Abstract
Esophagolymphadenectomy is the cornerstone of multimodality treatment for resectable esophageal cancer. The preferred surgical approach is transthoracic, with a two-field lymph node dissection and gastric conduit reconstruction. A minimally invasive approach has been shown to reduce postoperative complications and increase quality of life. Robot-assisted minimally invasive esophagectomy (RAMIE) was developed to facilitate this complex thoracoscopic procedure. RAMIE has been shown to be safe with good oncologic results and reduced morbidity. The use of RAMIE opens new indications for curative surgery in patients with T4b tumors, high mediastinal tumors, and lymph node metastases after neoadjuvant treatment.
 Video online
Video online
The online version of this article (doi:10.1007/s00104-016-0200-7) contains a video, which is available to authorized users.
Keywords: Esophageal cancer; Esophagectomy; Multimodal treatment; Surgery, robot-assisted; Thoracoscopy
Zusammenfassung
Die Ösophagolymphadenektomie ist der Eckpfeiler der multimodalen Behandlung des resektablen Ösophaguskarzinoms. Der chirurgische Zugang erfolgt bevorzugt transthorakal, mit 2-Feld-Lymphadenektomie und Rekonstruktion durch Magenhochzug. Für einen minimal-invasiven Ansatz wurde gezeigt, dass er die postoperativen Komplikationen reduziert und die Lebensqualität erhöht. Die roboterassistierte minimal-invasive Ösophagektomie (RAMIE) wurde mit dem Ziel entwickelt, dieses komplexe thorakoskopische Verfahren zu erleichtern. Die Sicherheit der RAMIE ist belegt, die onkologischen Ergebnisse sind gut, die Morbidität wird verringert. Die Anwendung eröffnet neue Indikationen für die kurative chirurgische Behandlung von Patienten mit T4b-Tumoren, Tumoren im oberen Mediastinum und Lymphknotenmetastasen nach neoadjuvanter Therapie.
Video online
The online version of this article (doi:10.1007/s00104-016-0200-7) contains a video, which is available to authorized users.
Background
Annually, 482,300 patients are diagnosed with esophageal cancer worldwide, and 406,800 patients die of this disease [1]. Esophagolymphadenectomy is the cornerstone of multimodality treatment for locally advanced esophageal cancer [2, 3]. The preferred curative treatment for esophageal cancer is transthoracic esophagectomy with a two-field lymph node dissection and gastric conduit reconstruction [4]. This procedure allows for en bloc resection of the esophagus and extensive mediastinal lymphadenectomy [5].
Minimally invasive surgery was introduced to reduce surgical trauma and postoperative morbidity. Minimally invasive esophagectomy (MIE) has several advantages over open esophagectomy, such as diminished blood loss, reduced morbidity, and shorter hospital stay [6, 7]. However, conventional thoracolaparoscopic techniques are limited by a two-dimensional view, a disturbed eye–hand coordination, and a reduced freedom of movement. These technical limitations may compromise the feasibility of MIE and its worldwide adaptation [8–10].
In 2003, the robot-assisted thoracolaparoscopic approach was developed at the University Medical Center Utrecht (UMC Utrecht) in The Netherlands [10]. Robot-assisted minimally invasive esophagectomy (RAMIE) reduces the limitations of thoracoscopic esophagectomy by offering a stable three-dimensional (3D) view, 10-times-enlarged image, restored eye–hand axis, and excellent dexterity with the endowristed instruments. In this article we present our technique, give suggestions for successful implementation, discuss recent developments, and look to the future directions of RAMIE.
RAMIE at UMC Utrecht
Preparation
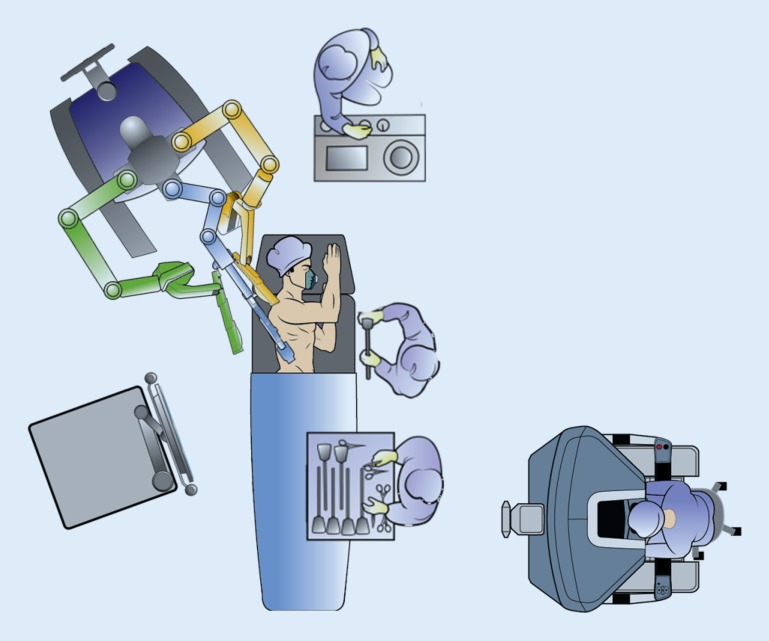
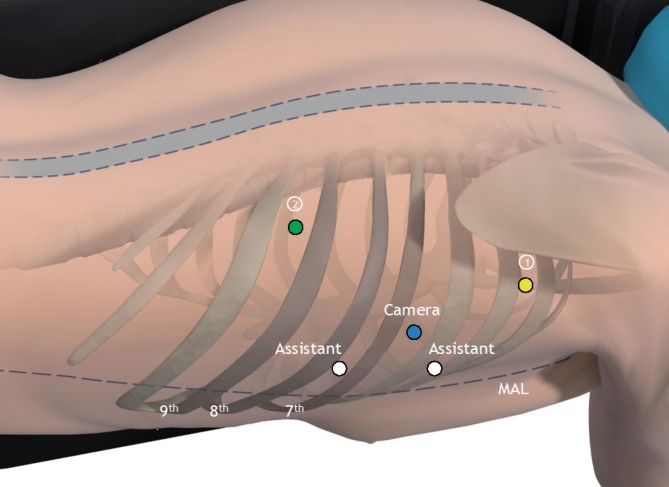
General and thoracic epidural anesthetics are combined to ensure sufficient intraoperative and postoperative analgesia. Recently we started using single-dose and bilateral paravertebral block combined with sufentanil in the context of our enhanced recovery after esophagectomy program. This may provide similar postoperative analgesia and allow for early discharge avoiding the disadvantages of epidural anesthesia such as catheter malposition and hypotension [11]. The patient is intubated with a left-side double-lumen tube and is positioned in the left lateral decubitus position, tilted 45° to the prone position. The robotic system (da Vinci Si System, Intuitive Surgical Inc., Sunnyvale, Calif.) is positioned on the dorsocranial side of the patient (Fig. 1). Three ports are placed for the robotic system as well as two ports for the assisting surgeon (Fig. 2), whereafter the right lung is desufflated. Through one of the assistant ports, CO2 at 6 mmHg is insufflated to keep the lung out of the operative field.
Fig. 1.
Operating room set-up. (© 2014 Intuitive Surgical Inc., used with permission)
Fig. 2.
Trocar placement in the thoracic phase. Robotic arms 1 (yellow) and 2 (green), camera (blue), and two assisting ports (white). MAL (midaxillary line) (©2014 Intuitive Surgical Inc., used with permission)
Surgical procedure
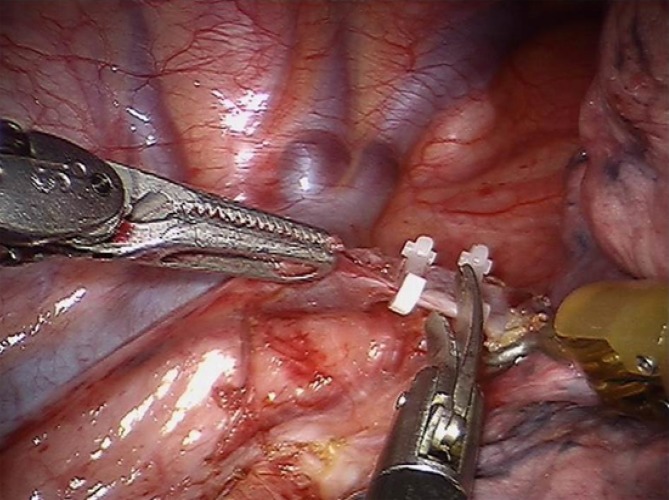
The right pulmonary ligament is divided and the parietal pleura is dissected at the anterior side of the esophagus from the diaphragm up to the level of the azygos arch. The azygos arch is ligated with robotic Hem-o-lok clips (Teleflex Medical, Durham, N.C.) and divided. These clips are endowristed facilitating precise positioning (Fig. 3). To establish dissection of the right paratracheal lymph nodes, dissection of the parietal pleura is continued above the azygos arch. The right vagal nerve is dissected below the level of the carina to preserve its pulmonary branches [12, 13]. Dissection of the parietal pleura is continued from cranially to caudally along the azygos vein at the posterior side of the esophagus. Paratracheally left, the left recurrent nerve is identified and carefully protected. At the level of the diaphragm, the thoracic duct is clipped with the robotic Hem-o-lok clips to prevent leakage. At this level, a Penrose drain is placed around the esophagus to facilitate traction and esophageal mobilization. The esophagus is then resected from the diaphragm up to the thoracic inlet en bloc with the surrounding mediastinal and subcarinal lymph nodes and the thoracic duct. The aortoesophageal arteries are identified and clipped.
Fig. 3.
Clipping and dissection of the azygos vein. (From [30])
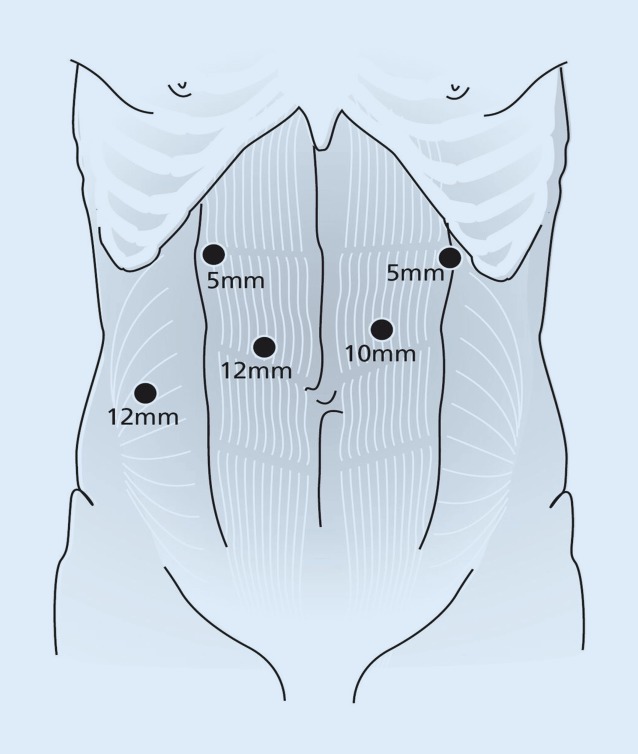
The patient is put in supine position for the abdominal phase. After introduction of the five trocars (Fig. 4), the hepatogastric ligament is opened and transection of the lesser omentum is continued until the left crus of the diaphragm. Hereafter, the greater gastric curvature is dissected using a harmonic ace (Ethicon, Cincinnati, Ohio). Abdominal lymphadenectomy is performed including lymph nodes surrounding the celiac trunk, along the left gastric and splenic arteries and the lesser omental lymph nodes. The left gastric artery is ligated with Hem-o-lok and transected at its origin. Through a left-sided vertical incision along the sternocleidomastoid muscle, the cervical phase of the esophagectomy is initiated to facilitate mobilization of the cervical esophagus. No formal cervical lymph node dissection is carried out. The cervical esophagus is transected and a cord is attached to the specimen. The dissected esophagus with the surrounding lymph nodes en bloc are pulled down through the mediastinum under laparoscopic view. Hereafter, the left para-umbilical trocar port is widened to a 5–7-cm transverse incision. The resection specimen is removed through this incision with a wound drape (3M, St. Paul, Minn.) to create the gastric conduit extracorporeally. A linear stapler (GIATM 80, 3.8 mm; Medtronic, Minneapolis, Minn.) is used to create a 4-cm-wide gastric conduit, which is routinely oversewn with a 3/0 polydioxanone single-layer running suture. The gastric conduit is pulled up through the mediastinum along the original anatomic tract of the esophagus with the aid of a plastic tube (laparoscopic camera bag). A cervical hand-sewn end-to-side anastomosis is created between the gastric tube and the cervical esophagus using a 3/0 polydioxanone single-layer running suture. A jejunostomy feeding tube is placed in the second loop after the ligament of Treitz for postoperative feeding. The abdomen is closed in layers with a PDS loop for the fascia and skin intracutaneously with Monocryl.
Fig. 4.
Trocar placement in the abdominal phase. (From [8], by kind permission of John Wiley and Sons)
Pushing the limits of esophagectomy with RAMIE
Since the introduction of RAMIE, we have gained considerable experience with the use of the da Vinci robot in over 300 cases. After a learning curve of 120 cases, a plateau was reached with a steady level of performance. Utilizing the full capabilities of the da Vinci robot, we are currently investigating new indications in patients who were deemed inoperable with conventional surgery.
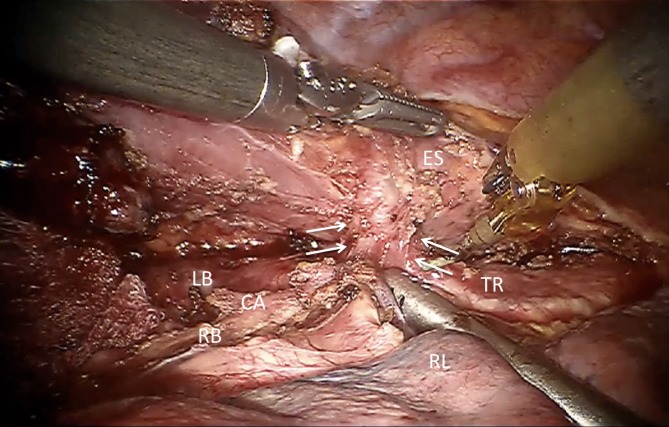
Until recently, patients with cT4b tumors were considered inoperable and guidelines recommend definitive chemoradiotherapy as the treatment of choice [14]. However, cT4 status is a negative predictor of locoregional control [15]. Definitive chemoradiotherapy is associated with a high rate of esophageal stenosis and perforation, the latter occurring in 25 % of patients, and most often these perforations are lethal. Furthermore, functional results are poor and there is frequently recurrence in up to 41 % of cases [15, 16]. Therefore, we started salvage surgery with RAMIE in patients with cT4b esophageal tumors after long-course chemoradiotherapy. The patients are restaged with positron emission tomography–computed tomography and endobronchial ultrasound to determine the actual situation, and they are selected for salvage surgery if tracheal ingrowth has been reduced (Fig. 5). The enlarged 3D image allows for very precise dissection of the irradiated tumor tissue from the trachea, bronchi, and aorta. The level of precision makes dissection in downstaged T4b tumors feasible. So far, we have treated ten patients using this strategy, leading to radical resections in 90 % of cases (unpublished data). We are awaiting the long-term oncologic and functional results with this approach before it can be recommended for all patients.
Fig. 5.
T4b esophageal tumors, tracheal involvement. ES esophagus, CA carina, LB left bronchus, RB right bronchus TR trachea, RL right lung
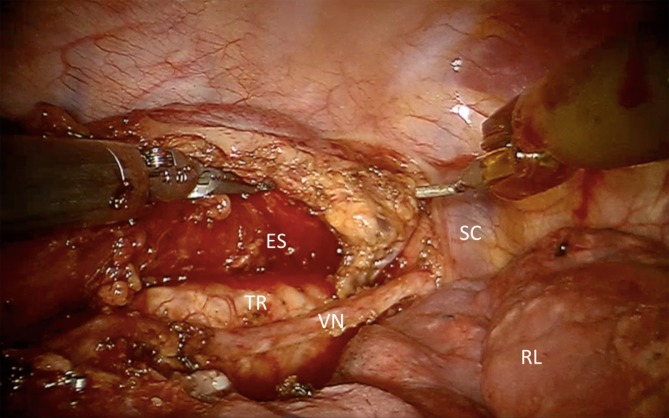
Moreover, the upper mediastinum and thoracic aperture can be reached with an excellent 3D view and magnified observation of the operative field (Fig. 6; [17]). In this way, we were able to achieve an R0 resection in 28 out of 29 patients (97 %) with upper esophageal tumors and paratracheal lymph node involvement (unpublished data).
Fig. 6.
Upper mediastinum. ES esophagus, VN vagal nerve, TR trachea, RL right lung, SC subclavian artery
Future directions include the use of the robotic platform for image-guided surgery and fluorescence detection of lymph nodes and tumor margins. The integration of optical imaging modalities that specifically visualize the areas of interest may reduce complications and improve the surgical accuracy of lymph node dissection [18].
Another application of intravascular fluorescence dye imaging is the intraoperative assessment of the vascularization of the gastric conduit. This may guide one to the optimal site of the anastomosis and thereby contribute to reducing anastomotic leakage [19].
Surgical training program
We have developed a structured training program that enables esophageal surgeons to be guided through the learning curve in 20 cases. The proctored surgeons should have basic minimally invasive skills and knowledge of esophageal surgery. The program starts with three case observations in a RAMIE expert center, followed by a basic robotic course and a cadaveric laboratory course. Thereafter, the proctor supervises the surgeon for the first three to ten cases and reviews his skills after the first 20.
Optimal performance during technically complex procedures requires a dedicated team [20]. Therefore, at least two motivated surgeons, a dedicated anesthetist, and a scrub nurse should be involved in the program. A sufficient case load (>20/year) and guaranteed access to a robotic system are of crucial importance [21, 22].
In urology, proctoring has proven to be an essential mechanism for the successful implementation of robot-assisted radical prostatectomy, and this approach is comparable in RAMIE [23].
Discussion
RAMIE offers a 3D, magnified surgical view combined with a high degree of freedom of the articulating instruments. This facilitates meticulous dissection from the diaphragm to the thoracic inlet. Especially for the thoracic phase, where the esophagus is surrounded by delicate vital structures such as the trachea, pulmonary veins, aorta, and recurrent nerves, these features are of great value. Moreover, these features allow the exploration of salvage surgery in patients with previously inoperable T4b and upper mediastinal tumors.
Robotic assistance may also facilitate the use of an intrathoracic anastomosis [24]. Until recently, we performed a three-stage esophagectomy (McKeown procedure) with a hand-sewn esophagogastric anastomosis in the left side of the neck as stated previously. However, the incidence of anastomotic leakage after cervical esophagogastrostomy remains relatively high (10–30 %) and intrathoracic manifestation occurs in more than half of patients with cervical anastomotic leakage [25, 26]. The incidence of leakage from intrathoracic anastomosis is reported to be lower [27]. Therefore, we started performing a two-stage (Ivor Lewis) procedure with a robotic hand-sewn intrathoracic anastomosis for distal esophageal tumors. Constructing an intrathoracic anastomosis during conventional thoracoscopy is technically challenging. By working in the upper thoracic aperture, the instruments have to reach deep into the thorax, imposing problems in manipulation through the fulcrum effect at the ribs [28]. The robot nullifies these problems owing to the endo-wristed intracorporeal instruments, tremor filtering, and its 3D view of the surgical field. Therefore, in our opinion the robot contributes to a high-quality intrathoracic anastomosis. In our initial experience, we have found the hand-sewn robotic technique to be very controlled and reliable. Larger numbers of patients have to be treated with this technique before the success rates and long-term results can be reported.
So far, we have chosen to perform the abdominal aspect of our procedure via a laparoscopic approach, since the da Vinci Si Surgical System (Intuitive Surgical Inc.) was not constructed to facilitate multiquadrant surgery. The new da Vinci Xi Surgical System (Intuitive Surgical Inc.) has its four arms mounted on an overhead boom enabling multiquadrant surgery without the need to redock the system. Results of the robotic abdominal phase are awaited in the near future.
Conclusion
In conclusion, robotic-assisted minimally invasive esophagectomy with two-field lymphadenectomy is an excellent minimally invasive technique for dissecting the esophagus from the mediastinum with radical lymphadenectomy. Additionally, it may provide new curative options in patients deemed inoperable with conventional surgery. Robotic assistance reduces the limitations of MIE while retaining its advantages over open esophagectomy. Therefore, we initiated a randomized controlled trial, comparing robot-assisted and open three-phase esophagectomy to measure the additional value of RAMIE over open esophagectomy [29]. Short-term results are expected in the course of 2016.
Acknowledgments
Open access funding provided by University Medical Center Utrecht.
Caption video online
Compliance with ethical guidelines
Conflict of interest
R. van Hillegersberg, M.F.J. Seesing, H.J.F. Brenkman, and J.P. Ruurda state that there are no conflicts of interest.
The accompanying manuscript does not include studies on humans or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Footnotes
The German version of this article can be found under doi: 10.1007/s00104-016-0239-5.
Video online
The online version of this article (doi:10.1007/s00104-016-0200-7) contains a video, which is available to authorized users.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246(6):992–1001. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 3.Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 4.Haverkamp L, Seesing MFJ, Ruurda JP, Boone J, van Hillegersberg R. Dis Esophagus. 2016 [Google Scholar]
- 5.Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347(21):1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 6.Biere SS, Maas KW, Bonavina L, Garcia JR, van Berge Henegouwen MI, Rosman C, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial) BMC Surg. 2011;11(1):2. doi: 10.1186/1471-2482-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhage RJ, Hazebroek EJ, Boone J, van Hillegersberg R. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir. 2009;64(2):135–146. [PubMed] [Google Scholar]
- 8.Ruurda JP, van der Sluis PC, van der Horst S, van Hillegersberg R. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol. 2015;112(3):257–265. doi: 10.1002/jso.23922. [DOI] [PubMed] [Google Scholar]
- 9.Camarillo DB, Krummel TM, Salisbury JK., Jr. Robotic technology in surgery: past, present, and future. Am J Surg. 2004;188(4A Suppl):2S–15S. doi: 10.1016/j.amjsurg.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 10.van Hillegersberg R, Boone J, Draaisma WA, Broeders IA, Giezeman MJ, Borel Rinkes IH. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc. 2006;20(9):1435–1439. doi: 10.1007/s00464-005-0674-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Fang C, Li J, Geng QT, Wang S, Kang F, et al. Single-dose, bilateral paravertebral block plus intravenous sufentanil analgesia in patients with esophageal cancer undergoing combined thoracoscopic-laparoscopic esophagectomy: a safe and effective alternative. J Cardiothorac Vasc Anesth. 2014;28(4):966–972. doi: 10.1053/j.jvca.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Weijs TJ, Ruurda JP, Luyer MD, Nieuwenhuijzen GA, van Hillegersberg R, Bleys RL. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J Anat. 2015;227(4):431–439. doi: 10.1111/joa.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weijs TJ, Ruurda JP, Luyer MD, Nieuwenhuijzen GA, van der Horst S, Bleys RL, et al. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy. Surg Endosc. 2015 doi: 10.1007/s00464-015-4683-y. [DOI] [PubMed] [Google Scholar]
- 14.Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(2):194–227. doi: 10.6004/jnccn.2015.0028. [DOI] [PubMed] [Google Scholar]
- 15.Versteijne E, van Laarhoven HW, van Hooft JE, van Os RM, Geijsen ED, van Berge Henegouwen MI, et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus. 2015;28(5):453–459. doi: 10.1111/dote.12215. [DOI] [PubMed] [Google Scholar]
- 16.Gkika E, Gauler T, Eberhardt W, Stahl M, Stuschke M, Pöttgen CI. Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis Esophagus. 2014;27(7):678–684. doi: 10.1111/dote.12146. [DOI] [PubMed] [Google Scholar]
- 17.Suda K, Ishida Y, Kawamura Y, Inaba K, Kanaya S, Teramukai S, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg. 2012;36(7):1608–1616. doi: 10.1007/s00268-012-1538-8. [DOI] [PubMed] [Google Scholar]
- 18.van der Poel HG, Buckle T, Brouwer OR, Valdés Olmos RA, van Leeuwen FW. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur Urol. 2011;60(4):826–833. doi: 10.1016/j.eururo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Zehetner J, DeMeester SR, Alicuben ET, Oh DS, Lipham JC, Hagen JA, et al. Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after esophagectomy. Ann Surg. 2015;262(1):74–78. doi: 10.1097/SLA.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCulloch P, Morgan L, New S, Catchpole K, Roberston E, Hadi M, et al. Combining systems and teamwork approaches to enhance the effectiveness of safety improvement interventions in surgery: the safer delivery of surgical services (S3) program. Ann Surg. 2015;10(9):e0138490. doi: 10.1097/SLA.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 21.Good DW, Stewart GD, Laird A, Stolzenburg JU, Cahill D, McNeill SA. A critical analysis of the learning curve and postlearning curve outcomes of two experience- and volume-matched surgeons for laparoscopic and robot-assisted radical prostatectomy. J Endourol. 2015;29(8):939–947. doi: 10.1089/end.2014.0810. [DOI] [PubMed] [Google Scholar]
- 22.Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut. 2014;63(9):1393–1400. doi: 10.1136/gutjnl-2013-306074. [DOI] [PubMed] [Google Scholar]
- 23.Zorn KC, Gautam G, Shalhav AL, Clayman RV, Ahlering TE, Albala DM, et al. Training, credentialing, proctoring and medicolegal risks of robotic urological surgery: recommendations of the society of urologic robotic surgeons. J Urol. 2009;182(3):1126–1132. doi: 10.1016/j.juro.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg. 2013;145(1):90–96. doi: 10.1016/j.jtcvs.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 25.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 26.van Rossum PS, Haverkamp L, Carvello M, Ruurda JP, van Hillegersberg R. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus. 2016 doi: 10.1111/dote.12472. [DOI] [PubMed] [Google Scholar]
- 27.Biere SS, Maas KW, Cuesta MA, van der Peet DL. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg. 2011;28(1):29–35. doi: 10.1159/000322014. [DOI] [PubMed] [Google Scholar]
- 28.Ruurda JP, Draaisma WA, van Hillegersberg R, Borel Rinkes IH, Gooszen HG, Janssen LW, et al. Robot-assisted endoscopic surgery: a four-year single-center experience. Dig Surg. 2005;22(5):313–320. doi: 10.1159/000088628. [DOI] [PubMed] [Google Scholar]
- 29.van der Sluis PC, Ruurda JP, van der Horst S, Verhage RJ, Besselink MG, Prins MJ, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial) Trials. 2012;13(1):230. doi: 10.1186/1745-6215-13-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Hillegersberg R. Esophagectomy for Cancer. In: Spinoglio G, editor. Robotic Surgery. Current Applications and New Trends. Mailand: Springer; 2015. [Google Scholar]