine-I aldehyde and resulted in accumulation of primarily the preceding intermediate chanoclavine-I39, 40 (also see Supplementary Information). Knockout of easM resulted in the accumulation of large quantities of festuclavine and prevented the formation of downstream fumigaclavines41 (Fig. 1). Our objective in this current study was to utilise these ergot alkaloid pathway mutants in virulence assays conducted with the larvae of G. mellonella to investigate the role of ergot alkaloids in virulence of N. fumigata.
Results
Contribution of ergot alkaloids to virulence of N. fumigata FGSC A1141
Because the ergot alkaloid pathway gene knockout strains available for study were prepared previously in two different genetic backgrounds of N. fumigata (Table 1), data were collected and analysed separately for the two sets of modified strains. Comparison of survival data from the dmaW knockout (a mutant that lacks all ergot alkaloids38; Table 2) and its wild-type, parental isolate N. fumigata FGSC A1141 indicated that ergot alkaloids were required for full virulence of N. fumigata in G. mellonella. When experiments were conducted at 22 °C, the rate of mortality caused by the dmaW knockout strain was significantly lower than that caused by the wild-type strain FGSC A1141 (P < 0.0001; Fig. 2A). Ectopic complementation with a functional allele of dmaW restored full virulence, demonstrating the change in virulence was due to the dmaW mutation (Fig. 2). The virulence of the dmaW knockout strain was reduced to the point where it was not statistically different from that of the PBS control (P = 0.94), but larvae infected with dmaW knockout appeared to be more strongly melanised and more lethargic than did larvae injected with the PBS control. To further investigate this issue, larvae were challenged with the same set of treatments but incubated at 37 °C (a temperature more favorable for N. fumigata) following inoculation. The dmaW knockout strain was again significantly less virulent than the parent strain FGSC A1141 (P < 0.0001) (Fig. 2B), but under these conditions the dmaW knockout strain was intermediate in virulence between FGSC A1141 and the sterile PBS control (Fig. 2B). These data demonstrate that factors other than ergot alkaloids also contribute to virulence of N. fumigata. As in the room-temperature experiment, the strain complemented with the functional dmaW allele did not differ significantly in virulence compared to its parent strain FGSC A1141 (P = 0.54). The times required for each strain to kill 50% of inoculated larvae (i.e., LT50 values) were calculated as 21 hr for both FGSC A1141 and the dmaW complemented strain and 48 hr for the dmaW knockout strain.
Table 1.
Strains of Neosartorya fumigata included in this study.
| Strain | Source/derivation | Most abundant ergot alkaloid(s) | Reference |
|---|---|---|---|
| FGSC A1141 | Clinical | festuclavine/fumigaclavine C | 45 |
| dmaW ko | Gene knockout in FGSC A1141 | no ergot alkaloids | 38 |
| dmaW ct | Complemented dmaW ko in FGSC A1141 | festuclavine/fumigaclavine C | 38 |
| Af293 | Clinical | festuclavine/fumigaclavine C | 50 |
| easM ko | Gene knockout in Af293 | festuclavine | 41 |
| easA/G ko | Gene knockout in Af293 | chanoclavine-I | current study |
Table 2.
Mean ergot alkaloid concentrations (μM; mean ± standard error) in Neosartorya fumigata conidial extracts injected into Galleria mellonella larvae.
| Straina | Background | Chanoclavine-I | Festuclavine | Fumigaclavine C | Summed ergot alkaloids |
|---|---|---|---|---|---|
| FGSC A1141 | FGSC A1141 | n.d.b | 6.1 ± 1.2 | 2.0 ± 0.7 | 8.1 ± 1.5 |
| dmaW ko | FGSC A1141 | n.d. | n.d. | n.d. | n.d. |
| dmaW ct | FGSC A1141 | n.d. | 2.1 ± 0.4 | 1.1 ± 0.4 | 3.2 ± 0.7 |
| filtrate | FGSC A1141 | n.d. | 1.0 ± 0.1 | 0.1 ± 0.03 | 1.1 ± 0.2 |
| Af293 | Af293 | n.d. | 1.8 ± 0.8 | 1.0 ± 0.5 | 2.9 ± 1.3 |
| easA/G ko | Af293 | 0.3 ± 0.03 | n.d. | n.d. | 0.3 ± 0.03 |
| easM ko | Af293 | n.d. | 9.3 ± 1.8 | n.d. | 9.3 ± 1.8 |
ako, knockout; ct, complemented; filtrate, conidia removed by filtration prior to addition of methanol.
bn.d., not detected; limit of detection 0.07 μM.
Figure 2.
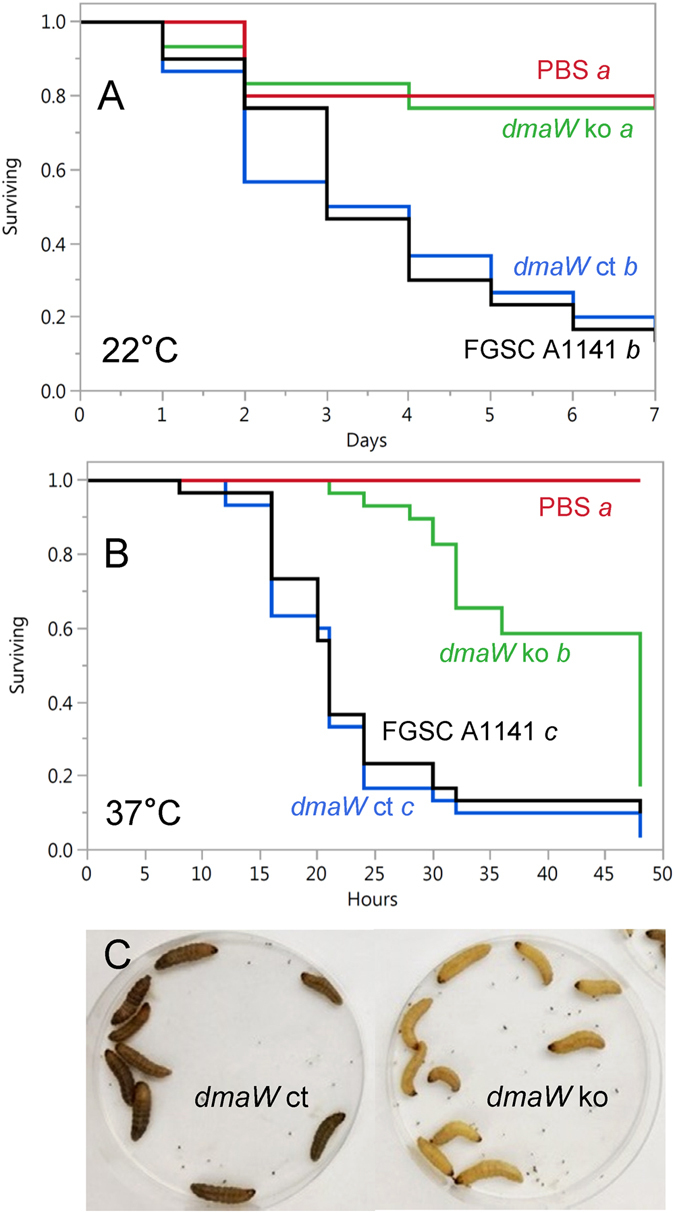
Survival curves for G. mellonella larvae injected with conidial suspensions of N. fumigata FGSC A1141, an ergot alkaloid-deficient dmaW knockout (ko) mutant of that strain, an ergot alkaloid restored strain complemented with a wild-type allele of dmaW (dmaW ct), or phosphate-buffered saline control (PBS). In panel A, inoculated larvae were incubated at 22 °C for seven days. In panel B, inoculated larvae were incubated at 37 °C for 48 hours. Curves were derived from data from three trials with 10 larvae per treatment per trial. Treatment labels followed by different italicised letters have survival curves that differ significantly in a log-rank test with Bonferroni-adjusted alpha value set at 0.0083 (derived from 0.05/six pairwise comparisons). Panel C shows larvae incubated at 22 °C for 5 days in one trial.
Larval response to conidium-free fungal extracts
To test whether ergot alkaloids or other soluble factors in the N. fumigata FGSC A1141 conidial suspension solution were insecticidal in the absence of the fungus, 20 μL aliquots of conidial suspension solution that had been filtered through a 0.2-μm filter were assayed in G. mellonella larvae. The filtrate lacking conidia did not induce significantly greater mortality than did the PBS control (P = 0.06, with Bonferroni-adjusted alpha at 0.016). The filtered, conidium-free extract induced significantly less mortality than the unfiltered conidial suspension (P < 0.0001) (Fig. 3). These data indicate that mortality resulted primarily after infection with live conidia in the presence of ergot alkaloids and not as a result of acute toxicity of the conidial suspension solution. Comparison by HPLC of ergot alkaloid concentrations in N. fumigata FGSC A1141 conidial suspensions with filtrate prepared from those conidial suspensions indicated that only 12% of the ergot alkaloids were soluble in the filtrate, whereas the remainder must have been associated with the conidia themselves (Table 2). The observed percentage of water-soluble ergot alkaloids was consistent with previously published data42.
Figure 3.
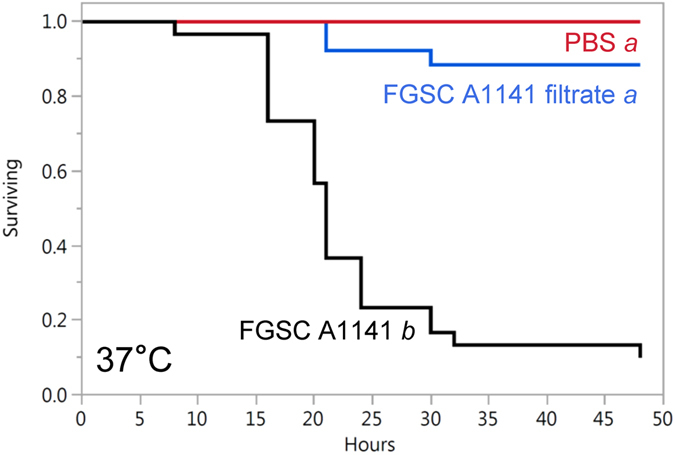
Survival curves for G. mellonella larvae injected with conidial suspensions of N. fumigata FGSC A1141, conidium-free, filtered suspensions of FGSC A1141, or phosphate-buffered saline control (PBS). Curves were derived from data from three trials with 10 larvae per treatment per trial. Treatment labels followed by different italicised letters have survival curves that differ significantly in a log-rank test with Bonferroni-adjusted alpha value set at 0.016 (derived from 0.05/three pairwise comparisons).
Virulence of mutants accumulating different ergot alkaloid pathway intermediates
Ergot alkaloid mutants in the N. fumigata Af293 background also demonstrated reduced virulence. An easA/G knockout strain, which is blocked after chanoclavine-I aldehyde (Fig. 1; Supplementary Information), and an easM knockout strain, which is blocked after festuclavine (Fig. 1), were significantly less virulent in log-rank tests than their parent strain Af293 (P < 0.003) (Fig. 4). These data indicate that ergot alkaloids downstream of the mutated genes were required for full virulence. LT50 values for the easA/G and easM mutant were 30 hr and 27 hr, respectively, whereas the LT50 value for Af293 was 21 hr. The lack of significant difference between the survival curves obtained with the easA/G knockout strain and the easM knockout strain (P = 0.32) indicates that festuclavine does not contribute significantly to mortality of larvae, or that the increase in chanoclavine-I detected in the easA/G knockout strain (Table 2) compensated for the loss of festuclavine. This result is underscored by the great abundance of festuclavine present in the easM knockout (Table 2).
Figure 4.
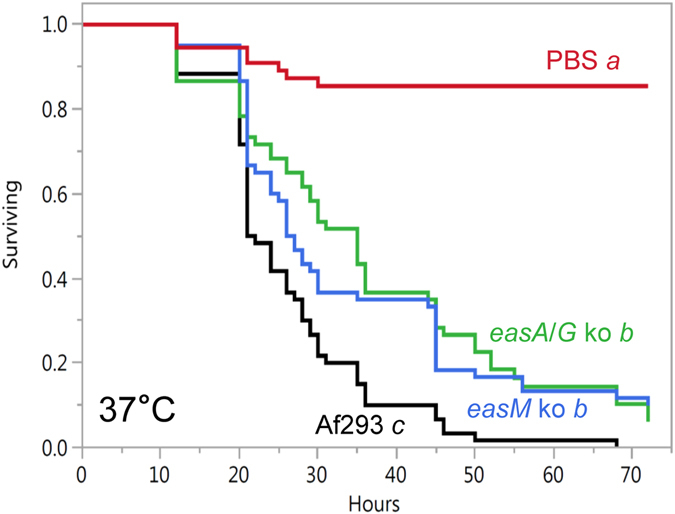
Survival curves for G. mellonella larvae injected with conidial suspensions of N. fumigata Af293, an easM knockout (ko) of strain Af293, an easAG ko of strain Af293, or phosphate-buffered saline control (PBS). Curves are derived from data from four trials with 15 larvae per treatment per trial. Treatment labels followed by different italicised letters have survival curves that differ significantly in a log-rank test with Bonferroni-adjusted alpha value set at 0.0083 (derived from 0.05/six pairwise comparisons).
Correlation of mortality with concentrations of individual ergot alkaloids
To further investigate the relative contributions of the abundantly accumulating intermediate festuclavine compared to the pathway end product fumigaclavine C, we correlated the concentrations of ergot alkaloids in each separate spore suspension sample with the percentage of larvae killed at 21 hr by injection of that same sample. This particular time point was selected because 21 hr was the LT50 value for both wild-type strains (Af293 and FGSC A1141) and the dmaW-complemented strain in experiments conducted with 37 °C incubations. The concentration of fumigaclavine C in individual samples correlated positively with larval mortality (P = 0.02) (though the R 2 value is low, indicating a linear response is not the best fit for the data), whereas the concentration of festuclavine did not correlate with mortality (Fig. 5). When correlation analyses were repeated with data with Af293-derived strains and FGSC A1141-derived strains tested separately, the correlation of fumigaclavine C with percent mortality was still evident (P = 0.04 for Af293-derived strains, and P = 0.06 for FGSC A1141-derived strains), whereas festuclavine did not correlate well with percent mortality (P = 0.78 for Af293-derived strains, and P = 0.10 for FGSC A1141-derived strains).
Figure 5.
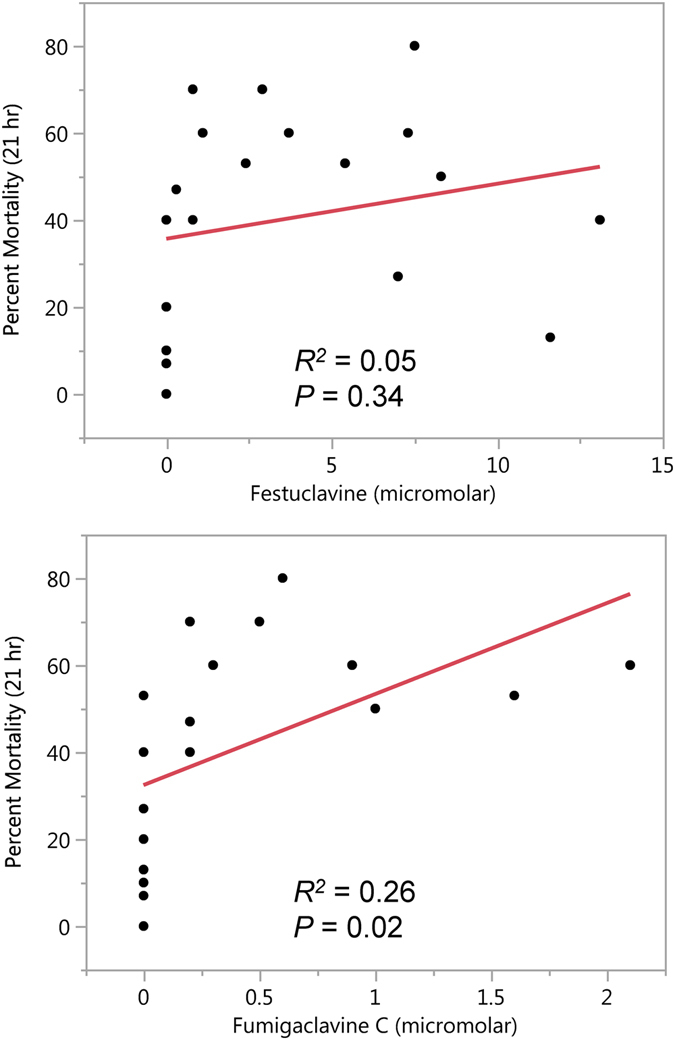
Correlation of ergot alkaloid concentrations in individual N. fumigata conidial extracts with larval mortality resulting from injection of that extract. Mortality was assessed at 21 hours post inoculation because both wild-type strains (Af293 and FGSC A1141) required 21 hours to kill 50% of inoculated larvae when those larvae were incubated at 37 °C. Data points represent all trials conducted at 37 °C with Af293 and FGSC A1141 and the ergot alkaloid mutants derived from those strains.
Discussion
Disruption of the ergot alkaloid pathway in two different genetic backgrounds reduced the virulence of the opportunistic human pathogen N. fumigata, when tested in the invertebrate model G. mellonella. Since we made use of existing mutants, one of the mutants tested (dmaW knockout) was in a N. fumigata FGSC A1141 background, whereas two other mutants (easA/G knockout and easM knockout) were in a N. fumigata Af293 background. Both parent strains produce fumigaclavine C as their pathway end point and have similar ergot alkaloid profiles43 but presumably contain other genetic differences. Because of this difference in backgrounds, we did not compare the survival curves of the dmaW knockout with the easM and easA/G knockouts by a log-rank test. Previous studies have shown that different environmental isolates vary in their virulence30, 31, 44. Adhering to within-isolate limits, we can conclude that elimination of all ergot alkaloids from N. fumigata isolate FGSSC A1141 significantly reduced virulence and that elimination of alkaloids downstream from chanoclavine-I aldehyde and festuclavine in strains derived from N. fumigata Af293 also reduced virulence. The abundantly accumulating intermediate festuclavine (which was the end product of the pathway in the easM knockout) did not appear to be a significant virulence factor because the easM knockout (which accumulated high concentrations of festuclavine) did not differ in virulence from the easA/G knockout whose pathway was blocked prior to festuclavine. Moreover, larval mortality in individual trials did not correlate with festuclavine concentration but did correlate significantly with concentration of fumigaclavine C, which appears to be a significant virulence factor.
The effect of temperature on mortality rate among strains is probably multifaceted. The observation that the dmaW knockout strain appeared more debilitated at ambient temperature as opposed to 37 °C may have resulted from the effects of temperature on fungal growth rate, production of undetermined fungal metabolites or enzymes, the insect’s immune system, or a combination of these factors. Certainly, the increase in temperature to 37 °C favored the fungus from a growth-rate perspective. Previous studies with C. neoformans also documented an increased mortality rate at 37 °C as opposed to 30 °C33. Irrespective of the cause, at the higher temperature the residual virulence of the ergot alkaloid-deficient, dmaW knockout strain was distinguishable from the background mortality associated with injection of the PBS control.
The approach to ergot alkaloid analysis that we took in this current study enabled us to document the concentrations of ergot alkaloids in each individual inoculum sample; however, this approach limited us to detecting only the most abundant ergot alkaloid in each sample. Ergot alkaloids are closely associated with conidia of N. fumigata 42, 45, 46 and typically are extracted with methanol to acquire sufficient quantities for accurate quantification47. In this present study, ergot alkaloids were extracted with 50% methanol from aqueous conidial suspensions. Use of an initially aqueous solution was necessary to maintain viability of conidia that were to be used as inoculum and to avoid toxicity of injecting an organic solvent into larvae. A second major limitation on our ability to detect and quantify ergot alkaloid intermediates in this present study was that we extracted only 50,000 conidia per µL (derived from the concentration injected into larvae), whereas extracts in our previously published analyses typically contained ten to 100 times more conidia per µL. As a result of these constraints on alkaloid analysis, roles for other intermediates and alternate products of the ergot alkaloid pathway are more difficult to assess. Other ergot alkaloids were probably present in the injected conidial suspension but were below the limit of detection. Ergot alkaloid pathways in general are inefficient in that several intermediates typically accumulate to measurable levels48. Accumulation of several ergot alkaloid pathway intermediates including chanoclavine-I, fumigaclavine A, and fumigaclavine B have been measured in N. fumigata strains Af293 and FGSC A114138, 43.
Previous studies with N. fumigata and G. mellonella provided indirect evidence of the importance of fumigaclavine C in virulence30, 31. In these studies, a gene knockout strain deficient in peptide synthetase PesL had reduced virulence to G. mellonella 30, whereas the contribution of peptide synthetase Pes1 to virulence varied by genetic background of the knockout strain30, 31. The exact functions of these peptide synthetases are unknown but, unexpectedly, both appear to be required for the final step in the biosynthesis of fumigaclavine C in N. fumigata 30. The data of O’Hanlon et al.30 suggest that the lack of virulence in the PesL mutant resulted at least in part from the lack of fumigaclavine C. The role of Pes1 in virulence was less clear, since knockout of pes1 in an Af293.1 background reduced virulence in the G. mellonella model, but knocking out this same gene in an ATCC 46645 background did not significantly reduce virulence. The effects of Pes1 and PesL on fumigaclavine C biosynthesis could not be separated from the effects of these peptide synthetases on other metabolites and processes (such as increased sensitivities to oxidative stress and antifungal compounds30, 31). Interestingly, earlier steps in the ergot alkaloid pathway appeared to be unaffected by the pesL mutation30, indicating that alkaloids upstream of fumigaclavine C were less important for virulence.
The data presented herein provide direct evidence of a role for ergot alkaloids in virulence of N. fumigata to animals. Ergot alkaloids are well known for their bioactivity in mammals and have been exploited as pharmaceuticals and illicit drugs. They are best known from plant-associated fungi in the Clavicipitaceae (Claviceps species and Epichloë species) where they affect herbivores, but the alkaloids have no known effects on the plants themselves5, 6. Ergot alkaloids such as lysergic acid derivatives produced by Epichloë species do have anti-insect activities upon ingestion14, 49, but the ergot alkaloids of N. fumigata did not induce significant mortality upon injection at the concentrations used in this present study. Since N. fumigata has no mechanism for making ingress into an insect, it is unlikely that its ergot alkaloids affect insects in nature. The means by which ergot alkaloids contribute to animal pathogenesis is not known, but based on their close association with conidia one potential mechanism would be protection of inhaled conidia from cells of the host’s innate immune system.
Methods
Experimental organisms
Two wild-type isolates of N. fumigata (FGSC A1141 and Af293) that contained a complete functional pathway to fumigaclavine C43 and ergot alkaloid mutants derived from these wild-type isolates were studied. All fungi were cultivated on malt extract agar (per L: 6 g malt extract, 1.8 g maltose, 6 g dextrose, 1.2 g yeast extract, 15 g agar). The dmaW knockout (strain FGSC A1141 background), which lacks the capacity to produce any ergot alkaloids, and its ectopically complemented derivative were characterised previously38. Two ergot alkaloid mutants in the N. fumigata Af293 background also were studied. Strain easM knockout contains a replacement in the gene easM which encodes a P450 monooxygenase required to oxidise festuclavine to fumigaclavine B41; as a result, the easM knockout accumulates large quantities of festuclavine and lesser quantities of chanoclavine-I. Strain easA/G knockout contained a replacement of a fragment that spanned adjacent genes easA and easG (Supplementary Information). Since easA immediately precedes easG in the ergot alkaloid pathway, the easA/G knockout mutant has the phenotype of the previously characterised easA knockout39, meaning that it accumulates smaller quantities of chanoclavine-I aldehyde and larger quantities of the immediate precursor chanoclavine-I39, 40.
Galleria mellonella larvae were purchased from a local franchise of Petco and were inoculated the same day they were purchased. Larvae that were active, light colored (without any dark markings), and weighed at least 200 mg were chosen for inoculation.
Pathogenicity assays
Pathogenicity assays were derived from methods described previously28, 32–36. Conidia from cultures of the indicated strains grown on malt extract agar for two to four weeks were harvested by tapping an inverted culture such that large numbers of conidia collected on the inside of the Petri dish lid. Conidia were suspended in a modified PBS solution (pH 7.4) containing 9 mM Na2PO4, 1.6 mM KH2PO4, 123 mM NaCl, 0.01% wt/vol tween 20, and 10 μg/mL rifampin. Spore concentration was determined with the aid of a hemocytometer by counting dilutions made in 0.1% aqueous tween 20. Concentrations were adjusted with the modified PBS solution to obtain final concentrations of 2.5 × 105 conidia/μL for experiments conducted at 22 °C and 1 × 105 conidia/μL for experiments conducted at 37 °C. Once the desired conidial concentration was obtained, 20 μL of conidial suspension (containing 5 million conidia for experiments at 22 °C or 2 million conidia for experiments at 37 °C) was injected with a disposable 29.5-gauge hypodermic needle through the hindmost left proleg of a G. mellonella larva. A fresh hypodermic needle and syringe was used for each treatment. To test for acute toxicity of ergot alkaloid-containing conidial suspensions, aliquots of conidial suspensions of FGSC A1141 were spin-filtered through a 0.2-μm nylon filter to remove conidia prior to injection. Ten larvae per treatment were injected with each treatment or control for experiments involving strains derived from FGSC A1141, and 15 larvae per treatment were injected for experiments involving strains derived from N. fumigata Af293. Experiments involving strains derived from FGSC A1141 were repeated three times at 22 °C and three times at 37 °C, whereas those involving Af293-derived strains were repeated four times at 37 °C. Potential sources of variation included batch-to-batch differences in quality of G. mellonella larvae, quantification of hydrophobic spores in an aqueous solution, and quality of conidia and quantities of alkaloids present when harvesting conidia from cultures of slightly different ages. For these reasons, each experiment was conducted three times (for FGSC A1141 derivatives) or four times (for Af293 derivatives) with fresh batches of larvae and freshly-prepared conidial suspensions in each trial, and all results were included. Mortality was assessed by failure of larvae to move in response to being rolled over their back and typically was accompanied by melanisation. Observations were made twice daily for trials run at 22 °C and every two to eight hours for trials conducted at 37 °C. Slight differences in the timing of observations among trials resulted in stair-steps at unequal intervals along the x axis of Kaplan-Meier plots.
Statistical analyses
Results were analysed by Kaplan-Meier survival plots, and differences among survival curves were tested by log-rank tests. To compensate for multiple pairwise comparisons in a given experiment, Bonferroni corrections were applied such that the critical alpha value (0.05) was divided by the number of comparisons made in each experiment. The Bonferroni-adjusted alpha value is indicated in the legends of relevant figures. To correlate larval mortality with concentrations of certain ergot alkaloids in injected conidial suspensions, percent mortality values for all fungal treatments incubated at 37 °C were correlated with molar concentrations of individual ergot alkaloids in each injected conidial suspension. Mortality was assessed at 21 hr in these correlation analyses, because that was the time required for both wild-type isolates to kill 50% of inoculated larvae. When fungal isolate genetic background (i.e., Af293 vs. FGSC A1141 as origin of strains) was considered as a factor in ANOVA, background did not have a significant effect (P = 0.48) on percent mortality at 21 hr; thus, data from both backgrounds were included in the correlation analyses. All statistical analyses were performed with JMP (SAS, Cary, NC, USA). Data are available upon request to the corresponding author.
Analysis of ergot alkaloids
Ergot alkaloids in each extract were assayed by diluting the extract with an equal volume of HPLC-grade methanol, incubating at room temperature for 30 min, and centrifuging for 5 min to remove conidia and any precipitate prior to analysis by high performance liquid chromatography (HPLC). Ergot alkaloids were analysed by HPLC with fluorescence detection as described previously47. Briefly, 20 μL of alkaloids were separated on a reverse-phase C18 column (Phenomenex Prodigy, Torrance, CA, USA; 5-μm particle size ODS3, 150 mm × 4.6 mm) with a multi-linear, binary gradient of from 5% (vol/vol) to 75% acetonitrile in aqueous 50 mM ammonium acetate. Analytes were monitored by fluorescence with excitation wavelength set at 272 nm and emission wavelength set at 372 nm.
Electronic supplementary material
Acknowledgements
We thank Tyson Currence for assistance in preparing the easA/easG knockout of N. fumigata and Sarah Robinson for critical reading of the manuscript. This work was supported by grant R15GM114774 from the National Institutes of Health, National Institute of General Medical Sciences and by Hatch funds from United States Department of Agriculture. The article was published with permission of the West Virginia Agriculture and Forestry Experiment Station as scientific article number 3323.
Author Contributions
D.G.P. designed the study, performed experiments, and wrote and revised the paper. S.L.A. performed experiments and revised the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09107-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wallwey C, Li S-M. Ergot alkaloids: structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat. Prod. Rep. 2011;28:496–510. doi: 10.1039/C0NP00060D. [DOI] [PubMed] [Google Scholar]
- 2.Gerhards N, Neubauer L, Tudzynski P, Li S-M. Biosynthetic pathways of ergot alkaloids. Toxins. 2014;6:3281–3295. doi: 10.3390/toxins6123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakubczyk D, Cheng JZ, O’Connor SE. Biosynthesis of the ergot alkaloids. Nat. Prod. Rep. 2014;31:1328–1338. doi: 10.1039/C4NP00062E. [DOI] [PubMed] [Google Scholar]
- 4.Robinson SL, Panaccione DG. Diversification of ergot alkaloids in natural and modified fungi. Toxins. 2015;7:201–218. doi: 10.3390/toxins7010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florea S, Panaccione DG, Schardl CL. Ergot alkaloids of the Clavicipitaceae. Phytopathology. 2017;107:504–518. doi: 10.1094/PHYTO-12-16-0435-RVW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haarmann T, Rolke Y, Giesbert S, Tudzynski P. Ergot: from witchcraft to biotechnology. Mol. Plant Pathol. 2009;10:563–577. doi: 10.1111/j.1364-3703.2009.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliff LD, Du Boulay GH, Marshall J, Russell RR, Symon L. Effect of nicergoline on cerebral blood flow. J. Neurol. Neurosurgery Psych. 1977;40:746–747. doi: 10.1136/jnnp.40.8.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jähnichen S, Horowski R, Pertz HH. Agonism at 5-HT 2B receptors is not a class effect of the ergolines. Euro. J. Pharmacol. 2005;513:225–228. doi: 10.1016/j.ejphar.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Baskys A, Hou AC. Vascular dementia: pharmacological treatment approaches and perspectives. Clin. Interv. Aging. 2007;2:327–335. [PMC free article] [PubMed] [Google Scholar]
- 10.Winblad B, et al. Therapeutic use of nicergoline. Clin. Drug Investig. 2008;28:533–552. doi: 10.2165/00044011-200828090-00001. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Lloret S, Rascol O. Dopamine receptor agonists for the treatment of early or advanced Parkinson’s disease. CNS Drugs. 2010;24:941–968. doi: 10.2165/11537810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Morren JA, Galvez-Jimenez N. Where is dihydroergotamine mesylate in the changing landscape of migraine therapy? Expert Opin. Pharmacother. 2010;11:3085–3093. doi: 10.1517/14656566.2010.533839. [DOI] [PubMed] [Google Scholar]
- 13.Panaccione DG, et al. Effects of ergot alkaloids on food preference and satiety in rabbits, as assessed with gene knockout endophytes in perennial ryegrass (Lolium perenne) J. Agric. Food Chem. 2006;54:4582–4587. doi: 10.1021/jf060626u. [DOI] [PubMed] [Google Scholar]
- 14.Potter DA, Stokes JT, Redmond CT, Schardl CL, Panaccione DG. Contribution of ergot alkaloids to suppression of a grass-feeding caterpillar assessed with gene-knockout endophytes in perennial ryegrass. Entomol. Exp. Appl. 2008;126:138–147. doi: 10.1111/j.1570-7458.2007.00650.x. [DOI] [Google Scholar]
- 15.Du RH, Li EG, Cao Y, Song YC, Tan RX. Fumigaclavine C inhibits tumor necrosis factor α production via suppression of toll-like receptor 4 and nuclear factor κB activation in macrophages. Life Sci. 2011;89:235–240. doi: 10.1016/j.lfs.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Wu XF, Fei MJ, Shu RG, Tan RX, Xu Q. Fumigaclavine C, a fungal metabolite, improves experimental colitis in mice via downregulating Th1 cytokine production and matrix metalloproteinase activity. Int. Immunopharmacol. 2005;5:1543–1553. doi: 10.1016/j.intimp.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Li YX, Himaya SWA, Dewapriya P, Zhang C, Kim SK. Fumigaclavine C from a marine-derived fungus Aspergillus fumigatus induces apoptosis in MCF-7 breast cancer cells. Mar. Drugs. 2013;11:5063–5086. doi: 10.3390/md11125063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denning DW. Invasive aspergillosis. Clin. Infect. Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 19.Latgé JP. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin RM, et al. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bok JW, et al. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot. Cell. 2005;4:1574–1582. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugui JA, et al. Role of laeA in the regulation of alb1, gliP, conidial morphology, and virulence in Aspergillus fumigatus. Eukaryot. Cell. 2007;6:1552–1561. doi: 10.1128/EC.00140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramer RA, et al. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell. 2006;5:972–980. doi: 10.1128/EC.00049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bok JW, et al. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 2006;74:6761–6768. doi: 10.1128/IAI.00780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupfahl C, et al. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low‐dose mouse infection model. Mol. Microbiol. 2006;62:292–302. doi: 10.1111/j.1365-2958.2006.05373.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugui JA, et al. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell. 2007;6:1562–1569. doi: 10.1128/EC.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fallon JP, Reeves EP, Kavanagh K. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology. 2011;157:1481–1488. doi: 10.1099/mic.0.043786-0. [DOI] [PubMed] [Google Scholar]
- 29.Unsöld IA, Li SM. Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: gene expression, purification, and characterization of fumigaclavine C synthase FGAPT1. Chembiochem. 2006;7:158–164. doi: 10.1002/cbic.200500318. [DOI] [PubMed] [Google Scholar]
- 30.O’Hanlon KA, et al. Nonribosomal peptide synthetase genes pesL and pes1 are essential for fumigaclavine C production in Aspergillus fumigatus. Appl. Environ. Microbiol. 2012;78:3166–3176. doi: 10.1128/AEM.07249-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves EP, et al. A nonribosomal peptide synthetase (Pes1) confers protection against oxidative stress in Aspergillus fumigatus. FEBS J. 2006;273:3038–3053. doi: 10.1111/j.1742-4658.2006.05315.x. [DOI] [PubMed] [Google Scholar]
- 32.Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004;28:101–112. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Mylonakis E, et al. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 2005;73:3842–3850. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs BB, O’Brien E, El Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence. 2010;1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- 35.Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002;34:153–157. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 36.Slater JL, Gregson L, Denning DW, Warn PA. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med. Mycol. 2011;49:S107–S113. doi: 10.3109/13693786.2010.523852. [DOI] [PubMed] [Google Scholar]
- 37.Jackson JC, Higgins LA, Lin X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coyle CM, Panaccione DG. An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus. Appl. Environ. Microbiol. 2005;71:3112–3118. doi: 10.1128/AEM.71.6.3112-3118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coyle CM, Cheng JZ, O’Connor SE, Panaccione DG. An old yellow enzyme gene controls the branch point between Aspergillus fumigatus and Claviceps purpurea ergot alkaloid pathways. Appl. Environ. Microbiol. 2010;76:3898–3903. doi: 10.1128/AEM.02914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng JZ, Coyle CM, Panaccione DG, O’Connor SE. A role for old yellow enzyme in ergot alkaloid biosynthesis. J. Amer. Chem. Soc. 2010;132:1776–1777. doi: 10.1021/ja910193p. [DOI] [PubMed] [Google Scholar]
- 41.Bilovol Y, Panaccione DG. Functional analysis of the gene controlling hydroxylation of festuclavine in the ergot alkaloid pathway of Neosartorya fumigata. Curr. Genet. 2016;62:853–860. doi: 10.1007/s00294-016-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulinti P, et al. Accumulation of ergot alkaloids during conidiophore development in Aspergillus fumigatus. Curr. Microbiol. 2014;68:1–5. doi: 10.1007/s00284-013-0434-2. [DOI] [PubMed] [Google Scholar]
- 43.Robinson SL, Panaccione DG. Chemotypic and genotypic diversity in the ergot alkaloid pathway of Aspergillus fumigatus. Mycologia. 2012;104:804–812. doi: 10.3852/11-310. [DOI] [PubMed] [Google Scholar]
- 44.Kowalski CH, et al. Heterogeneity among isolates reveals that fitness in low oxygen correlates with Aspergillus fumigatus virulence. mBio. 2016;7:e01515–16. doi: 10.1128/mBio.01515-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panaccione DG, Coyle CM. Abundant respirable ergot alkaloids from the common airborne fungus Aspergillus fumigatus. Appl. Environ. Microbiol. 2005;71:3106–3111. doi: 10.1128/AEM.71.6.3106-3111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coyle CM, Kenaley SC, Rittenour WR, Panaccione DG. Association of ergot alkaloids with conidiation in Aspergillus fumigatus. Mycologia. 2007;99:804–811. doi: 10.1080/15572536.2007.11832512. [DOI] [PubMed] [Google Scholar]
- 47.Panaccione DG, Ryan KL, Schardl CL, Florea S. Analysis and modification of ergot alkaloid profiles in fungi. Methods Enzymol. 2012;515:267–290. doi: 10.1016/B978-0-12-394290-6.00012-4. [DOI] [PubMed] [Google Scholar]
- 48.Panaccione DG. Origins and significance of ergot alkaloid diversity in fungi. FEMS Microbiol. Lett. 2005;251:9–17. doi: 10.1016/j.femsle.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 49.Clay K, Cheplick GP. Effect of ergot alkaloids from fungal endophyte-infected grasses on fall armyworm (Spodoptera frugiperda) J. Chem. Ecol. 1989;15:169–182. doi: 10.1007/BF02027781. [DOI] [PubMed] [Google Scholar]
- 50.Pain A, et al. Insight into the genome of Aspergillus fumigatus: analysis of a 922 kb region encompassing the nitrate assimilation gene cluster. Fungal Genet. Biol. 2004;41:443–453. doi: 10.1016/j.fgb.2003.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


