Abstract
Astrocytes have diverse, remarkably complex shapes in different brain regions. Their branches closely associate with neurons. Despite the importance of this heterogeneous glial cell type for brain development and function, the molecular cues controlling astrocyte branch morphogenesis and positioning during neural circuit assembly remain largely unknown. We found that in the Drosophila visual system, astrocyte-like medulla neuropil glia (mng) variants acquire stereotypic morphologies with columnar and layered branching patterns in a stepwise fashion from mid-metamorphosis onwards. Using knockdown and loss-of-function analyses, we uncovered a previously unrecognized role for the transmembrane leucine-rich repeat protein Lapsyn in regulating mng development. lapsyn is expressed in mng and cell-autonomously required for branch extension into the synaptic neuropil and anchoring of cell bodies at the neuropil border. Lapsyn works in concert with the fibroblast growth factor (FGF) pathway to promote branch morphogenesis, while correct positioning is essential for mng survival mediated by gliotrophic FGF signaling.
How glial cells, such as astrocytes, acquire their characteristic morphology during development is poorly understood. Here the authors describe the morphogenesis of astrocyte-like glia in the Drosophila optic lobe, and through a RNAi screen, they identify a transmembrane LRR protein–Lapsyn–that plays a critical role in this process.
Introduction
The architecture of brain regions is defined by the connectivity of diverse neurons and their partnership with glia. Astrocytes actively shape neural circuits at short and long range by influencing their wiring and function1. For instance, they locally regulate sensorimotor circuit formation by expressing guidance cues in the spinal cord2. Moreover, astrocytes promote and remove synapses3–5, and engulf axon fragments during developmental pruning6–8. In addition to providing tensile strength and metabolic support to neurons, astrocytes influence neuronal activity by regulating extracellular ion and neurotransmitter concentrations at synapses9, 10. To fulfill these roles, their processes closely associate with neuronal cell bodies, dendritic and axonal arbors and synaptic contacts10, 11. Vertebrate astroglia display a considerable regional heterogeneity1, 12, 13. While protoplasmic astrocytes in the cortex are star-shaped, Müller glia in the retina or Bergmann glia in the cerebellum have elongated morphologies14. Astrocyte branches can be sparse or highly ramified, resembling filopodia or fine veil-like sheaths14. However, the question as to how astrocytes acquire their characteristic shapes at specific locations has so far received little attention.
Like their vertebrate counterparts, glia in the central nervous system (CNS) of Drosophila melanogaster comprise several populations10. Surface-associated glia, consisting of perineurial and subperineurial glia, cover the entire CNS and form the blood-brain barrier. Cortex glia extend thin processes around individual or groups of neuronal somata15. Finally, neuropil-associated glia include ensheathing and astrocyte-like glia16, 17. The former cover neuropil surfaces and compartments, whereas the latter share morphological and molecular similarities with mammalian protoplasmic astrocytes16. They extend highly ramified processes deep into synaptic neuropils, express transporters for neurotransmitter clearance, and share core electrophysiological features with vertebrate astrocytes5, 10, 18–22.
The fibroblast growth factor (FGF) signaling pathway plays a remarkably conserved role in controlling glial development. For instance, FGF8/FGF receptor 3 signaling promotes the elaboration of complex branches in developing murine astrocytes23. In the postembryonic fly CNS, FGF signaling is required for generating perineurial and cortex glia24. In the eye-antennal imaginal disc, this pathway controls glial proliferation, migration, differentiation, and axonal wrapping25. Furthermore, astrocytes in the larval ventral nerve cord (VNC) require activation of the FGF receptor Heartless (Htl) to extend processes into the neuropil26. Considering the pleiotropic functions of FGF signaling, other mechanisms are expected to collaborate with this pathway in controlling astrocyte branch morphogenesis. As with neurons, the acquisition of complex branching patterns by glia may require the action of so far elusive cell surface molecules.
In the Drosophila visual system, photoreceptors (R-cells, R1-R8) in the retina extend axons into the optic lobe, consisting of the lamina, medulla, lobula plate, and lobula27 (Fig. 1a). Consistent with their function in visual information processing, these ganglia are structured into reiterated columnar units. The medulla, lobula plate, and lobula neuropils are additionally organized into parallel synaptic layers. Throughout development and in adults, R-cell axons and higher-order neurons closely associate with different glial cell subtypes28, 29. In the VNC and antennal lobe, astrocyte-like glia adopt asymmetric stellate shapes16, 26. However in the visual system, we and others previously described an astrocyte-like subtype among medulla neuropil glia (mng) with a columnar branching pattern29–32.
Fig. 1.
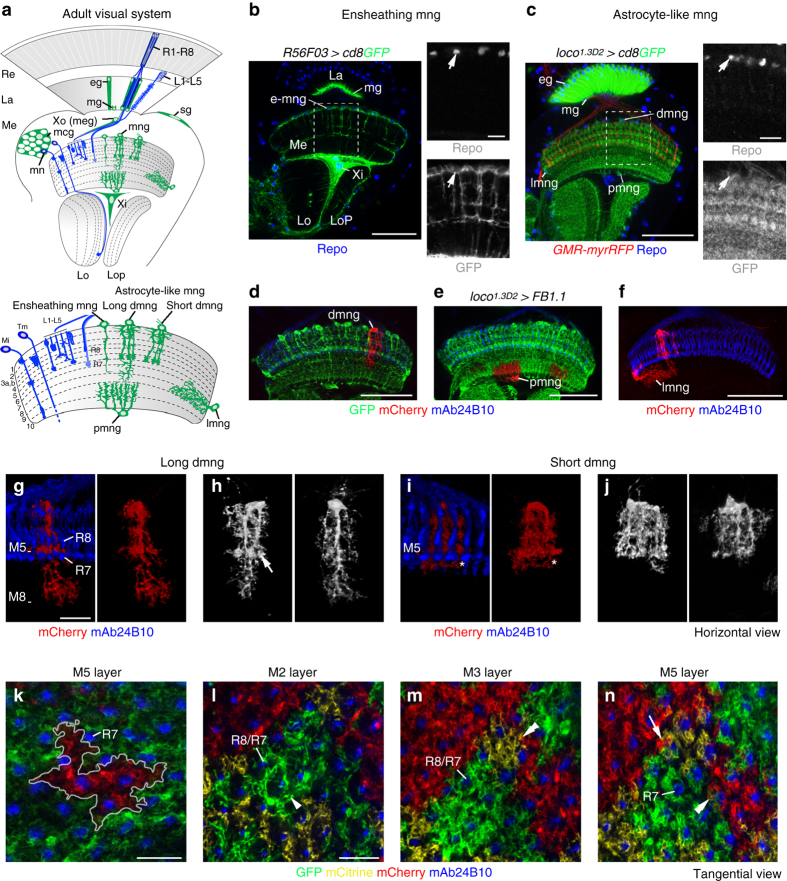
Four astrocyte-like glial variants in the medulla. a Schematic of glial subtypes in the adult Drosophila optic lobe. dmng distal medulla neuropil glia, eg epithelial glia, L1-L5 lamina neurons, La lamina, lmng lateral mng, Lo lobula, Lop lobula plate, mcg medulla cortex glia, Me medulla, mg marginal glia, Mi medulla intrinsic neurons, mn medulla neurons, pmng proximal mng, R1-R8, R8/R7 R-cell and axon subtypes, Re retina, sg surface glia, Tm transmedullary neurons, Xi inner chiasm glia, Xo or meg outer chiasm glia or medulla glia. R56F03-Gal4 (b) and loco 1.3D2 -Gal4 (c) drive expression of UAS-cd8GFP (green) in ensheathing mng (e-mng) and astrocyte-like mng, respectively. R-cell axons were labeled with GMR-myrRFP (c, red), and glial nuclei with Repo (b, c, blue, arrows). d–n Single astrocyte-like mng were visualized using loco 1.3D2 -Gal4 and UAS-FB1.1. Processes of dmng infiltrate medulla neuropil layers M1-M8 (d), pmng layers M9 and M10 (e) and lmng layers M7 and M8 (f). dmng visualized with volocity include long (g, h) and short (i, j) variants with stereotypic morphologies terminating in layers M8 and M5, respectively. dmng form microdomains in layer M5 (h, arrow). Asterisks in i indicate branches in layer M5 in a deeper focal plane. k Processes of single dmng (outlined) associate with 7–8 columns in layer M5. l–n Glial branches accumulate in the column periphery in layer M2 (l, arrowhead), evenly spread through columns in M3 (m) and aggregate in the column center adjacent to R7 axons in M5 (n). mng branches show a tiled organization (l–n). Processes of several mng converge into the same column (n, arrow, similar to microdomains in h) and marginally overlap (m, n, double arrowheads). R-cell axons were labeled with mAb24B10 (blue). Panels b–f, g–n show single optical sections, panels g–j show projections. For genotypes and sample numbers, see Supplementary Table 1. Scale bars, 50 μm (b–f), 20 μm (g–n), 10 μm (small panels b, c)
In this study, we followed the development of astrocyte-like mng from birth to adulthood. This glial subtype is initially highly motile and acquires its final branching pattern in a stepwise manner. To gain insights into the molecular mechanisms controlling mng development, we conducted a RNA interference (RNAi) screen for secreted and cell surface proteins. Genetic analyses revealed that the transmembrane molecule leucine-rich repeat activity-regulated protein at synapses (Lapsyn)33 is expressed in glia and essential for branch extension and anchorage of mng at the neuropil border. Branch formation required both Lapsyn and Htl FGF receptor activation, whereas Lapsyn-mediated positioning of mng at the neuropil border ensured exposure to gliotrophic FGF signals and consequently their survival. Finally, we provide evidence that lapsyn controls branch morphogenesis of astrocytes in other CNS regions.
Results
Four astrocyte-like glial variants in the medulla
To visualize mng in the Drosophila visual system, we tested several Gal4 insertions for specific activity (Fig. 1b–c and Supplementary Fig. 1a–d). mng were identified by co-expression of membrane-bound green fluorescent protein (GFP) and the glial-specific homeodomain transcription factor reversed polarity (Repo)34, and cell body position at the medulla neuropil border. R56F03-Gal4 and NP6520-Gal4, markers for ensheathing glia17, 32, 35, 36, labeled a mng subset that extended sparsely branched processes into the neuropil (Fig. 1b and Supplementary Fig. 1b). By contrast, a 2.2 kb-enhancer fragment of the locomotion defects (loco) gene upstream of Gal4 (loco 1.3D2 -Gal4)37drove strong expression in astrocyte-like mng, whose processes abundantly infiltrated the neuropil layers M1-M10 (Fig. 1c). Finally we combined R56F03-Gal4, loco 1.3 -lexA,UAS-FB1.1B 260b and lexAop-myrmCherry transgenes with immunolabeling using antibodies against Repo or the homeodomain transcription factor Prospero, a marker for astrocyte-like glia in the VNC36 (Supplementary Fig. 1g, h). This confirmed that as their VNC counterparts, astrocyte-like mng express Prospero and that above drivers label closely associated, yet genetically and morphologically distinct glial subtypes.
To image astrocyte-like mng with single cell resolution, we next combined loco 1.3D2 -Gal4 with the FlybowUAS-FB1.1 transgene31 (Fig. 1d–n). This uncovered four astrocyte-like mng variants: short and long distal mng (dmng), whose processes infiltrated neuropil layers M1-M5 and M1-M8, respectively (Fig. 1d, g–j); proximal mng (pmng or chandelier glia29) mainly covering layers M9 and M10 (Fig. 1e); and lateral mng (lmng) with tangential processes primarily extending in layers M7 and M8 (Fig. 1f). dmng and pmng displayed stereotypic morphologies, with a small number of main branches giving rise to a rich meshwork of secondary processes (Fig. 1g–j). dmng contributed branches to 6–10 columns (n = 8 for layer M5; Fig. 1k). Similar to other astrocytes26, 38, the processes of astrocyte-like mng showed a tiled organization, but their density and distribution varied between synaptic layers, and peripheral branches from several neighboring cells converged into single columns where they slightly intermingled (Fig. 1g-j, l–n).
Further probing the molecular profile of astrocyte-like mng (Supplementary Fig. 2), we observed expression of the GABA transporter (GAT)26, the high-affinity glutamate transporter dEAAT118, 19, 36 and the glutamate recycling enzyme glutamate synthase 2 (Gs2)39. Thus, despite their distinct morphologies, astrocyte-like glia in the medulla are functionally related to their counterparts in other CNS regions.
Key steps of astrocyte-like mng development
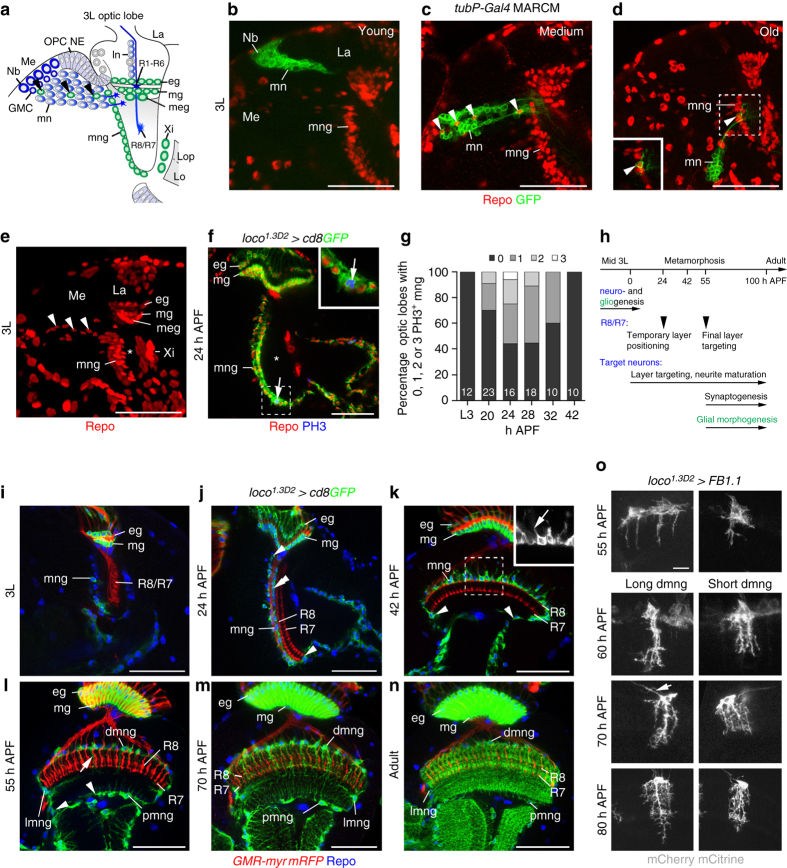
R-cells and target neurons are primarily generated during larval development. In the optic lobe, medial neuroepithelial cells in the outer proliferation center (OPC) sequentially transform into neuroblasts (Fig. 2a). These transition through five temporal identity transcription factors, producing a string of neurons directed toward the medulla neuropil. The oldest neuroblasts transform into neuroglioblasts, switching to the production of glia during their final division40, 41. Combining mosaic analysis with a repressible cell marker (MARCM)42 and Repo labeling, we confirmed that mng originate from OPC neuroglioblasts and migrate to and stop at the most anterior cortex-neuropil interface (Fig. 2b–e). Individual clones on average gave rise to 1.56 mng (±0.7 95% confidence interval, n = 9). Quantification of phosphoHistone 3 (PH3) labeling indicated that OPC neuroglioblasts are the primary source for mng because these do not undergo mitosis during the third instar larval stage and only divide occasionally between 20 and 30 h after puparium formation (APF; Fig. 2f, g).
Fig. 2.
Development of astrocyte-like medulla neuropil glia. a Schematic of a third instar larval (3L) optic lobe. mng medulla neuropil glia, eg epithelial glia, GMC ganglion mother cell, La lamina, Lo lobula, Lop lobula plate, ln lamina neurons, Me medulla, meg medulla glia, mg marginal glia, mn medulla neurons, Nb neuroblast/neuroglioblast, OPC NE outer proliferation center neuroepithelium, R1-R6, R8/R7, R-cell axon subtypes, Xi inner chiasm glia. Arrowheads, migratory mng. b–d MARCM clones were generated using tubP-Gal4 and hs-FLP transgenes. Young GFP-labeled clones (green) closest to the OPC neuroepithelium contain Nb and neurons (b, n = 18). Medium (c, n = 6) and older (d, n = 11) clones include Repo-positive glial cells (arrowheads, red). e A projection of optical sections illustrates the migratory path of OPC-derived mng (arrowheads) through the cortex toward the anterior medulla neuropil edge (asterisk). f In 24 h APF optic lobes, mng were labeled with loco 1.3D2 -Gal4 UAS-cd8GFP (green) and Repo (red), and cells undergoing mitosis with phosphoHistone 3 (PH3, blue). g Percentage of optic lobes containing PH3-positive mng during early metamorphosis (n = optic lobes: 12, 23, 16, 18, 10, and 10). h Timeline of key steps underlying R-cell, target neuron, and glial development. i–n mng were labeled with loco 1.3D2 -Gal4UAS-cd8GFP (green) and Repo (blue). During 3L (i), at 24 h (j) and 42 h APF (k), mng cell bodies at the neuropil border (j, double arrowhead) migrate around the neuropil (j–l, arrowheads). Distal mng (dmng) extend short processes into the cortex at 42 h APF (k, insert) and into the neuropil from 55 h APF onwards (l, arrows). These become highly branched at 70 h APF and in adults (m, n). o Long and short dmng variants were labeled with UAS-FB1.1 (white). mng extend filopodia-like processes into the neuropil at 55 h APF. Primary processes project to final layers at 60 h APF. Increasingly branched secondary processes arise at 60, 70, and 80 h APF. Some dmng send thin processes along axon tracts into the cortex (arrow at 70 h APF). Panels f, i–n show single optical sections, b–e, o projections. For genotypes and sample numbers, see Supplementary Table 1. Scale bars, 50 μm (b–f, i–n), 10 μm (o)
During metamorphosis, R8 and R7 axons initially reside in temporary layers and target to their final layers M3 and M6 around 55 h APF27. Target neuron subtypes acquire their characteristic branching patterns throughout pupal development27, 43. Generation of mature synapses in the visual system is initiated during mid-pupal development44 (Fig. 2h). We observed that larval mng begin to express UAS-cd8GFP under the control of loco 1.3D2 -Gal4 after they completed migration to the neuropil border (Fig. 2i). During the first half of metamorphosis, cell bodies spread to the lateral and proximal medulla neuropil border (Fig. 2j–l). Astrocyte-like mng send transient short processes into the cortex at 42 h APF and longer branches into the neuropil from 55 h APF onwards (Fig. 2k–n). Flybow labeling (Fig. 2o) revealed that dmng initially form filopodia-like processes, which transform into main columnar branches characteristic for long and short variants to then generate fine layered secondary branches. Thus, stepwise branch morphogenesis of astrocyte-like mng is spatially and temporally controlled, and takes place after R-cell axons have reached their final layers, in parallel with target neuron arbor maturation and synapse formation.
A screen for factors regulating mng branch formation
Glial branch morphogenesis is likely mediated by cell-cell interactions. We therefore sought to identify secreted and cell surface proteins that control branch formation of astrocyte-like mng in a high-resolution confocal-based RNA interference (RNAi) screen. loco 1.3D2 -Gal4, UAS-dicer2 (dcr2) and UAS-cd8GFP were crossed with selected UAS-RNAi transgenes to achieve efficient knockdown and to visualize astrocyte-like mng in pharate adults. Screening 522 UAS-RNAi lines directed against the mRNA of 306 genes, we uncovered 24 genes whose RNAi-mediated knockdown caused defects (Fig. 3a).
Fig. 3.
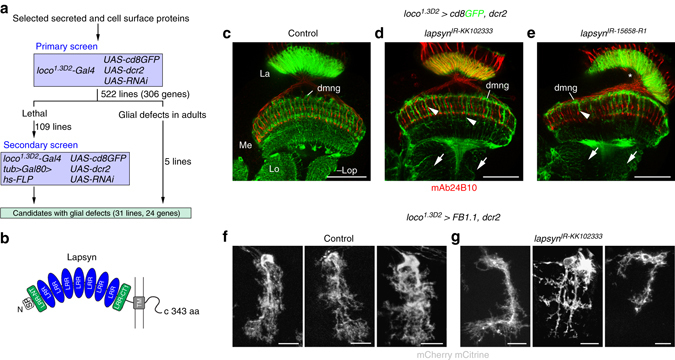
A RNA interference screen for factors mediating glial branch morphogenesis. a Schematic representation of screen strategy. To knockdown genes that encode selected secreted and cell surface molecules, UAS-RNAi and UAS-dcr2 were expressed in astrocyte-like glia under the control of loco 1.3D2 -Gal4. A secondary screen was performed for UAS-RNAi lines causing early lethality. To achieve mosaic expression of UAS-RNAi transgenes in astrocyte-like glia using loco 1.3D2 -Gal4, a heat shock-FLP (hs-FLP) recombinase transgene was induced transiently to promote the random excision of the FRT site flanked Gal4-repressor Gal80 encoding cDNA downstream of the widely active tubulin enhancer (tub > Gal80>)68. b Lapsyn encodes a transmembrane (TM) cell adhesion protein containing seven leucine-rich repeat (LRR) domains flanked by N-terminal LRR-NT and C-terminal LRR-CT1domains. SP signal peptide. c–e loco 1.3D2 -Gal4 drives expression of UAS-cd8GFP, UAS-dcr2, and two independent RNAi constructs, UAS-lapsyn IR-KK102333 and UAS-lapsyn IR-15658-R1. f, g loco 1.3D2 -Gal4 drives expression of UAS-FB1.1 (white) in controls (f) and UAS-lapsyn IR-KK102333, UAS-dcr2 in lapsyn knockdown animals (g). La lamina. Compared to controls (c, f), processes of astrocyte-like mng are reduced following lapsyn knockdown (d, e, g) in the medulla (Me) (d, e, arrowheads), lobula (Lo), and lobula plate (Lop) (d, e, arrows). Some brains displayed a medulla rotation defect (e, asterisk). Panels c–e show single optical sections, f, g projections. For genotypes and sample numbers, see Supplementary Table 1. Scale bars, 50 μm (c–e) and 10 μm (f, g)
Candidates with strong branching defects included a member of the extracellular leucine-rich repeat (LRR) protein superfamily encoded by lapsyn (leucine-rich repeat activity-regulated protein at synapses)33. The 343 amino acid (aa) protein consists of an extracellular domain with seven central LRR motifs flanked by N-terminal LRR-NT and C-terminal LRR-CT1 motifs, followed by a transmembrane region and a short 45 aa intracellular sequence of low complexity that lacked homology with known domains45 (Fig. 3b). lapsyn has been identified as a gene whose transcript levels were altered in seizure- and learning and memory-mutants33. We found that lapsyn knockdown in astrocyte-like mng severely impaired the infiltration of the adult medulla neuropil by glial processes of dmng, pmng, and lmng (Fig. 3c–e). Phenotypes were highly penetrant for two independent UAS-RNAi transgenes (UAS-lapsyn IR-KK102333: 74%, n = 19; UAS-lapsyn IR-15658-R1: 62%, n = 13). dmng, individually labeled with Flybow (n = 61 in 22 samples), showed variable phenotypes (Fig. 3f, g): this included abnormally thick or fasciculated primary and secondary branches (24.5%; Fig. 3g, left panel), main processes with reduced secondary branches (37.8%; Fig. 3g, middle panel) or short unbranched primary processes (26.2%; Fig. 3g, right panel).
Next, we examined the cytoskeleton of adult astrocyte-like mng. Using loco 1.3D2 -Gal4, we co-expressed membrane-bound GFP or mCherry with fluorescent protein-tagged cytoskeletal markers (Supplementary Fig. 3). In control astrocyte-like mng, the microtubule (MT) marker mCherry-α-Tubulin (ChRFP-Tub) and the MT plus-end tracking fusion protein Eb1-GFP were mainly detected in primary branches, whereas nod-GFP, a minus-end MT marker, accumulated in cell bodies. LifeAct-GFP revealed actin-rich secondary branches. Thus, similar to VNC astrocyte-like glia26, mng showed a differential distribution of MT and actin in their processes. Following lapsyn knockdown, actin expression was severely reduced, whereas MT appeared less affected, consistent with the loss of mainly secondary branches.
Lapsyn is expressed in astrocyte-like mng
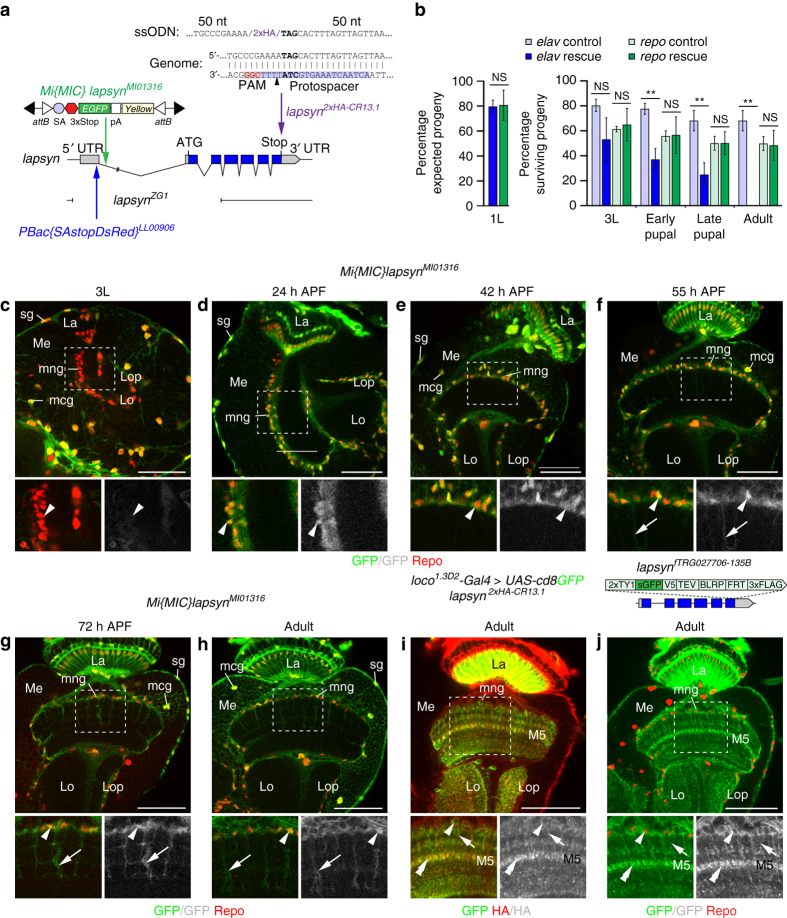
To distinguish whether lapsyn acts in glia and neurons or solely in glia, we conducted cell-type specific rescue experiments monitoring the viability of progeny during development. lapsyn ZG1, a null allele carrying a ~4.7 kb deletion, is homozygous embryonic lethal33 (Fig. 4a). Overexpression of lapsyn with the pan-neuronal driver elav C155 -Gal4 partially rescued the lethality of lapsyn ZG1 mutants up to the third instar larval stage, but these larvae barely moved (Fig. 4b). Their viability strongly decreased in pupae, and no adults emerged. In line with previous findings33, suggesting a non-neuronal requirement of lapsyn, expression with the pan-glial driver repo-Gal4 substantially restored viability of progeny throughout development and resulting adults could be maintained as a fertile stock.
Fig. 4.
lapsyn is expressed in glia. a The schematic shows the lapsyn genomic locus, the lapsyn ZG1 deletion, and Mi{MIC}lapsyn MI01316 and PBac{SAstopDsRed} LL00906 insertions. pA polyadenylation site, SA splicing acceptor site. To generate lapsyn 2xHA-CR13.1, two C-terminal HA epitope tags were inserted into the endogenous locus following a Cas9-induced DSB and HDR with a single-stranded oligo donor nucleotide (ssODN) containing flanking 50-nt homology arms. b For whole animal rescue experiments of lapsyn ZG1embryonic lethality, UAS-lapsyn was expressed in neurons (elav C155 -Gal4, blue bars, 224 recovered first instar (1L) larvae of 1210 embryos) or in glia (repo-Gal4, green bars, 529 recovered 1L larvae of 2380 embryos). GFP-negative rescue flies were compared to controls carrying CyO Dfd-YFP balancer chromosomes, corresponding to all other surviving progeny (heterozygous, or heterozygous and overexpressing lapsyn, light blue and green bars). Rescue of viability of 1L larvae (left panel) is shown as percentage of expected progeny. Rescue of viability of third instar (3L) larvae, early and late pupae and adults is shown as percentage of surviving progeny in “rescue” and “control” populations followed from the 1L stage onwards. Histograms show data points as means ± standard deviation error bars (n = 3 independent experiments). Unpaired, two-tailed Student’s t-test assuming normality but unequal standard deviation (1L: P = 0.879; 3L: P = 0.1027, P = 0.6796; early pupae: P = 0.006, P = 0.9369; late pupae: P = 0.0042, P = 0.9921; adult: P = 0.0047, P = 0.8668). NS not significant, **P < 0.01. c–h Mi{MIC}lapsyn MI01316 is inserted into the first non-coding intron. In 3L larvae, Repo-positive (red) surface glia (sg) and medulla cortex glia (mcg), but not mng (c) are labeled with GFP. mng express GFP at 24, 42, 55, and 72 h APF, and in adults (arrowheads). mng processes are labeled from 55 h APF onwards (f–h, arrows). i, j lapsyn 2xHA-CR13.1 and the fosmid lapsyn fTRG027706 report abundant lapsyn protein localization in mng cell bodies (arrowheads), primary (arrows), and secondary processes (double arrowheads), particularly in layer M5, using anti-HA (red) and GFP labeling (green), respectively. La lamina, Lo lobula, Lop lobula plate, Me medulla. Panels c–j show single optical sections. For genotypes, sample numbers and additional statistical values, see Supplementary Tables 1 and 2. Scale bars, 50 μm
Next, we examined the expression of Lapsyn. Because previously generated33and our own antisera were not suitable for tissue immunolabeling, we tested a Minos-mediated integration cassette (MiMIC) insertion46 into lapsyn. Mi{MIC}lapsyn MI01316 is inserted in a 5′ to 3′ orientation within the first intron between 5′UTR sequences, thus reporting GFP expression in the cells producing Lapsyn (Fig. 4a). We detected expression in glia but not neurons. Repo-positive surface and cortex glia were labeled throughout development, whereas neuropil-associated glial cell types, including mng, showed expression from 24 h APF onwards (Fig. 4c–h).
To determine the localization of Lapsyn protein, we initially used a ϕC31 recombinase-mediated cassette exchange (RMCE) strategy46, replacing Mi{MIC}lapsyn MI01316 with lapsyn cDNA and two C-terminal hemagglutinin (HA) epitope-tag encoding sequences. While the resulting insertion Mi{MIC}lapsyn lapsyn-2xHA-24A reported protein expression in astrocyte-like mng (Supplementary Fig. 4a–c), levels were low. We therefore pursued a Crispr/Cas9-mediated protein-tagging approach47. We inserted two in-frame C-terminal HA-encoding sequences into the lapsyn locus by inducing a Cas9-mediated double-stranded break (DSB) upstream of the stop codon while promoting homology directed repair (HDR) with a single-stranded oligo donor nucleotide. The template consisted of two 50-nt homology arms flanking the HA sequences (Fig. 4a). In parallel, we generated a transgenic line carrying a C-terminally tagged lapsyn fosmid from the FlyFos library48 (Fig. 4j). In the resulting lapsyn 2xHA-CR13.1 Crispr-Cas9 and lapsyn fTRG027706 fosmid lines, endogenous Lapsyn tagged with HA and GFP, respectively, was clearly detectable in the processes of adult astrocyte-like mng (Fig. 4i, j). Finally, we observed punctate Lapsyn in adult mng processes when overexpressing a UAS-lapsyn-GFP transgene33 (Supplementary Fig. 4d). Together, these approaches consistently report that Lapsyn is glial-specific in the optic lobe and located in the processes of astrocyte-like mng.
lapsyn cell-autonomously controls mng branch formation
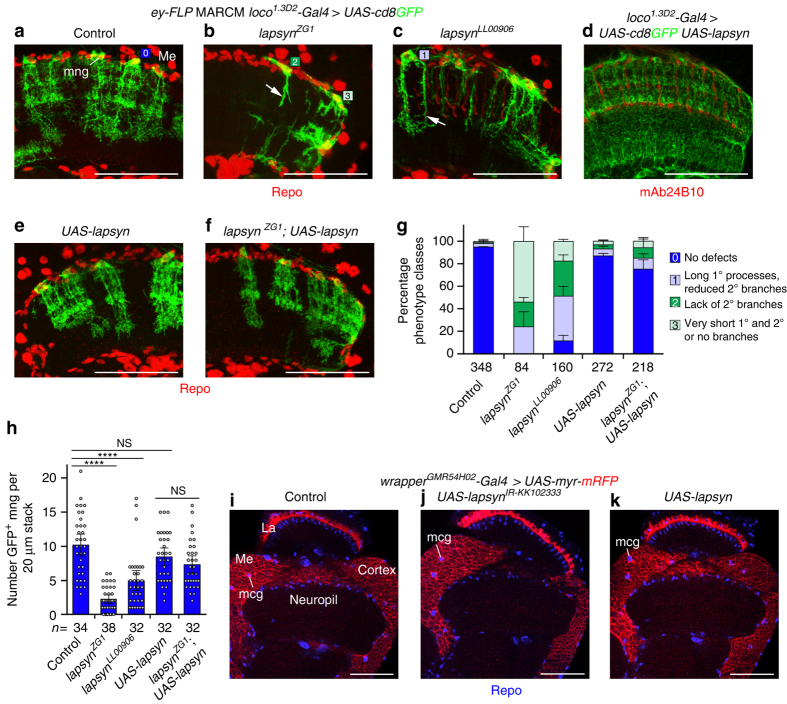
To test whether or not lapsyn is cell-autonomously required in astrocyte-like mng, we generated MARCM clones with two homozygous embryonic lethal alleles, lapsyn ZG1 and lapsyn LL00906, a 5′UTR piggyBAC insertion49 (Fig. 4a). eyeless-FLP (ey-FLP) served as the recombinase source in OPC neuroblasts to remove lapsyn in newborn mng. loco 1.3D2 -Gal4 was used to unambiguously identify astrocyte-like mng. In line with knockdown experiments, adult lapsyn-deficient mng displayed three classes of defects: failure to extend processes into the neuropil, formation of short unbranched primary processes and extension of long primary processes with reduced secondary branches (Fig. 5a–c, g).
Fig. 5.
lapsyn is cell-autonomously required for branch morphogenesis of astrocyte-like mng. a–c Control, lapsyn ZG1 and lapsyn LL00906 mutant astrocyte-like mng clones were generated by combining ey-FLP and MARCM. Clones were labeled with loco 1.3D2 -Gal4 UAS-cd8GFP (green) and all glial nuclei with Repo (red). Unlike control clones displaying a highly elaborate morphology (a, square-0), lapsyn mutant astrocyte-like mng showed severe defects: primary and secondary processes were very short (b, square-1), secondary branches were missing (b, square-2) or reduced (c, square-3). d, e Overexpression of UAS-lapsyn with loco 1.3D2 -Gal4 in all astrocyte-like glia (d) or in MARCM clones (e) did not affect general mng morphology. f Overexpression of UAS-lapsyn rescued morphological defects in lapsyn mng clones. g Quantification of phenotype classes. 100% stacked bar histograms show data points as means plus standard deviation error bars (n = independent experiments assessing individual mng in 20-µm optical stacks/optic lobes: 3 [34/19], 3 [38/24], 2 [32/18], 3 [32/17], 3 [31/16]). Color code same as in a–c. h Quantification of GFP-positive astrocyte-like mng numbers. In adults, fewer clones were recovered in lapsyn mosaics compared to controls. Overexpression of UAS-lapsyn does not affect cell numbers and rescues the reduction of clones in lapsyn ZG1. The scatter plot with bars shows data points and means ± 95% confidence intervals (n = 20-μm stacks of optical sections from 19,24,18,17, and 16 optic lobes). Unpaired, two-tailed Student’s t-test not assuming equal variance: P = 2.93 × 10−12, P = 6.44 × 10−6, P = 0.0882, P = 0.2131. NS not significant, ****P < 0.0001. i–k Knockdown and overexpression of lapsyn in medulla cortex glia (mcg) using wrapper GMR54H02 -Gal4 to express UAS-lapsyn IR-KK102333 (j) or UAS-lapsyn (k) does not affect their morphology compared to controls (i). La lamina, Me medulla. Panels d, i–k show single optical sections, a–c, e, f projections. For genotypes, sample numbers and additional statistical values, see Supplementary Tables 1 and 2. Scale bars, 50 μm (a–f, i–k)
Because clones included glia and neurons, we next conducted rescue experiments. Expression of untagged Lapsyn33 in all astrocyte-like mng or in MARCM clones did not affect dmng branch formation or territory size (Fig. 5d, e, g). Expression of Lapsyn in lapsyn ZG1 mutant mng substantially rescued the morphological defects caused by lapsyn loss (Fig. 5f, g). The number of clones at the medulla neuropil border was lower for lapsyn ZG1 and lapsyn LL00906 mutants than controls. Overexpression of lapsyn rescued also this phenotype (Fig. 5h). Hence, lapsyn is cell-autonomously required in astrocyte-like mng.
Monitoring the behavior of cells adjacent to lapsyn-deficient astrocyte-like mng, we detected processes of heterozygous mng in close vicinity of the sparse branches of mutant mng (Supplementary Fig. 5; 69/69 cells in 20 samples). This suggests that astrocyte-like mng maintain their numbers and/or adjust branch growth for complete neuropil coverage. To assess a potential role in other glia, we manipulated lapsyn in medulla cortex glia using wrapper GMR54H02 -Gal4 35. Knockdown did not reduce branch formation, and overexpression did not enhance branch extension within the cortex or direct processes into the medulla neuropil (Fig. 5i–k). Thus, lapsyn is neither required in cortex glia nor sufficient to control branch morphogenesis, suggesting that co-factors, found in mng, may be absent in this glial subtype.
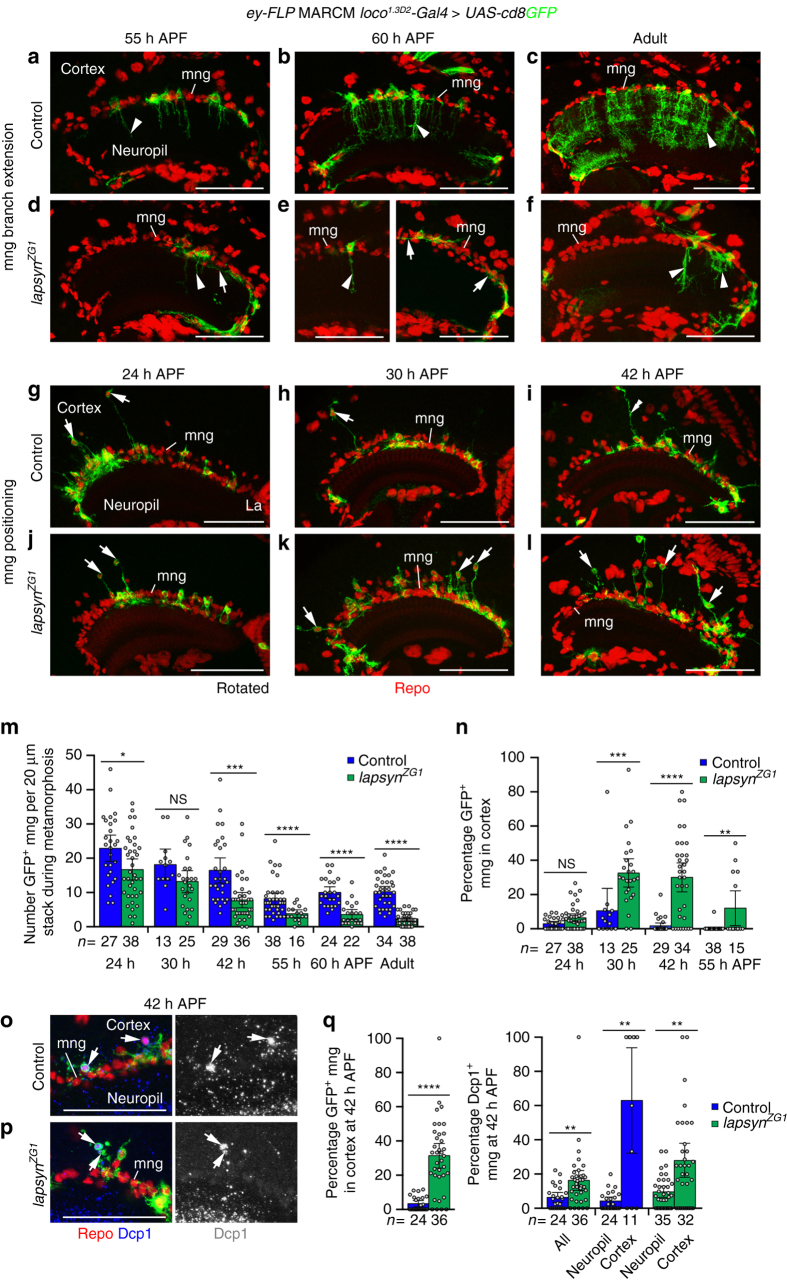
To determine the developmental time window during which lapsyn is required in astrocyte-like mng for branching, we compared wild type and mutant clones during metamorphosis (Fig. 6a–f). At 55 h APF, the first wild-type mng began to extend columnar processes into the medulla neuropil. By contrast, lapsyn-deficient mng either extended very short branches into the neuropil or formed branches that projected between glial cell bodies across the neuropil surface (Fig. 6a, d). Defects persisted at 60 h APF, when all control mng have sent branches into the neuropil (Fig. 6b, e) and in adults (Fig. 6c, f). Hence, lapsyn controls branch formation during mid-pupal development rather than maintenance at later stages.
Fig. 6.
lapsyn controls astrocyte-like mng branch formation and positioning during development. Clones were generated using ey-FLP MARCM and visualized with loco 1.3D2 -Gal4 UAS-cd8GFP (green). Repo-labeled glial nuclei (red). a–f Assessment of branch formation. Unlike in controls, lapsyn ZG1 mng extended reduced branches into the neuropil (arrowheads) or ectopic branches between glial cell bodies at the neuropil border (arrows) at 55 and 60 h APF. Defects persisted in adults. g–l Assessment of positioning. Compared to controls, more lapsyn ZG1 mng cell bodies were detected in the cortex at 30 and 42 h than at 24 h APF (arrows). These sent long protrusions to the neuropil border. mng at the border extended long processes into the cortex (i, double arrowhead). Quantifications assessing numbers of GFP-positive mng (m) and percentages in the cortex of control and lapsyn ZG1 mosaics (n) during metamorphosis. Scatter plots with bars show data points and means ± 95% confidence intervals (n = 20-μm stacks of optical sections from 19,27,6,12,18,21,22,12,13,13,19, and 23 (m) and 19, 27, 6, 12, 18, 19, 22, and 12 optic lobes (n). m Unpaired, two-tailed Student’s t-test not assuming equal variance: P = 0.0129, P = 0.0678, P = 0.0001, P = 5.47 × 10−5, P = 4.38 × 10−7, P = 2.03 × 10−12. n Two-tailed Mann-Whitney U-test corrected for ties: P = 0.0557, P = 0.0002, P = 4.0 × 10−7, P = 0.0010. o, p Dcp1 (blue) labeling reveals that control (o) and lapsyn ZG1 (p) mng in the cortex undergo apoptosis (arrows) at 42 h APF. q Comparison of percentages of control and lapsyn ZG1 clones in the cortex (left) and Dcp1-positive clones in the cortex and at the neuropil border (right) at 42 h APF. Scatter plots with bars show data points and means ± 95% confidence intervals (n = 20-μm stacks from 21, 29 (left), 21, 29, 21, 11, 28, and 26 optic lobes). Two-tailed Mann-Whitney U-test corrected for ties: P = 2.4 × 10−7, P = 0.0016, P = 0.0029, P = 0.0025. NS not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Panels a–l show projections. For genotypes, sample numbers and additional statistical values, see Supplementary Tables 1 and 2. Scale bars, 50 μm
Lapsyn controls mng positioning at the neuropil border
To shed light on the potential causes for the reduction in recovered adult lapsyn mutant clones (Fig. 5h), we next examined the distribution and numbers of astrocyte-like mng during metamorphosis. Unexpectedly, in both control and lapsyn ZG1 mosaics, we observed GFP-positive mng cell bodies in the medulla cortex at 24, 30, and 42 h APF (Fig. 6g–l), but not in adults (Fig. 6c, f). At 24 h APF, mng numbers at the distal medulla neuropil border were only slightly lower in lapsyn ZG1 mosaics than in controls, suggesting that lapsyn is not essential for their differentiation or initial migration to the neuropil border (Fig. 6m). In control and experimental mosaics, the number of recovered clones decreased until ~55 h APF and then remained constant. This decrease was more pronounced in lapsyn ZG1 mosaics. In the cortex at 24 h APF, the percentages of control and lapsyn-deficient mng were low and similar. However, at 30, 42, and 55 h APF, they were considerably higher for mutant mng than controls (Fig. 6n). Cortical localization correlated with a higher percentage of cells labeled with an antibody against cleaved Drosophila death caspase-1 (Dcp1) (Fig. 6o–q). While overexpression of the anti-apoptotic Baculo virus gene p35 in lapsyn ZG1 clones did not rescue mng numbers at the neuropil border (Supplementary Fig. 6a, b), we detected 8% surviving mng in the adult cortex compared to 0% in controls (lapsyn ZG1, UAS-p35: 100 mng in 13 samples; UAS-p35: 293 mng in 12 samples). These findings suggest that after their initial migration to the medulla neuropil border, mng retain motile properties and require lapsyn for anchoring their cell bodies in this location to ensure their survival.
FGF signaling but not lapsyn controls mng survival
Does lapsyn mediate mng survival directly or indirectly by ensuring exposure to a gliotrophic signal through correct positioning? To address this question, we focused on FGF signaling because of its central conserved role in controlling glial development23–26 and because we had identified the Htl receptor as one candidate in our screen. In Drosophila, binding of the FGF8-like ligands Pyramus and Thisbe (Ths) activates Htl followed by tyrosine phosphorylation of the receptor and the constitutively bound adaptor protein Downstream of FGF (Dof)50. Since the function of FGF signaling in optic lobe neuropils was not known, we first examined the expression of pathway components in the medulla. The htl fTRG482 fosmid transgenic line48 and an antibody against Dof25 reported strong expression of both in mng cell bodies throughout development and in processes extending into the neuropil from 55 h APF onwards (Fig. 7a, b). By contrast, expression analysis of Mi{MiC}ths MI01564, a 5′-3′ insertion into the first intron between 5′ UTR sequences, indicates that the ths ligand is generated by neurons (Fig. 7c).We then assessed the requirement of htl using RNAi-mediated knockdown in astrocyte-like mng. Expression of UAS-htl IR-GD6692 or UAS-htl IR-GD27180 using loco 1.3D2 -Gal4 at 29 °C from 24 h after egg laying severely affected mng survival (Supplementary Fig. 6c–f). To delay the onset of knockdown, progeny were initially raised at 18 °C and switched to 29 °C at a stage equivalent to 21 h APF at 25 °C. While this allowed many mng to survive to adulthood, they displayed severely reduced primary and secondary branches similar to phenotypes observed following lapsyn knockdown or loss (Fig. 7d–f, h). In a converse experiment, we increased FGF signaling in all astrocyte-like mng by overexpressing a constitutively activated form of htl (UAS-htl λ)51. Remarkably, many mng relocated into the cortex under these conditions, consistent with a pro-migratory role of FGF signaling50. The total number of mng and the number of mng at the neuropil border were increased in adults (Fig. 7g, h). Quantifications of optic lobes at 24 and 30 h APF labeled with PH3 and at 42 h APF with Dcp1 showed that higher mng numbers were due to increased mitosis and reduced apoptosis levels (Fig. 7i–l). Hence, Htl plays an additional role in the medulla by promoting glial survival.
Fig. 7.
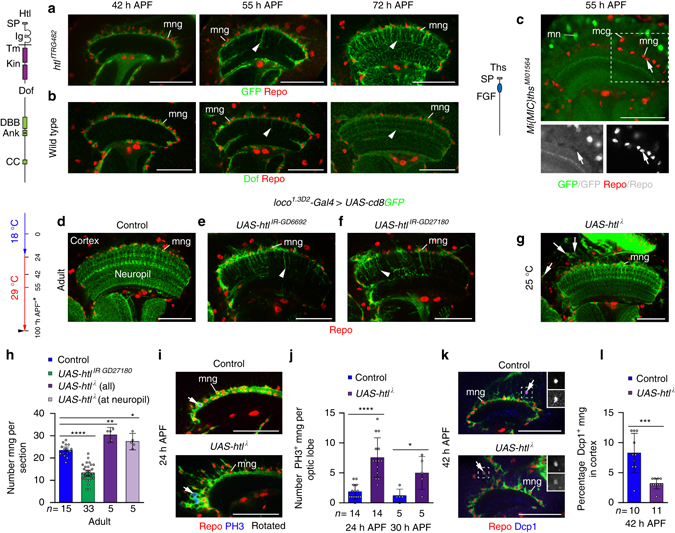
FGF signaling regulates astrocyte-like mng development. Repo labeled glial nuclei (red). a htl fTRG482 reports Htl expression (green) at 42, 55, and 72 h APF in mng cell bodies and processes (arrowheads). b Dof (green) labeling of wild-typeOreR optic lobes showed a similar expression pattern. c At 55 h APF, Mi{MIC}ths MI01564 reports Ths production in medulla neurons (MN), but not glia (arrows) including mng or medulla cortex glia (MCG). Ank ankyrin repeat, DBB Dof-BVAP-BANK domain CC coiled-coil domain, Ig immunoglobulin domain, Kin kinase, SP signal peptide, Tm transmembrane domain. d–g, I, k mng were labeled with loco 1.3D2 -Gal4 UAS-cd8GFP (green). Following expression of UAS-htl IR-GD6692 (e) and UAS-htl IR-GD27180 (f) adult mng showed reduced primary and secondary processes in the neuropil (arrowheads) compared to controls (d). Samples were shifted to 29 °C at 21 h APF. Asterisk, time points are adjusted to h APF at 25 °C. g Expression of constitutively active htl λ in mng did not affect branch extension into the neuropil. Surviving mng are ectopically positioned in the medulla cortex (g, arrows). h Quantification of mng numbers. Htl knockdown decreases and over-activation increases mng numbers. The scatter plot with bars shows data points and means ± 95% confidence intervals (n = optic lobes (single optical sections)). i, j Numbers of mitotic PH3-positive mng (blue) increased following Htl over-activation at 24 and 30 h APF. k, l The percentage of apoptotic Dcp1-positive mng (blue, bottom insets) per optic lobe in the cortex decreased following Htl over-activation at 42 h APF. The scatter plots with bars j, l show data points and means ± standard deviation (n = entire optic lobes). Unpaired, two-tailed Student’s t-test not assuming equal variance were: P = 9.84 × 10−13, P = 0.0017, P = 0.0372 (h), P = 1.39 × 10−5, P = 0.0327 (j), and P = 0.0007 (l). *P < 0.05, **P < 0. 01, ***P < 0.001, ****P < 0.0001. All image panels show single optical sections. For genotypes, sample numbers and additional statistical values, see Supplementary Tables 1 and 2. Scale bars, 50 μm
We next sought to understand the involvement of lapsyn in regulating survival in the context of FGF pathway roles. We observed that phenotypes in astrocyte-like mng expressing solely UAS-htl IR-GD27180 or both UAS-lapsyn and UAS-htl IR-GD27180 were indistinguishable (Fig. 8a–c). This suggests that lapsyn expression alone cannot rescue cell loss or branching defects caused by reduced FGF signaling. When overexpressing UAS-htl λ in clones, we observed a high percentage of ectopic mng in the cortex at 24 and 42 h APF (43 and 75%) that increased to 81% in adults (Fig. 8d–g ). Thus, clonal expression of htl λ provided us with a tool to generate ectopic mng in the cortex and to test the contribution of lapsyn in regulating survival following abnormal positioning. We observed that lapsyn loss in UAS-htl λ expressing clones did not alter the percentage of ectopic mng in the cortex. This suggests that FGF signaling but not lapsyn controls mng survival. When we co-expressed UAS-lapsyn and UAS-htl λ, the percentage of mng in the cortex was reduced by 53% (Supplementary Fig. 7). This further supported the notion that the role of lapsyn in anchoring mng, possibly by counter-acting the pro-migratory role of FGF signaling, is pivotal. Surviving htl λ-expressing mng formed extensive stellate branches in the cortex. Moreover these, as well as mng at the neuropil border, extended short tufted arbors into superficial medulla neuropil layers. mng clones that expressed UAS-htl λ but lacked lapsyn showed substantially less branching in the cortex and in the neuropil compared to clones overexpressing UAS-htl λ (Fig. 8e–f). Together, these findings support the model (Supplementary Fig. 8) that lapsyn mediates mng anchorage at the neuropil border, and thus indirectly survival, which depends on gliotrophic FGF signaling. Moreover, lapsyn acts downstream of or in parallel with FGF signaling to control branch morphogenesis of astrocyte-like mng, revealing the robust control of this developmental step through independent molecular mechanisms.
Fig. 8.
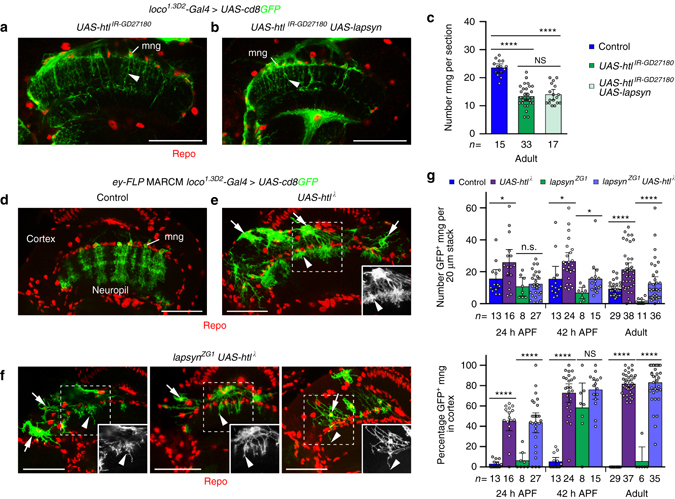
lapsyn does not directly mediate survival of astrocyte-like mng. Repo-stained glial nuclei (red). a, b mng were labeled with loco 1.3D2 -Gal4 UAS-cd8GFP (green). htl knockdown with UAS-htl IR-GD27180 disrupted mng branch morphogenesis in adults (a, arrowhead). UAS-lapsyn expression did not rescue these defects (b, arrowhead). c Quantification of mng numbers. UAS-lapsyn expression did not rescue the decrease of cell numbers following htl knockdown. The scatter plot with bars shows data points and means ± 95% confidence intervals (n = optic lobes (one optical section)). Unpaired, two-tailed Student’s t-test not assuming equal variance: P = 9.84 × 10−13, P = 1.82 × 10−9, P = 0.5166. d–f UAS-htl λ was expressed in mng clones using ey-FLP MARCM and loco 1.3D2 -Gal4 UAS-cd8GFP. Unlike in controls (d), wild-type clones (e), and lapsyn ZG1 mutant clones (f) expressing UAS-htl λ migrated into the medulla cortex and survived until adulthood (arrows). UAS-htl λ expressing clones extended short bushy processes into the neuropil (e, arrowhead). In lapsyn ZG1 mng clones expressing UAS-htl (f), branching is reduced in the cortex (arrow) and neuropil (arrowheads). g Quantification of ey-FLP MARCM clone numbers and localization at 24 and 42 h APF, and in adults. UAS-htl λ expression increased the number of glial cell clones compared to wild type and promoted lapsyn ZG1 clone survival. High percentages of wild type and lapsyn ZG1 clones overexpressing UAS-htl λ were found in the medulla cortex. Scatter plots with bars show data points and means ± 95% confidence intervals. Unpaired, two-tailed Student’s t-test not assuming equal variance: P = 0.0396, P = 0.5881, P = 0.0241, P = 0.0144, P = 2.49 × 10−6, P = 1.04 × 10−5 (top: n = 20-μm stacks of optical sections from 4,7,2,9,6,10,3,6,14,16,4 and 14 optic lobes); P = 4.25 × 10−8, P = 2.92 × 10−7, P = 7.97 × 10−15, P = 0.1432, P = 2.11 × 10−34, P = 6.59 × 10−22 (bottom: n = 20-μm stacks from 4,7,2,9,6,10,3,6,14,15,4 and 14 optic lobes). NS, not significant, *P < 0.05, ****P < 0.0001. All image panels show projections. For genotypes, sample numbers and additional statistical values, see Supplementary Tables 1 and 2. Scale bars, 50 μm
Requirement of lapsyn in other CNS regions
Finally, we asked whether lapsyn mediates astrocyte branch morphogenesis beyond the visual system. The lapsyn fTRG027706-135B fosmid reported protein expression in astrocyte populations in the adult antennal lobe, the central brain, and mushroom body lobes, as well as in the second and third instar larval and adult VNC (Fig. 9a, d, g and Supplementary Fig. 9a, b). loco 1.3D2 -Gal4 was active in all regions examined and therefore suitable for driving UAS-lapsyn IR-KK102333 expression (Fig. 9b, e, h and Supplementary Fig. 9c). Because astrocytic branches in mushroom body lobes were sparse, effects following lapsyn knockdown in astrocyte-like glia were less conspicuous in these neuropils. However, branch morphogenesis in the antennal lobes, the central brain and adult and third instar larval VNC was severely reduced (Fig. 9c, f, i and Supplementary Fig. 9d). Together, these findings indicate that lapsyn plays a general role in regulating astrocyte branch formation in the Drosophila CNS regardless of their developmental origins.
Fig. 9.
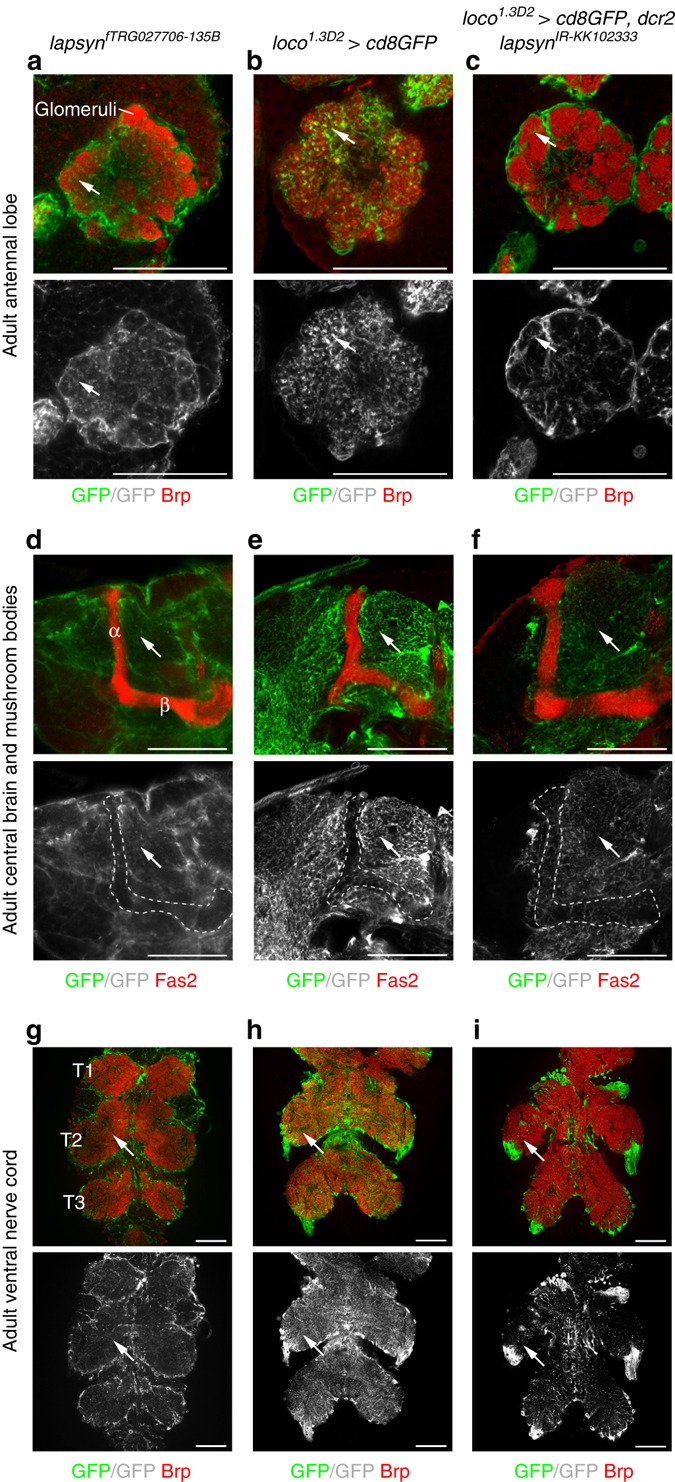
lapsyn is widely required for branch morphogenesis of astrocyte-like glia in the CNS. Brp was used to visualize synaptic neuropils (red, a–c, g–i). Fas2 labeled the mushroom body lobes (d–f, red). a, d, g The fosmid lapsyn fTRG027706 reported lapsyn expression (green, arrows) in adult astrocyte-like glia extending into glomeruli within the antennal lobe (a), the neuropils of the central brain (c), and the VNC (g) in addition to other glia. b, e, h loco 1.3D2 -Gal4 UAS-cd8GFP (green) labeled branches of astrocyte-like glia in all examined CNS areas. In mushroom body α and β lobes (d, e, f, outlined), lapsyn expression was weak, in line with the low density of astrocyte-like glial branches labeled with loco 1.3D2 -Gal4 UAS-cd8GFP, limiting assessment of defects following lapsyn knockdown. Compared to controls b, e, h, the knockdown of lapsyn in astrocyte-like glia resulted in reduced branch morphogenesis (arrows) in the adult antennal lobe, the central brain and VNC (c, f, i). All panels show single optical sections. For genotypes and sample numbers, see Supplementary Table 1. Scale bars, 50 μm
Discussion
Vertebrate astrocytes in different brain regions are considered as developmentally and functionally diverse cell types2, 12, 13. In Drosophila, recent studies elucidated the development and function of astrocyte-like glia in the VNC, mushroom bodies, and antennal lobes5, 6, 16, 21, 22, 26. Our characterization of mng in the visual system further supports the notion that Drosophila astrocyte-like glia constitute a heterogeneous population with distinct origins, developmental behaviors, and morphologies. Larval VNC astrocyte-like glia are derived from embryonic longitudinal glioblasts, while their counterparts in the central brain originate from embryonic glioblasts and dedicated postembryonic type II neuroblasts. These astrocyte subtypes expand by mitotic divisions as they migrate over neuropil surfaces52, 53. By contrast, OPC neuroepithelial-derived neuroblasts mature into neuroglioblasts to generate astrocyte-like mng that rarely divide40, 41 (this study). During early metamorphosis VNC and brain astrocyte-like glia lose their processes and transform into phagocytes that engulf synapses and neuronal fragments before regrowing branches during late pupal development8 or are removed by apoptosis53. However, astrocyte-like mng—similar to their antennal lobe counterparts5—associate with a newly arising, initially synapse-free neuropil. Interestingly, antennal and optic lobe astrocyte-like glia extend processes in a similar time window that coincides with the onset of synaptogenesis5. Finally, unlike the asymmetric stellate VNC and brain astrocyte-like glia, dmng variants are elongated and associate with distinct medulla neuropil layers. Their stereotypic morphology argues that branch formation is controlled by layered and intracolumnar patterning mechanisms, which may include differential adhesive interactions.
Extracellular LRR superfamily members control axonal and dendritic targeting, as well as synapse formation and plasticity of neurons54. Our expression and functional analyses in Drosophila uncovered a central role for the LRR transmembrane protein Lapsyn33 in regulating the development of astrocyte-like glia. Compared to neuronal LRR proteins, only very few members such as the secreted astrocytic leucine-rich repeat molecule (Alrm) in Drosophila and Oligodendrocyte-myelinating glycoprotein (OMgp) in vertebrates have been associated with glia16, 54. Tandem arrays of LRR motifs form arc or horseshoe-shaped structures. These serve as versatile protein recognition motifs that promote cell-cell interactions by binding to specific, albeit structurally unrelated ligands54, 55. Each repeat consists of 20–30 residues subdivided into a highly conserved and a variable segment, whose consensus sequences and length help to subdivide LRR motifs into classes55. LRR proteins underwent substantial expansion and diversification in different animal species45. Lapsyn contains no other recognizable motifs than the LRR and transmembrane domains. In classifications based on the presence of non-LRR domains, unrelated members lacking any other motifs tend to group. However, categorizations assessing arrays based on LRR class composition helped to cluster proteins with potentially similar functional structures56. In line with previous reports33, our searches using SmartBLAST and CATH/Gene3D programs57 predicted leucine-rich repeat-containing protein 4 (LRRC4), also known as Netrin-G ligand (NGL), family members as remotely related proteins of Lapsyn in vertebrates with 29% identity. LRRC4C (NGL-1), LRRC4 (NGL-2), and LRRC4B (NGL-3) contain one immunoglobulin domain in addition to the LRR chain, and regulate neurite outgrowth, laminar targeting and synapse formation58. Interestingly, NGL-2 also has been linked to astrocyte differentiation and glioma59. While NGL-1 and NGL-2 bind to mammalian-specific Netrin-G1 and 2, NGL-3 interacts via the LRR domain with the highly conserved leukocyte-antigen-related-like (LAR) and other protein tyrosine phosphatases60, underscoring the diversity of non-conserved and conserved binding partners for this LRR subfamily. However, to further elucidate Lapsyn function and its possible link to NGL family members, it is essential to identify its molecular partners using unbiased approaches in the future.
lapsyn is expressed in astrocyte-like mng and other glia, but not in neurons as previously described61. The tiled organization and minimal overlap of processes in adult mng suggests that—similarly to VNC glia26—branches stop growing once they infiltrated the entire neuropil and come in contact with those of neighboring glia. Because emerging processes from adjacent wild-type astrocyte-like mng barely touch and loss of lapsyn negatively affects process extension, this cell surface molecule is unlikely to mediate contact-dependent homotypic repulsive interactions. While astrocyte-like and ensheathing mng cell bodies are interspersed at the neuropil border, the branches of astrocyte-like mng are primarily in contact with neurons and only slightly with the sparse branches of ensheathing mng. It is therefore conceivable that Lapsyn is part of a signaling complex in astrocyte-like mng membranes that promotes cell-cell recognition events or adhesive interactions with as yet elusive heterotypic neuronal ligands to enable anchorage at the neuropil border and branch elaboration within the neuropil. At present, we cannot exclude additional interactions with ensheathing mng for positioning and initial branch extension, or an independent lapsyn requirement in this glial subtype. Persistent clonal overexpression of lapsyn did not affect astrocyte-like mng morphology, suggesting that this determinant does not promote growth and the tight temporal regulation of expression levels is not essential for its function.
Different from other CNS areas, some astrocyte-like mng leave the neuropil border and migrate into cortex during early pupal development, where they subsequently undergo apoptosis. Consistently, the number of wild-type mng clones decreases during metamorphosis. The medulla and lobula complex undergo a 90° rotation with respect to the lamina62, and medulla neuron somata extensively relocate to new positions in the cortex during early metamorphosis63. Therefore, mng find themselves in an environment particularly conducive to cell migration. Additionally, mng may compete for space and potential survival factors at the neuropil border, and excess cells may become expelled. Thus, lapsyn may be part of a mechanism ensuring that sufficient numbers of astrocyte-like mng are retained at the neuropil border.
The motility and survival of astrocyte-like mng depend on FGF signaling levels. Increased FGF receptor activity causes mng to relocate from the neuropil border into the cortex, consistent with the well-established pro-migratory role for this pathway50. Early htl knockdown reduced, while over-activation increased mng numbers. Importantly, in addition to some increase in proliferation, the percentage of apoptotic htl λ expressing astrocyte-like mng in the pupal medulla cortex was reduced and ectopic cells persisted into adulthood. This argues that htl signaling regulates survival. This less explored function of FGF signaling50 could either be primary or involve secondary pathways such as epidermal growth factor receptoractivation64.
Higher levels of Htl signaling increase the territories occupied by VNC astrocyte clones26. By contrast, enhanced Htl signaling in mng clones results in the formation of tufted arbors with short densely packed branches that are unable to extend into deeper neuropil layers. Hence, htl in mng may be required for promoting branch growth rather than acquiring specific patterns. lapsyn overexpression fails to rescue branch extension defects or cell loss caused by htl knockdown, suggesting that FGF signaling is not epistatic to lapsyn. However, mng—overexpressing constitutively active Htl while lacking lapsyn—survive into adulthood and display severely reduced branches. These genetic data support the notion that lapsyn acts downstream of or in parallel with FGF signaling to regulate branch formation, whereas FGF signaling, but not lapsyn, mediates survival. lapsyn overexpression is likely insufficient to rescue htl knockdown because additional factors may be affected that govern robust glial branch morphogenesis. Higher lapsyn levels reduced htl λ-induced migration of mng into the cortex. The differential effects on branch extension and positioning argue that lapsyn may not simply enhance or attenuate FGF signaling.
The neuron-derived FGF8-like ligand Ths promotes ensheathment of R-cell axon bundles by wrapping glia in the larval eye imaginal disc25 and astrocyte branch extension into the VNC neuropil26. Similarly, we observed that Ths is produced by neurons in the optic lobe. Together, this suggests a model, whereby parallel signals control the balanced development of astrocyte-like mng: neuronal FGF could function as a gliotrophic factor, and promote motility and branch growth, whereas other as yet unidentified neuron-derived factors could interact with Lapsyn to achieve the correct extension and ramification of processes within the neuropil. These or additional ligands may also mediate neuropil recognition and anchorage of mng at the neuropil border.
In summary, we identified potentially related roles for the transmembrane protein Lapsyn in regulating branch extension and positioning of astrocyte-like mng. Their complex adult morphology indicates that further factors await discovery to explain stepwise branch formation at astrocyte-neuron interfaces. Because astrocytes have been linked to several neurodevelopmental disorders in humans11, this detailed knowledge could ultimately contribute to our better understanding of underlying disease mechanisms.
Methods
Genetics
Drosophila melanogaster strains were maintained on standard medium at 25 °C, if not specified otherwise. The following stocks or crosses were used to label different glial cell subtypes: (1) repo-Gal4, (2) R56F03-Gal4 32, 35, 36, (3) NP6520-Gal4 17, (4) loco 1.3D2 -Gal4 37, (4) second and third chromosomal alrm-Gal4 insertions16 (by M. Freeman, University of Massachusetts), and (5) dEAAT1-Gal4 (ref. 18; by S. Birman, ESPCI) were crossed to UAS-cd8GFP. A homozygous loco 1.3D2 -Gal4; UAS-cd8GFP stock was used in some experiments. The stock GMR-myr mRFP; loco 1.3D2 -Gal4; UAS-cd8GFP, UAS-dcr2 was used to genetically label glia and R-cell axons in the same sample. Individual glial cells were visualized using the Flybow approach31 by crossing loco 1.3D2 -Gal4; hs-mFLP5/TM6B to UAS-FB1.1 260b. Progeny were subjected to two 45 min heat shocks in a 37 °C water bath at 48 and 72 h after egg laying (AEL) and dissected during indicated stages. To visualize ensheathing and astrocyte-like glia in the same sample, the stock UAS-FB1.1B 260b; loco 1.3 -lexA lexAop-myr mCherry was crossed to R56F03-Gal4. The loco 1.3 -lexA insertion on the third chromosome was generated for this study (see below).
Lineage analysis experiments were performed using MARCM42 by crossing (1) FRT19A tubP-Gal80 hs-FLP 1 ; UAS-lacZ nls UAS-cd8GFP; tub-Gal4/TM6B to (2) FRT19A/Y. To induce clones in the optic lobe, 12 h embryo collections were heat shocked for 10–20 min at 60–72 h AEL in a 37 °C water bath. Third instar larval brains were dissected during the following 24–48 h. MARCM clones were generated using the ubiquitous tubP-Gal4 driver and hs-FLP as a recombinase source to label all cell types with GFP in a clone. Other clone configurations than those described in Fig. 2b–d that included mng were not detected, supporting the notion that OPC neuroblasts maturing into neuroglioblasts are the primary source for mng in the medulla.
For the RNAi-based screen, loco 1.3D2 -Gal4; UAS-cd8GFP, UAS-dcr2 flies were crossed to 522 UAS-RNAi lines obtained from the Vienna Drosophila Resource Center (VDRC)65 and National Institutes of Genetics (Japan) collections (http://www.shigen.nig.ac.fly/nigfly). We focused on lines affecting genes that encode secreted and cell surface proteins with potential expression or functions in the nervous system. Candidates were selected based on information provided in refs. 45, 66, 67. Progeny of crosses were raised either at 29 °C or if lethal at 25 °C. UAS-RNAi lines that caused lethality under either condition were retested in a second screen, in which heat shock-induced FLP (hs-FLP) led to mosaic expression by excision of the FRT site flanked Gal4-repressor Gal80 downstream of a widely active tubulin enhancer68 (tub>Gal80>). Specifically, RNAi lines were combined with hs-FLP 122 , tub>Gal80>; loco 1.3D2 -Gal4; UAS-cd8GFP, UAS-dcr2. Progeny were shifted to 29 °C at 24–48 h AEL and exposed to a 10-min heat shock at 37 °C between 48 and 72 h AEL to achieve a spatially and temporally restricted knockdown. Lapsyn knockdown in astrocyte-like glia was achieved by crossing loco 1.3D2 -Gal4; UAS-cd8GFP, UAS-dcr2 to (1) UAS-lapsyn IR-KK102333 and (2) UAS-lapsyn IR-15658R1 or by crossing loco 1.3D2 -Gal4; UAS-cd8GFP to UAS-lapsyn IR-KK102333; UAS-dcr2. Progeny were shifted to 29 °C at 24–48 h AEL to achieve optimal knockdown.
loco 1.3D2 -Gal4, UAS-cd8GFP was combined with UAS-ChRFP-Tub to visualize microtubules. loco 1.3D2 -Gal4,UAS-cd8mCherry 260b transgenes were combined with (1) UAS-Eb1-GFP to track plus-end microtubules, (2) UAS-nod-GFP to label minus-end microtubules, and (3) UAS-LifeAct-GFP to visualize actin. To assess the effect of lapsyn knockdown on the cytoskeleton, UAS-lapsyn IR-KK102333; UAS-dcr2 was crossed to (1) loco 1.3D2 -Gal4, UAS-cd8mCherry 260b ; UAS-Eb1-GFP and (2) loco 1.3D2 -Gal4, UAS-cd8mCherry 260b; UAS-LifeAct-GFP.
To identify the cell types producing Lapsyn, optic lobes of heterozygous Mi{MIC}lapsyn MI01316 /CyO progeny46 were analyzed. Lapsyn protein expression was assessed in Mi{MIC}lapsyn lapsyn2xHA-24A and Mi{MIC}lapsyn lapsyn2xHA-73B transgenic flies, generated by injection-based RMCE. Furthermore, endogenous Lapsyn protein expression was determined in fly lines containing the Crispr/Cas9 allele lapsyn 2XHA-CR13.1 and the lapsyn fTRG027706-135B fosmid48 (see below). The former were crossed to loco 1.3D2 -Gal4; UAS-cd8GFP. Lapsyn localization in glial processes in gain-of-function studies was assessed by crossing loco 1.3D2 -Gal4 to UAS-lapsyn-GFP (ref. 33; by T. Littleton (Massachusetts Institute of Technology)).
For whole animal neuron- and glial cell-specific rescue experiments, lapsyn ZG1, a null allele carrying a ~4.7 kb deletion of the 5′UTR and the first two coding exons was used33 (by T. Littleton). (1) elav C155 -Gal4; lapsyn ZG1 /CyO Dfd-YFP and (2) lapsyn ZG1 /CyO Dfd-YFP; repo-Gal4/TM6B were crossed to lapsyn ZG1 /CyO Dfd-YFP; UAS-lapsyn. Rescue experiments were conducted in independent triplicates, using overnight grape juice agar plate collections of a total of 1210 and 2360 embryos, respectively. Rescue of viability at the first instar larval stage was calculated as percentage of expected progeny. Subsequently, hatched first instar larvae in “rescue” and “control” populations from above egg collections were monitored for survival as third instar larvae, as young (0–24 h APF) and old pupae with dark appearing wings development, and as adults. “Rescue” animals have the following genotypes: elav C155 -Gal4; lapsyn ZG1 /lapsyn ZG1 ; UAS-lapsyn/+ and lapsyn ZG1 /lapsyn ZG1 ; repo-Gal4/UAS-lapsyn. “Control” animals included all genotypes that were heterozygous for lapsyn ZG1, or heterozygous for lapsyn ZG1 while overexpressing lapsyn. The UAS-lapsyn transgene33 was generated by T. Littleton. The lapsyn fTRG027706-135B transgene reported Lapsyn expression in second and third instar larval VNCs solely in glia. elav-Gal4 C155 is known to be transiently active in glia during early development69. These low levels may enable very unhealthy animals to survive during early larval development.
To conduct loss- and gain-of-function, as well as rescue experiments in clones, the ey-FLP transgene was combined with MARCM (ey-FLP MARCM): ey-FLP; FRT42B tubP-Gal80 was crossed to (1) loco 1.3D2 -Gal4, FRT42B; UAS-cd8GFP (control), as well as (2) loco 1.3D2 -Gal4, FRT42B lapsyn ZG1 /CyO Dfd-YFP; UAS-cd8GFP, and (3) loco 1.3D2 -Gal4, FRT42B lapsyn LL00906 /CyO Dfd-YFP; UAS-cd8GFP (lapsyn loss-of-function); ey-FLP; FRT42B tubP-Gal80; UAS-lapsyn/TM6B was crossed to (1) loco 1.3D2 -Gal4, FRT42B; UAS-cd8GFP (gain-of-function) and (2) loco 1.3D2 -Gal4, FRT42B lapsyn ZG1 /CyO, Dfd-YFP; UAS-cd8GFP (rescue in astrocyte-like mng clones). For overexpression in all astrocyte-like mng, loco 1.3D2 -Gal4; UAS-cd8GFP was crossed to UAS-lapsyn. lapsyn LL00906 is a mutagenic piggyBac element inserted into the lapsyn locus 260 bp after the transcriptional start site on a chromosome also carrying a FRT42B site49. While we observed qualitatively similar defects for lapsyn LL00906 and lapsyn ZG1, the penetrance was slightly lower for lapsyn LL00906. For lapsyn knockdown and overexpression in medulla cortex glia, wrapper GMR54H02 -Gal4 35 was recombined with UAS-myrmRFP and subsequently crossed to (1) UAS-lapsyn IR-KK102333 ; UAS-dcr2 and (2) UAS-lapsyn. For rescue experiments by overexpression of p35, ey-FLP; FRT42B tubP-Gal80; UAS-p35 was crossed to (1) loco 1.3D2 -Gal4, FRT42B; UAS-cd8GFP (control) and (2) loco 1.3D2 -Gal4, FRT42B lapsyn ZG1 /CyO Dfd-YFP; UAS-cd8GFP.
To visualize control or mutant clones and surrounding heterozygous astrocyte-like mng, ey-FLP; FRT42B tubP-Gal80; loco 1.3 -lexA lexAop-myr mCherry/TM6B was crossed to (1) loco 1.3D2 -Gal4, FRT42B; UAS-cd8GFP and (2) loco 1.3D2 -Gal4, FRT42B lapsyn ZG1 /CyO Dfd-YFP; UAS-cd8GFP.
For expression analysis of FGF receptor pathway components, (1) htl fTRG482 (ref. 48), (2) wild type OreR, and (3) Mi{MIC}ths MI01564 were used. To assess interactions with the FGF signaling pathway, loco 1.3D2 -Gal4; UAS-cd8GFP was crossed to (1) UAS-htl IR-GD6692 and (2) UAS-htl IR-GD27180 (knockdown; refs. 26, 65), (3) UAS-htl IR-GD27180 ; UAS-lapsyn (rescue) and (4) UAS-htl λ (over-activation; ref. 51). Additionally, GMR-myr RFP; loco 1.3D2 -Gal4; UAS-cd8GFP, UAS-dcr2 was crossed to UAS-htl IR-GD27180. Because early htl knockdown by continuous expression at 29 °C severely interfered with the survival of astrocyte-like mng, progeny were maintained at 18 °C until 42 h APF (equivalent to 21 h APF at 25 °C) and then transferred to 29 °C. To increase FGF signaling in wild type or lapsyn ZG1 astrocyte-like mng clones, ey-FLP; FRT42B tubP-Gal80; UAS-htl λ /TM6B was crossed to (1) loco 1.3D2 -Gal4, FRT42B; UAS-cd8GFP and (2) loco 1.3D2 -Gal4, FRT42B lapsyn ZG1 /CyO Dfd-YFP; UAS-cd8GFP. To increase FGF signaling in wild type or UAS-lapsyn overexpressing astrocyte-like mng, loco 1.3D2 -Gal4; UAS-cd8GFP was crossed to (1) UAS-htl λ and (2) UAS-htl λ, UAS-lapsyn. Supplementary Table 3 and references provide additional information for Drosophila stocks used in this study. If not otherwise indicated, lines were obtained from the Bloomington Drosophila Stock Center and assembled into above described stocks. Generally, crosses involved about 5–8 males and 10–15 unfertilized females. To avoid overcrowding, parents were transferred to fresh vials every day for crosses at 25 °C and every three days at 18 °C.
Molecular biology
To generate the lapsyn 2xHA construct for insertion into the attP site containing Mi{MIC}lapsyn MI01316 line using ϕC31 integrase-mediated RMCE by injection46, the lapsyn ORF was PCR amplified from the Gold collection clone GH22922 (Drosophila Genomics Resource Center) with the forward primer lapsyn F1 and the reverse primer lapsyn R2 5′-GAGGCTAGCGGCGTAATCGGGCACATCGTAGGGGTATTTTCGGGCAATATCACAGTG-3′, that added a NheI site and the first HA-tag encoding sequence. This fragment was subcloned into the pTV vector70 using EcoRI and NheI restriction enzyme sites. The lapsyn 3′UTR was PCR amplified using the forward primer lapsyn F2 5′-AAGCTAGCTACCCCTACGATGTGCCCGATTACGCCTAGCACTTTAGTTAGTTAATTAGTTGCTTAG-3′ that added a NheI site and a second HA-tag encoding sequence, as well as the reverse lapsyn R1 primer. This fragment was inserted into the pTV vector containing the lapsyn-HA fragment using NheI and BamHI restriction enzyme sites. The full-length lapsyn 2xHA 3′UTR construct was subcloned into pBS-KS-attB1-2-GT-SA (Addgene; ref. 46) using EcoRI and BamHI sites. Injections and crosses to generate Mi{MIC}lapsyn lapsyn2xHA and alleles were performed as described in ref. 46. Testing resulting lines for the correct orientation of RMCE events, we identified two lines (Mi{MIC}lapsyn lapsyn2xHA-24A and Mi{MIC}lapsyn lapsyn2xHA 73B) as correct insertions with the help of the following primers: RMCE 1 5′-TAGAGTGATGTGTGTGTAGTGAACTAAC-3′, RMCE A 5′-CGGAAGAGAGATAAATCGGTTG-3′, RMCE B2 5′-GCCCAGAAACGCCATCAAC-3′, and RMCE 2.2 5′-TCCCTATTCTCGAGGCTTTGAT-3′.
To generate the lapsyn 2xHA-CR13.1 allele, a PAM site and guide sequence closest to the lapsyn stop codon were selected (Fig. 4). Forward and reverse oligonucleotides of the target sequence (Oligotop 5′-gtcGACTAACTAAAGTGCTATTTT-3′ and Oligobottom 5′-aaacAAAATAGCACTTTAGTTAGT-3′) with BbsI-compatible ends were annealed and ligated into the pCFD3 plasmid digested with BbsI (ref. 47; by S. Bullock (MRC LMB) and C. Alexandre (Francis Crick Institute)). A single-stranded oligo donor nucleotide (ssODN, 169-nt) that contained two 50-nt homology arms flanking the sequences of two HA epitope tags separated by a GGGS spacer, was synthesized by IDT (Integrated DNA Technologies): 5′-ACAACCGTCAGCCGGAGGACGAGCCTCTGCACTGTGATATTGCCCGAAAATACCCATACGACGTCCCTGACTATGCGGGAGGTGGGAGTTATCCCTATGATG TGCCCGATTACGCTTAGCACTTTAGTTAGTTAATTAGTTGCTTAGTTAGTTAGTTAGTCTTAGGTGC-3′. The ssODN (250 ng/μl) and pCFD3 plasmid (300 ng/μl) were co-injected into y 1 M{vasa-Cas9.RFP-}ZH-2A w 1118 (BDSC 55821) embryos. Thirty-five viable and fertile recovered male and female flies were crossed individually to 3–4 yw; GlaBc/CyO female or male flies, respectively. In the next generation, 1–8 male offspring from each vial were crossed individually to 3–4 yw; Gla Bc/CyO females. Once progeny were visible, these males were sacrificed to prepare genomic DNA for PCR screening using the following primers: VasC9 seq2F: 5′-GACGAAAGAGGAATGCCCAGAAG-3′ and VasC9 seq2R: 5′-GGTAAAGGATTCAAGGCAGTTTG-3′. In 2 of 131 candidates, the HA epitope tag was inserted in frame upstream of the lapsyn stop codon. The line lapsyn 2xHA-CR13.1 was used for further analysis.
The tagged fosmid lapsyn fTRG027706 clone (Source BioScience) was amplified and sequence-verified following the protocol of Sarov et al.48. The fosmid (250 ng/μl) was injected into y 1 w P{nos- ϕC31\int.NLS}X; PBac{y + -attP-3B}VK00033 flies (BDSC: 32542). We recovered two independent insertions on the third chromosome. The line lapsyn fTRG027706-135B was used for further analysis.
To generate loco 1.3 -LexA, the 2.2 kb “1.3” loco enhancer fragment was removed from the p221-4 plasmid (ref. 37; by C. Klämbt (University of Münster)) and inserted into the Gateway® selection vector pENTR 1A (InvitrogenTM) using EcoRI restriction enzyme sites. This fragment was subcloned into the plasmid pBPLexA::p65Uw (Addgene ♯26231) using standard Gateway cloning technology. Transgenic flies with insertions in the attP2 site (68A4) on the third chromosome were generated by Rainbow Transgenic Flies, Inc. (Camarillo, CA, USA) using a ϕC31 integrase-based injection protocol.
Immunolabeling and imaging
Brains of larvae, pupae, and pharate adults were dissected in phosphate-buffered saline (PBS), fixed for 1 h at 20–24 °C in 2% paraformaldehyde (wt/vol) in 0.05 M sodium phosphate buffer (pH 7.4) containing 0.1 M L-lysine (Sigma-Aldrich) and washed in PBT (PBS containing 0.5% Triton X-100 (Sigma-Aldrich)). The following primary antibodies were diluted in 10% Normal Goat Serum (NGS) and PBT for immunolabeling over night at 4 °C: mouse antibody mAb24B10 (1:75, Developmental Studies Hybridoma Bank (DSHB)), mouse anti-Brp (1:10, DSHB nc82), rat antibody CadN (1:20, DSHBDN-Ex #8), rabbit antibody to Dcp1 (1:200, Cell Signaling Technology #9578), rabbit antibody to dEAAT1(1:5000, by D. Van Meyel, McGill University, ref. 36), rabbit antibody to Dof (1:200, shared by C. Klaembt, University of Münster), mouse antibody to Fas2 (1:10, DSHB, 1D4), rabbit antibody to GAT (1:2000, by M. Freeman, ref. 26), rabbit antibody to GFP (1:1000, Invitrogen A6455), rabbit antibody to PH3 (1:100, Millipore/Upstate #06-570), rabbit antibody to Gs2 (1:100, Merck Millipore MAB302), mouse antibody to Prospero (1:50, DSHB MR1A), mouse antibody to Repo (1:20, DSHB, 8D12), rabbit antibody to Repo (1:500, by J. Urban, University of Mainz, ref. 34) and rabbit antibody to RFP (1:500, Abcam ab62341). For immunofluorescence labeling, samples were incubated in the following secondary antibodies for 2.5 h at 20–24 °C: goat anti-mouse, anti-rabbit or anti-rat F(ab′)2 fragments coupled to Cy3 or Alexa Fluor®647 (1:400; Jackson ImmunoResearch Laboratories; antibody to mouse: Cy3 (115-166-003), Alexa Fluor®647 (115-606-003); antibody to rabbit: Cy3 (111-166-003), Alexa Fluor®647 (111-606-003); antibody to rat: Cy3 (112-166-003)). For immunolabeling of Mi{MIC}lapsyn lapsyn2xHA-24A optic lobes, over-night incubations were performed with primary rabbit antibody to HA-Tag (1:300, Cell Signaling Technology C29F4) and with secondary goat antibody to rabbit: Cy3 (111-166-003) for 4 h at 20–24 °C. For HA-immunolabeling of lapsyn 2XHA-CR13.1optic lobes, following the incubation in primary antibody over night at 4 °C, samples were washed for 15 min in PBT at 20–24 °C and overnight in NGS/PBT at 4 °C, and then transferred into secondary goat antibody to rabbit: Alexa Fluor®555 (Life Technologies, A21430) for 2 h at 20–24 °C. Images were collected using a Leica TCS SP5II laser scanning confocal microscope. Adobe Photoshop, Fiji and Volocity (Perkin Elmer) software programs were used for image analysis. mng clones are represented as projections of several optical sections to show their full extent of processes.
Quantifications
To quantify defects in glial branch morphogenesis, localization and survival, optic lobes of control and experimental animals were imaged in a horizontal orientation. GFP-positive astrocyte-like mng were assessed in one to three independent 20-µm stacks of optical sections per sample for all MARCM experiments. In quantifications shown in Figs. 7h and 8c, single optical sections were examined. In quantifications shown in Fig. 7j, l, 2-μm-image stacks of entire optic lobes were analyzed. Lateral, proximal, and distal astrocyte-like mng were assessed based on the position of their cell bodies relative to the neuropil with the exception of optic lobes at 24 h APF, when glial cells have not yet migrated to their final position and therefore were all considered as one group. Furthermore, positioning of astrocyte-like mng in the cortex or at the neuropil border was determined based on the following criteria. Cells were considered to be located at the neuropil border if part of the cell body (i) touches the neuropil labeled with an antibody against N-cadherin (CadN), (ii) is in close contact with other mng at the neuropil border, or (iii) is touching R-cell axons at 24 h APF. Otherwise, cells were considered to be localized in the medulla cortex regardless of long thin processes contacting the neuropil border. SmartBLAST and CATH/Gene3D57 programs were used to search for remote vertebrate homologs of Drosophila Lapsyn.
Statistics
In a pool of control or experimental animals, flies with unambiguously identifiable genotypes were selected randomly and independently from different vials at defined developmental stages. Data acquisition and analysis were not performed blindly because of the reliance on defined genotypes. Samples sizes were not pre-calculated using statistical tests but followed the standard of the field. Statistical calculations were performed using Prism 6 GraphPad and Microsoft Excel software. D’Agostino-Pearson omnibus normality tests indicated that data sets generally met the assumption for normality. P values were determined using the parametric unpaired two-tailed Student’s t-test (Welch corrected, not assuming equal variance or standard deviations). If a small set of data in a larger experiment did not pass this test (owing to few outlier data points or too small n numbers), normality was assumed and parametric Student’s t-tests were conducted. If the majority of data subsets did not meet the assumption of normality, non-parametric, two-tailed Mann-Whitney U-tests, corrected for ties, were performed. Quantifications are presented as stacked 100% bar graphs and as scatter plots and bar graphs. Data are presented as means ± 95% confidence interval error bars or as means ± standard deviation interval error bars. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. T values (t) and degrees of freedom (df), as well as U values are listed in Supplementary Table 2.
Data availability
Image data sets generated and analyzed in this study are available from the corresponding author on reasonable request. A source data file for quantifications shown in Figs. 2, 4–8, and Supplementary Fig. 7 is provided with the paper (Supplementary Data 1).
Electronic supplementary material
Acknowledgements
We are grateful to T. Littleton (Massachusetts Institute of Technology), C. Klämbt (University of Münster), M. Freeman (University of Massachusetts), D. Van Meyel (McGill University), S. Birman (ESPCI), B. Stramer (King’s College London), N. Tapon (Francis Crick Institute), S. Bullock (MRC LMB), and C. Alexandre (Francis Crick Institute) for sharing fly strains, antibodies, and constructs. We thank the Bloomington Drosophila Stock Center (NIH P40OD018537), the Vienna Drosophila Resource Center, the Japan National Institutes of Genetics fly stock center, the Kyoto Drosophila Genetic Resource Center, and the Developmental Studies Hybridoma Bank for fly strains and antibodies. We thank M. Pilatova and H. Apitz (Francis Crick Institute) for assistance with the verification and immunostaining of Mi{MIC}lapsyn lapsyn2xHA, E. Powell (Francis Crick Institute) for the loco 1.3D2 -Gal4 UAS-cd8mCherry 260b recombinant, C. Alexandre for the protocol and guidance with Crispr/Cas9 experiments, and W. Taylor (Francis Crick Institute) for advice on homology searches. We thank J.P. Vincent, F. Guillemot, H. Apitz, K. Dolan, R. Kaschula, and N. Shimosako (Francis Crick Institute), as well as E. Ober (DanStem) and B. Hofbauer (University of Würzburg) for critical reading of the manuscript. This work was supported by a fellowship of the Heinrich Hertz-Stiftung (S.M.), the Medical Research Council (U117581332 I.S.), and the Francis Crick Institute (FC001151 I.S.), which receives its core funding from Cancer Research UK, the Medical Research Council and the Wellcome Trust.
Author contributions
B.R. and I.S. conceived the project. B.R. and C.D.V. performed the experiments and analyzed the data. S.M. contributed to the lapsyn knockdown and lapsyn LL00906 mosaic analyses experiments. I.S. contributed to the characterization of the FGFR signaling pathway. I.S. wrote the manuscript with support of all authors.
Competing interests
The authors declare no competing financial interests.
Footnotes
Benjamin Richier and Cristina de Miguel Vijandi contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-00384-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fields RD, Woo DH, Basser PJ. Glial regulation of the neuronal connectome through local and long-distant communication. Neuron. 2015;86:374–386. doi: 10.1016/j.neuron.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molofsky AV, et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509:189–194. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthukumar AK, Stork T, Freeman MR. Activity-dependent regulation of astrocyte GAT levels during synaptogenesis. Nat. Neurosci. 2014;17:1340–1350. doi: 10.1038/nn.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr. Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Hakim Y, Yaniv SP, Schuldiner O. Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS ONE. 2014;9:e86178. doi: 10.1371/journal.pone.0086178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald RB, et al. Muller glia provide essential tensile strength to the developing retina. J. Cell Biol. 2015;210:1075–1083. doi: 10.1083/jcb.201503115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman MR. Drosophila central nervous system glia. Cold Spring Harb. Perspect. Biol. 2015 doi: 10.1101/cshperspect.a020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molofsky AV, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2:175–186. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res. Rev. 2010;63:11–25. doi: 10.1016/j.brainresrev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Dumstrei K, Wang F, Hartenstein V. Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J. Neurosci. 2003;23:3325–3335. doi: 10.1523/JNEUROSCI.23-08-03325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty J, Logan MA, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rival T, et al. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr. Biol. 2004;14:599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Stacey SM, et al. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J. Neurosci. 2010;30:14446–14457. doi: 10.1523/JNEUROSCI.1021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr. Biol. 2011;21:625–634. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacNamee SE, et al. Astrocytic glutamate transport regulates a Drosophila CNS synapse that lacks astrocyte ensheathment. J. Comp. Neurol. 2016;524:1979–1998. doi: 10.1002/cne.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, Z., Stork, T., Bergles, D. E.&Freeman, M. R. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature539, 428–432 (2016). [DOI] [PMC free article] [PubMed]
- 23.Kang K, Lee SW, Han JE, Choi JW, Song MR. The complex morphology of reactive astrocytes controlled by fibroblast growth factor signaling. Glia. 2014;62:1328–1344. doi: 10.1002/glia.22684. [DOI] [PubMed] [Google Scholar]
- 24.Avet-Rochex A, Kaul AK, Gatt AP, McNeill H, Bateman JM. Concerted control of gliogenesis by InR/TOR and FGF signalling in the Drosophila post-embryonic brain. Development. 2012;139:2763–2772. doi: 10.1242/dev.074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzdottir SR, et al. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- 26.Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR. Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron. 2014;83:388–403. doi: 10.1016/j.neuron.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadjieconomou D, Timofeev K, Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr. Opin. Neurobiol. 2011;21:76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Chotard C, Salecker I. Glial cell development and function in the Drosophila visual system. Neuron Glia Biol. 2007;3:17–25. doi: 10.1017/S1740925X07000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards TN, Nuschke AC, Nern A, Meinertzhagen IA. Organization and metamorphosis of glia in the Drosophila visual system. J. Comp. Neurol. 2012;520:2067–2085. doi: 10.1002/cne.23071. [DOI] [PubMed] [Google Scholar]
- 30.Tix S, Eule E, Fischbach KF, Benzer S. Glia in the chiasms and medulla of the Drosophila melanogaster optic lobes. Cell Tissue Res. 1997;289:397–409. doi: 10.1007/s004410050886. [DOI] [PubMed] [Google Scholar]
- 31.Hadjieconomou D, et al. Flybow: genetic multicolor cell labeling for neural circuit analysis in Drosophila melanogaster. Nat. Methods. 2011;8:260–266. doi: 10.1038/nmeth.1567. [DOI] [PubMed] [Google Scholar]
- 32.Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U. The glia of the adult Drosophila nervous system. Glia. 2017;65:606–638. doi: 10.1002/glia.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan Z, Buhl LK, Quinn WG, Littleton JT. Altered gene regulation and synaptic morphology in Drosophila learning and memory mutants. Learn. Mem. 2011;18:191–206. doi: 10.1101/lm.2027111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halter DA, et al. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development. 1995;121:317–332. doi: 10.1242/dev.121.2.317. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl Acad. Sci. USA. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peco E, et al. Drosophila astrocytes cover specific territories of the CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development. 2016;143:1170–1181. doi: 10.1242/dev.133165. [DOI] [PubMed] [Google Scholar]
- 37.Granderath S, Bunse I, Klambt C. gcm and pointed synergistically control glial transcription of the Drosophila gene loco. Mech. Dev. 2000;91:197–208. doi: 10.1016/S0925-4773(99)00304-4. [DOI] [PubMed] [Google Scholar]
- 38.Livet J, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 39.Thomas GB, van Meyel DJ. The glycosyltransferase Fringe promotes Delta-Notch signaling between neurons and glia, and is required for subtype-specific glial gene expression. Development. 2007;134:591–600. doi: 10.1242/dev.02754. [DOI] [PubMed] [Google Scholar]
- 40.Li X, et al. Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature. 2013;498:456–462. doi: 10.1038/nature12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki T, Takayama R, Sato M. Eyeless/Pax6 controls the production of glial cells in the visual center of Drosophila melanogaster. Dev. Biol. 2016;409:343–353. doi: 10.1016/j.ydbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/S0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 43.Ting CY, et al. Photoreceptor-derived activin promotes dendritic termination and restricts the receptive fields of first-order interneurons in Drosophila. Neuron. 2014;81:830–846. doi: 10.1016/j.neuron.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, et al. Cell-type-specific labeling of synapses in vivo through synaptic tagging with recombination. Neuron. 2014;81:280–293. doi: 10.1016/j.neuron.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolan J, et al. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics. 2007;8 doi: 10.1186/1471-2164-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venken KJ, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods. 2011;8:737–743. doi: 10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Port F, Chen HM, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl Acad. Sci. USA. 2014;111:E2967–2976. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarov, M. et al. A genome-wide resource for the analysis of protein localisation in Drosophila. Elife5, e12068 (2016). [DOI] [PMC free article] [PubMed]
- 49.Schuldiner O, et al. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev. Cell. 2008;14:227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muha V, Muller HA. Functions and mechanisms of fibroblast growth factor (FGF) signalling in Drosophila melanogaster. Int. J. Mol. Sci. 2013;14:5920–5937. doi: 10.3390/ijms14035920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michelson AM, Gisselbrecht S, Buff E, Skeath JB. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development. 1998;125:4379–4389. doi: 10.1242/dev.125.22.4379. [DOI] [PubMed] [Google Scholar]
- 52.Beckervordersandforth RM, Rickert C, Altenhein B, Technau GM. Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech. Dev. 2008;125:542–557. doi: 10.1016/j.mod.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Omoto, J. J., Yogi, P. &Hartenstein, V. Origin and development of neuropil glia of the drosophila larval and adult brain: two distinct glial populations derived from separate progenitors. Dev. Biol. 404, 2–20 (2015). [DOI] [PMC free article] [PubMed]
- 54.de Wit J, Hong W, Luo L, Ghosh A. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu. Rev. Cell Dev. Biol. 2011;27:697–729. doi: 10.1146/annurev-cellbio-092910-154111. [DOI] [PubMed] [Google Scholar]
- 55.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 2001;11:725–732. doi: 10.1016/S0959-440X(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 56.Ng AC, et al. Human leucine-rich repeat proteins: a genome-wide bioinformatic categorization and functional analysis in innate immunity. Proc. Natl Acad. Sci. USA. 2011;108:4631–4638. doi: 10.1073/pnas.1000093107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lam SD, et al. Gene3D: expanding the utility of domain assignments. Nucleic Acids Res. 2016;44:D404–409. doi: 10.1093/nar/gkv1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soto F, Watkins KL, Johnson RE, Schottler F, Kerschensteiner D. NGL-2 regulates pathway-specific neurite growth and lamination, synapse formation, and signal transmission in the retina. J. Neurosci. 2013;33:11949–11959. doi: 10.1523/JNEUROSCI.1521-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li P, Xu G, Li G, Wu M. Function and mechanism of tumor suppressor gene LRRC4/NGL-2. Mol. Cancer. 2014;13 doi: 10.1186/1476-4598-13-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J. Biol. Chem. 2010;285:13966–13978. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang KX, Tan L, Pellegrini M, Zipursky SL, McEwen JM. Rapid changes in the translatome during the conversion of growth cones to synaptic terminals. Cell Rep. 2016;14:1258–1271. doi: 10.1016/j.celrep.2015.12.102. [DOI] [PubMed] [Google Scholar]
- 62.Meinertzhagen, I. A.&Hanson, T. E. in The Development of Drosophila Melanogaster (eds Bate, M. & Martinez Arias, A.) 1363–1491 (Cold Spring Harbor Laboratory Press, 1993).
- 63.Hasegawa E, et al. Concentric zones, cell migration and neuronal circuits in the Drosophila visual center. Development. 2011;138:983–993. doi: 10.1242/dev.058370. [DOI] [PubMed] [Google Scholar]
- 64.Cruz J, Bota-Rabassedas N, Franch-Marro X. FGF coordinates air sac development by activation of the EGF ligand Vein through the transcription factor PntP2. Sci. Rep. 2015;5 doi: 10.1038/srep17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 66.Kurusu M, et al. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron. 2008;59:972–985. doi: 10.1016/j.neuron.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogel C, Teichmann SA, Chothia C. The immunoglobulin superfamily in Drosophila melanogaster and Caenorhabditis elegans and the evolution of complexity. Development. 2003;130:6317–6328. doi: 10.1242/dev.00848. [DOI] [PubMed] [Google Scholar]
- 68.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berger C, Renner S, Luer K, Technau GM. The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev. Dyn. 2007;236:3562–3568. doi: 10.1002/dvdy.21372. [DOI] [PubMed] [Google Scholar]
- 70.Baena-Lopez LA, Alexandre C, Mitchell A, Pasakarnis L, Vincent JP. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development. 2013;140:4818–4825. doi: 10.1242/dev.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Image data sets generated and analyzed in this study are available from the corresponding author on reasonable request. A source data file for quantifications shown in Figs. 2, 4–8, and Supplementary Fig. 7 is provided with the paper (Supplementary Data 1).